This is the first prospective cohort study to examine both active and passive smoking in relation to lung cancer incidence in US women. Active smoking was strongly linked to lung cancer, and quitting smoking decreased lung cancer risk. We found no significant relationship between overall passive smoking and lung cancer; however, elevated exposure as an adult at home tended to increase risk.
Keywords: smoking, lung cancer, passive smokers, never smokers, lung cancer histology
Abstract
Background
Lung cancer is the leading cause of worldwide cancer deaths. While smoking is its leading risk factor, few prospective cohort studies have reported on the association of lung cancer with both active and passive smoking. This study aimed to determine the relationship between lung cancer incidence with both active and passive smoking (childhood, adult at home, and at work).
Patients and methods
The Women's Health Initiative Observational Study (WHI-OS) was a prospective cohort study conducted at 40 US centers that enrolled postmenopausal women from 1993 to 1999. Among 93 676 multiethnic participants aged 50–79, 76 304 women with complete smoking and covariate data comprised the analytic cohort. Lung cancer incidence was calculated by Cox proportional hazards models, stratified by smoking status.
Results
Over 10.5 mean follow-up years, 901 lung cancer cases were identified. Compared with never smokers (NS), lung cancer incidence was much higher in current [hazard ratio (HR) 13.44, 95% confidence interval (CI) 10.80–16.75] and former smokers (FS; HR 4.20, 95% CI 3.48–5.08) in a dose-dependent manner. Current and FS had significantly increased risk for all lung cancer subtypes, particularly small-cell and squamous cell carcinoma. Among NS, any passive smoking exposure did not significantly increase lung cancer risk (HR 0.88, 95% CI 0.52–1.49). However, risk tended to be increased in NS with adult home passive smoking exposure ≥30 years, compared with NS with no adult home exposure (HR 1.61, 95% CI 1.00–2.58).
Conclusions
In this prospective cohort of postmenopausal women, active smoking significantly increased risk of all lung cancer subtypes; current smokers had significantly increased risk compared with FS. Among NS, prolonged passive adult home exposure tended to increase lung cancer risk. These data support continued need for smoking prevention and cessation interventions, passive smoking research, and further study of lung cancer risk factors in addition to smoking.
ClinicalTrials.gov
introduction
Lung cancer is the leading cause of worldwide cancer deaths [1]. Smoking, the primary lung cancer risk factor, is linked to 80%–85% of female cases and 90% of male cases [2]. Studies have established that smokers have greatly increased lung cancer risk [3, 4] and that lung cancer incidence and mortality increase in a dose-dependent manner with smoking [4–6]. Smoking cessation reduces the risk of lung cancer incidence and mortality [3, 5, 7]. Among an estimated 16 000–24 000 lung cancer cases occurring annually in US never smokers (NS) [8], women have higher incidence rates than men [9].
Passive smoking is also an established risk factor for lung cancer [10]. However, evidence is mixed regarding which settings and durations of passive exposure are linked to increased lung cancer risk. Some studies report a positive association between lung cancer incidence and passive smoking during childhood [11, 12], adulthood home [13–15], and work [16, 17], including dose-dependent relationships [14, 15, 18]; other studies have found these correlations only at extensive exposure levels (≥40–80 pack-years) [19–22] or not at all for certain exposure categories [6, 11, 20, 23].
Despite extensive literature on smoking and lung cancer, few prospective cohort studies contain data on both active and passive smoking; most studies on this relationship in women have been conducted in case–control settings. Therefore, we studied relationships among active and passive smoking with lung cancer incidence using data from a large, multiethnic prospective cohort, the Women's Health Initiative Observational Study (WHI-OS). To our best knowledge, this is the first study to investigate the effect of both active and passive smoking on lung cancer risk in a complete prospective cohort of US women.
methods
design, setting, and participants
The WHI-OS is a multiethnic prospective cohort study designed to study morbidity and mortality in postmenopausal women; the study design has been previously described [24]. In brief, 93 676 postmenopausal women aged 50–79 were enrolled between 1993 and 1998 at 40 US clinical centers. Excluded from the original cohort were 1351 women due to incomplete data on smoking and 16 021 due to missing covariate data, resulting in 76 304 women for the study analysis.
measurement of exposures and confounders
This study aimed to determine the relationship between active/passive smoking and lung cancer incidence. All information on exposures and confounders was collected at baseline. NS were defined by questionnaire as having smoked <100 cigarettