Abstract
Background. Pyrophosphate (PPi) is a potent inhibitor of vascular calcification and may be deficient in renal failure. We sought to determine whether plasma PPi is affected by dialysis or the mode of dialysis and whether it correlates with vascular calcification.
Methods. PPi was measured in plasma samples stored from a recent study of vascular calcification in 54 HD patients, 23 peritoneal dialysis (PD) patients and 38 patients with stage 4 chronic kidney disease (CKD). Calcification was quantified in a standardized section of the superficial femoral artery using computed tomography, and PPi was measured by enzyme assay, at both baseline and 1 year.
Results. Baseline plasma PPi was weakly correlated with age and serum phosphate, but not with alkaline phosphatase activity or other biochemical parameters, and did not differ between HD, PD and CKD patients. Both baseline calcification score and change in the calcification score at 1 year decreased with increasing quartiles of plasma PPi. In a multivariate analysis, plasma PPi was independently correlated with baseline calcification (P = 0.039) and the change in calcification (P = 0.029).
Conclusion. Plasma PPi is negatively associated with vascular calcification in end-stage renal disease (ESRD) and CKD but is not affected by dialysis, the mode of dialysis or nutritional or inflammatory status. Although these data are consistent with an inhibitory effect of PPi on vascular calcification, further studies are needed to establish a causal role.
Keywords: chronic kidney disease, end-stage renal disease, haemodialysis
Introduction
Vascular calcification is common in patients with advanced kidney disease and is thought to increase their risk of cardiovascular death [1,2]. Arterial calcification comprises two anatomically and pathophysiologically distinct disorders: medial calcification (also known as Monckeberg's arteriosclerosis) and intimal (or atherosclerotic) calcification. While both occur in patients with chronic kidney disease (CKD), the incidence of medial calcification is markedly increased in renal failure. In the absence of renal disease, medial calcification only occurs in diabetes, rare genetic disorders and with advanced ageing. Although circulating calcium and phosphate concentrations have correlated with the progression of vascular calcification in longitudinal studies of dialysis patients [3–6], they only partly account for the calcification. It is clear then that vascular calcification is controlled by factors other than circulating calcium and phosphate levels.
A large body of data indicate that medial vascular calcification is a thermodynamically favoured process under normal conditions that is actively inhibited by vascular smooth muscle [7]. This tissue produces a number of inhibitors of calcification, genetic deficiency of which produces extensive medial calcification in animals and humans [8–10]. This suggests that uraemic vascular calcification must be associated with a deficiency of one or more of these inhibitors. One such inhibitor is pyrophosphate (PPi), which directly blocks hydroxyapatite formation [11]. We have previously shown that PPi is produced by arterial smooth muscle and prevents calcification of rat aortas in culture [12]. The absence of the ectoenzyme that synthesizes extracellular PPi results in massive arterial calcification and very early death in humans [10]. PPi is hydrolyzed by alkaline phosphatase and addition of this enzyme to aortas in vitro induces calcification [12]. Furthermore, we have shown that alkaline phosphatase is upregulated in the vascular smooth muscle of rats with renal failure [13], suggesting that deficiency of PPi in the arterial wall may contribute to vascular calcification in advanced kidney disease.
The role of PPi in uraemic vascular calcification is unknown because of the difficulty in measuring PPi and the uncertain correlation between circulating levels and levels in the vascular wall. The assay involves several enzymes and must be performed in plasma since platelets release PPi. An initial study showed that PPi is removed by haemodialysis and that plasma PPi levels may be reduced in haemodialysis patients [14]. Whether plasma PPi levels are affected by dialysis or by renal failure and are related to vascular calcification remains unknown. We tested the hypothesis that plasma PPi levels are inversely related to vascular calcification in a previously described cohort of patients with advanced kidney disease undergoing haemodialysis, peritoneal dialysis or no dialysis [15]. In addition, we sought to determine the factors that affect PPi levels in these patients.
Subjects and methods
Subjects
One hundred and thirty-four patients (60 HD, 28 PD and 46 CKD stage 4) were recruited from Derby City General Hospital. The patients were eligible unless they had previously undergone renal transplantation or limb amputation. The CKD stage 4 patients were staged on the four-variable MDRD estimated glomerular filtration rate (eGFR) with values of 15–29 ml/min/1.73 m2. The Local Regional Ethics Committee granted project approval, and written consent was obtained from the participants. Control subjects were recruited from the faculty and staff of the Renal Division at Emory University.
Measurement of vascular calcification
Multi-slice computerized tomography (MSCT) and measurement of haemodynamic variables were performed at baseline, 12 months and 24 months by a single observer. Baseline data have been previously published with a full description of the methodology [15]. Briefly, MSCT was used to quantify calcification in a standardized section of the superficial femoral artery (SFA). Each slice was scored individually and a calcification score (CaSc) was generated. Calcification was considered to be present if an area ≥1 mm2 displayed a density >130 HF units [16]. Validation studies confirmed that the scoring technique is highly reproducible. Interobserver reproducibility between the investigator and a consultant radiologist was assessed in a 1 in 20 sample. The intraclass correlation was 1 (confidence interval 1–1) and the co-efficient of variation (CoV) was 3.9%. Repeatedly scored scans showed an intraobserver intraclass correlation of 1 (CI 1–1) and a CoV of 2.4%.
Biochemical studies
Monthly blood samples were collected for HD patients and at regular clinic visits for PD and CKD stage 4 patients. All biochemical measurements were performed in the same clinical laboratory. A time-averaged value was given for baseline (results averaged over the 6 months prior to the study), and for 12-month results (values averaged over the 12-month period between study visits). Single samples of plasma were obtained at baseline and 12 months. Although this was a 2-year study, plasma samples were not obtained at 24 months.
Measurement of plasma PPi
PPi was measured enzymatically using uridine-diphosphoglucose (UDPG) pyrophosphorylase as previously described [14] but with modifications. For each plasma sample, a blank was prepared by incubating 20 μl with 0.175 units of inorganic pyrophosphatase (from baker's yeast) for 1 h at 37°C. The blank and an equal volume of untreated plasma were then incubated with 100 μl of an assay solution containing 90 mM KCl, 5 mM MgCl2, 70 mM Tris–HCl (pH 7.60), 20 μM NADPH, 8 μM UDPG, 0.5 units/ml UDPG pyrophosphorylase (type X from baker's yeast), 3.7 units/ml phosphoglucomutase (from rabbit muscle), 0.1 units/ml glucose-6-phosphate dehydrogenase (type XV from baker's yeast) and 0.15 μCi/ml [14C]UDPG. The AMP was added to reduce background hydrolysis of UDPG, presumably by 5′-nucleotidase. After 30 min at 37°C, 200 μl of 3% activated charcoal was added on ice with occasional stirring to bind residual UDPG. After centrifugation, the radioactivity in 200 μl of supernatant was counted. For each sample, the blank was subtracted to obtain the true PPi concentration.
Statistical analysis
Results were reported as mean ± SD for normally distributed data. Comparisons of paired data were performed using paired sample t-tests for parametric data and Wilcoxon matched-pair test for non-parametric data. Mann–Whitney U-tests were used to compare skewed variables between two groups and Kruskal–Wallis tests for more than two groups. Chi-squared tests were used to compare categorical data. Multiple regression was performed using a linear model with backward stepwise subtraction using a P-value of 0.1 for removal. Mean values were substituted for missing values. Missing values never comprised more than 5% of the total. Testing was performed with the SPSS Version 17 software (SPSS Inc., Chicago, IL, USA).
Results
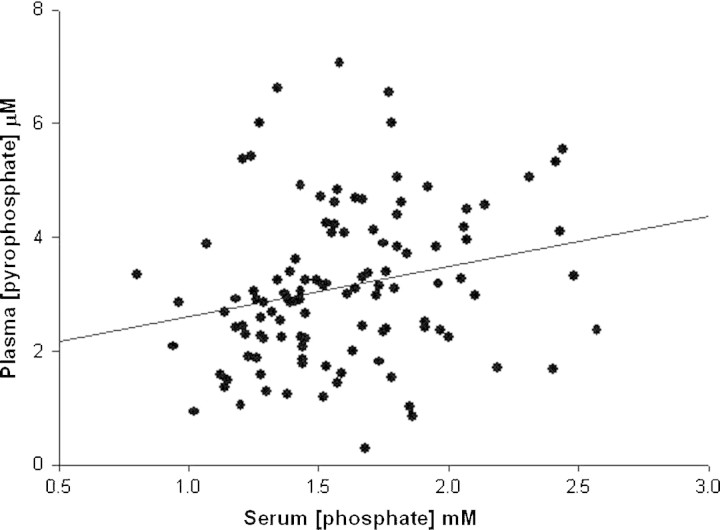
Plasma PPi values and calcification scores were available from 115 patients at baseline. The characteristics of these patients are shown in Table 1 and the biochemical values are shown in Table 2. Plasma PPi did not differ between HD, PD and CKD patients and was unrelated to duration of dialysis. The plasma level of PPi in 22 normal subjects was 2.63 ± 0.47 μM. There were weak but significant correlations between plasma PPi and serum phosphate (r = 0.21, P < 0.05; Figure 1) or age (r = 0.21; P < 0.05) in the patients but no correlation between serum alkaline phosphatase activity, albumin or high-sensitivity C-reactive protein (CRP). In a multivariate regression, only serum phosphate was significantly correlated with plasma PPi.
Table 1.
Characteristics of the patients at baseline
| CKD stage | PD, | HD, | |
|---|---|---|---|
| 4, n = 38 | n = 23 | n = 54 | |
| Age (years) | 62 ± 15 | 60 ± 15 | 61 ± 15 |
| Male gender | 24 (63%) | 14 (61%) | 38 (72%) |
| Dialysis duration (months) | 32 ± 22 | 38 ± 25 | |
| Diabetes mellitus | 8 (20%) | 7 (30%) | 14 (26%) |
| Number of patients calcified | 20 (51%) | 16 (70%) | 40 (75%) |
| Vitamin D | 16 (41%) | 13 (57%) | 33 (62%) |
| Non-calcium-based phosphate binders | 4 (10%) | 10 (43%) | 14 (26%) |
| Calcium-based phosphate bindersa | 11 (28%) | 12 (52%) | 37 (70%) |
| Dose of calcium (g/day) | 0.7 ± 1.0 | 1.1 ± 1.2 | 1.2 ± 1.5 |
aSome patients were prescribed both calcium-containing and non-calcium-containing phosphate binders.
Table 2.
Biochemical parameters at baseline
| CKD | PD | HD | |
|---|---|---|---|
| Serum Ca (mM) | 2.3 ± 0.1* | 2.5 ± 0.1 | 2.5 ± 0.1 |
| Serum P (mM) | 1.4 ± 0.27** | 1.6 ± 0.2 | 1.7 ± 0.4 |
| Serum Alk Phos (U/l) | 85 ± 37 | 118 ± 75 | 102 ± 51 |
| Serum PTH (pg/ml) | 195 ± 107 | 295 ± 291 | 291 ± 280 |
| Plasma PPi (μM) | 3.1 ± 1.1 | 3.3 ± 1.4 | 3.0 ± 1.1 |
Biochemical results except for PPi are time averaged. Results are expressed as mean ± SD.
*P < 0.001, **P = 0.005 versus other groups.
Fig. 1.
Correlation between serum phosphorus and plasma pyrophosphate at baseline. The correlation coefficient is 0.23, P < 0.02.
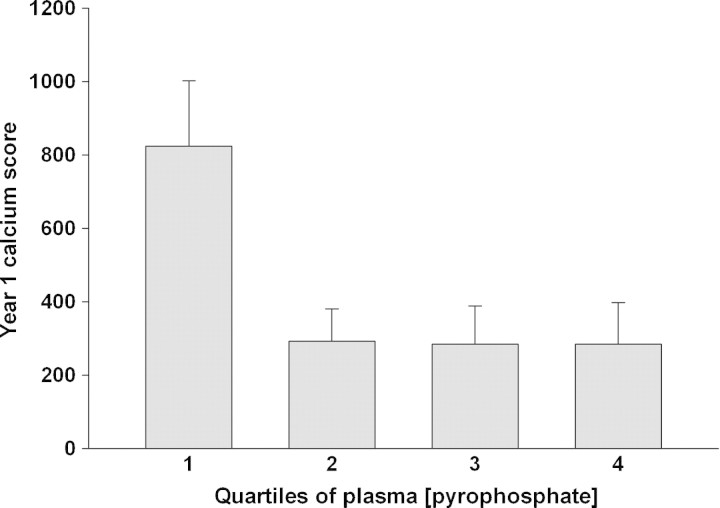
In HD (74%) and PD (70%) patients, the incidence of vascular calcification was similar and greater than in the CKD patients (50%). The calcification scores are given in Table 3. HD patients had higher calcification scores at baseline than the other groups. Although progression was also greater, this was not statistically significant. Because of the skewed distribution of the calcification scores, the relationship between plasma PPi and calcification score was analysed by quartile of plasma PPi rather than by linear regression. As shown in Figure 2, scores were greatest in the lowest quartile of plasma PPi and decreased with increasing quartiles, but the differences did not reach statistical significance. In the 91 patients in whom plasma PPi and vascular calcification were measured at 1 year, the calcification score was also highest in the lower quartile of plasma PPi and this difference was significant (Figure 3). In a multivariate regression model that included age, dialysis mode, diabetic status, dose of active vitamin D, calcium-based phosphate binder, and serum calcium, phosphorus, parathyroid hormone, albumin, and high-sensitivity CRP, baseline plasma PPi quartile was independently associated with the baseline calcification score along with haemodialysis, age and serum phosphorus (Table 4). There was no difference in PPi levels between patients with and without vascular calcification at baseline.
Table 3.
Calcification scores
| CKD | PD | HD | |
|---|---|---|---|
| Median baseline CaSc | 1 (0–131) | 22 (0–390) | 183 (0.5–646)* |
| Median 12-month CaSc | 7 (0–254) | 37 (2–512) | 267 (0–876) |
| Median change in CaSc | 1 (0–92) | 6 (0–122) | 30 (0–216) |
Values in parentheses are the interquartile range. *P = 0.005 versus other groups.
Fig. 2.
Mean baseline vascular calcification score in increasing quartiles of baseline plasma pyrophosphate. The mean PPi levels in each quartile were 1.49, 2.60, 3.34 and 5.04 μM. Error bars indicate standard error.
Fig. 3.
Mean 1-year vascular calcification score in increasing quartiles of 1-year plasma pyrophosphate. Error bars indicate standard error. P = 0.005 by the Kruskal–Wallis test.
Table 4.
Variables remaining as significant correlates of the baseline calcification score in a multivariate regression with backward elimination
| Parameter | B coefficient | P-value |
|---|---|---|
| Haemodialysis | 304 ± 84 | <0.001 |
| Age | 7.5 ± 3.0 | 0.015 |
| Plasma pyrophosphate | −80 ± 38 | 0.039 |
| Serum phosphorus | 270 ± 132 | 0.044 |
The R2 for the model was 0.249.
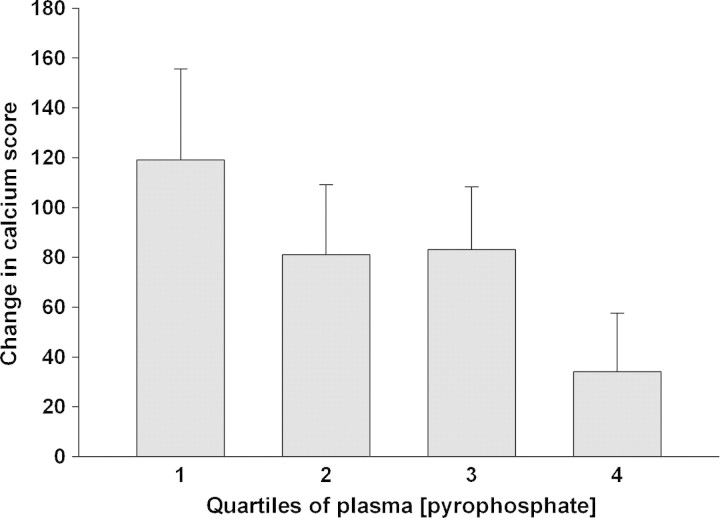
Repeat calcification scores were obtained in 100 patients after 12 months, and the change in calcification score ranged from a decrease of 236 to an increase of 670. The change was greatest in the lowest quartile and lowest in the highest quartile of baseline plasma PPi (Figure 4), but the differences did not reach statistical significance. In a multivariate linear regression model that included the same parameters as above, baseline PPi quartile was independently associated with the change in calcification score along with age and serum phosphorus (Table 5).
Fig. 4.
Mean change in vascular calcification score in increasing quartiles of baseline plasma pyrophosphate. Error bars indicate standard error.
Table 5.
Variables remaining as significant correlates of the change in the calcification score in a multivariate regression with backward elimination
| Parameter | B coefficient | P-value |
|---|---|---|
| Serum phosphorus | 146 ± 42 | 0.001 |
| Age | 3.2 ± 1.0 | 0.002 |
| Plasma pyrophosphate | −28 ± 13 | 0.029 |
The R2 for the model was 0.179.
Discussion
This study is the first comparison of circulating PPi levels and vascular calcification in any group of patients. Both baseline calcification and change in calcification were independently and inversely correlated with plasma PPi, consistent with an inhibitory effect of PPi. The fact that a separate set of measurements obtained at 1 year showed higher calcification scores in the lowest quartile of plasma PPi further supports the link between vascular calcification and PPi. However, the effect of plasma PPi was small, consistent with a multifactorial aetiology of uraemic vascular calcification. The weakness of the relationship may also be explained by the fact that plasma PPi levels may not correlate well with levels in the vascular wall. Furthermore, the technique used to quantitate vascular calcification cannot distinguish between intimal and medial calcification. Whereas PPi clearly inhibits medial calcification, its effect on intimal calcification is unknown. Thus, it is possible that atherosclerosis and intimal calcification could be obscuring the correlation between plasma PPi and medial calcification. Lastly, the plasma PPi assay is a difficult assay subject to large background values (necessitating measurements before and after adding pyrophosphatase) and release of PPi from platelets during and after venotomy.
Plasma PPi did not differ between HD, PD or CKD patients, indicating that it is not influenced by dialysis or mode of dialysis. The values were also slightly higher than those in the normal subjects. Using an earlier version of the PPi assay, we previously reported reduced plasma PPi levels in haemodialysis patients, due primarily to a group of patients with very low levels [14]. However, these dialysis patients were from different location than the current study (USA versus UK) and may well differ, particularly in the subpopulation with very low PPi levels. Interpretation of plasma PPi levels in renal failure is complicated by the relationship with phosphate levels previously reported in haemodialysis patients [14] and in normal subjects [17] and confirmed here. Thus, although the absolute value of plasma PPi may not be reduced in renal failure, it may be reduced relative to the circulating phosphate levels. The basis of the relationship between circulating PPi and phosphate levels is unknown but could be related to inhibition of alkaline phosphatase by phosphate [18]. In the current study, the correlation between plasma PPi and the calcification score was not improved by normalizing plasma PPi to serum phosphorus (not shown).
Factors that control tissue or circulating levels of PPi in renal failure are not understood but could include synthesis, hydrolysis and dialytic clearance. In our previous study, we demonstrated removal of PPi by haemodialysis with an associated decrease in the plasma level [14]. While this could influence calcification during dialysis and in the immediate postdialysis period, it cannot explain the reduced predialysis levels since the plasma levels in this study did not differ between dialysis patients and nondialysis patients. The greater degree of calcification in the haemodialysis patients therefore cannot be explained by lower PPi levels, indicating that other factors are involved. Synthesis of PPi has not been studied in vivo because it is difficult to measure. Hydrolysis of PPi into orthophosphate by tissue nonspecific alkaline phosphatase (TNAP) is an important determinant of PPi levels, as evidenced by the fact that humans lacking this enzyme (hypophosphatasia) have increased plasma levels [19]. Our recent data showing upregulation of TNAP in uraemic aortas with resulting increased hydrolysis of PPi [13] support this as a mechanism for PPi deficiency in renal failure. However, there was no correlation between serum alkaline phosphatase activity and plasma PPi levels in the current study. This is probably explained by the fact that circulating TNAP is derived primarily from bone and may not reflect levels in other tissues. TNAP is an ectoenzyme bound to the plasma membrane and the circulating form is probably released from cells during apoptosis [20].
While the correlation between plasma PPi and vascular calcification in this study suggests a role for PPi in uraemic vascular calcification, there may be other explanations. In particular, PPi binds avidly to hydroxyapatite, so that the reduced plasma levels may be a result of PPi sequestration by calcified vessels. However, the substantially greater amounts of hydroxyapatite in bone make it unlikely that changes in vascular hydroxyapatite could affect systemic PPi levels. Definitive demonstration of a role for PPi in uraemic vascular calcification will require interventions to alter circulating or tissue levels.
Conflict of interest statement. Dr O’Neill is a co-inventor of a pending patent owned by Emory University related to research described in this paper.
(See related article by M. Ketteler and P. H. Biggar. What dishwashers and humans have in common. Nephrol Dial Transplant 2010; 25: 4–6.)
References
- 1.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 2.Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in CKD 4 and 5 patients. Clin J Am.Soc.Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Raggi P, Chasan-Taber S, et al. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–1496. doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- 6.Stompor T, Pasowicz M, Sulowicz W, et al. Trends and dynamics of changes in calcification score over the 1-year observation period in patients on peritoneal dialysis. Am J Kidney Dis. 2004;44:517–528. [PubMed] [Google Scholar]
- 7.O’Neill WC. The fallacy of the calcium phosphorus product. Kidney Int. 2007;72:792–796. doi: 10.1038/sj.ki.5002412. [DOI] [PubMed] [Google Scholar]
- 8.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix Gla protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 9.Bucay N, Sarosi I, Dunstan C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutsch F, Vaingankar S, Johnson K, et al. PC-1 Nucleotide triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 12.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O’Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 13.Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC. Renal failure upregulates smooth muscle alkaline phosphatase and increases pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomashvili KA, Khawandi W, O’Neill WC. Reduced plasma pyrophosphate levels in hemodialysis patients. J Am Soc Nephrol. 2005;16:2495–2500. doi: 10.1681/ASN.2004080694. [DOI] [PubMed] [Google Scholar]
- 15.Sigrist MK, Bungay P, Taal MW, McIntyre CW. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707–714. doi: 10.1093/ndt/gfi236. [DOI] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Amer College Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Silcox DC, McCarty DJ. Measurement of inorganic pyrophosphate in biologic fluids. Elevated levels in some patients with osteoarthritis, pseudogout, acromegaly, and uremia. J Clin Invest. 1973;52:1863–1870. doi: 10.1172/JCI107369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coburn SP, Mahuren JD, Jain M, Zubovic Y, Wortsman J. Alkaline phosphatase (EC 3.1.3.1) in serum is inhibited by physiological concentrations of inorganic phosphate. J Clin Endocrinol Metab. 1998;83:3951–3957. doi: 10.1210/jcem.83.11.5288. [DOI] [PubMed] [Google Scholar]
- 19.Rachow JW, Ryan LM. Inorganic pyrophosphate metabolism in arthritis. Rheumatic Disease Clinics of North America. 1988;14:289–302. [PubMed] [Google Scholar]
- 20.Farley RJ, Stilt-Coffing B. Apoptosis may determine the release of skeletal alkaline phosphatase activity from human osteoblast-line cells. Calcif Tissue Int. 2001;68:43–52. doi: 10.1007/BF02685002. [DOI] [PubMed] [Google Scholar]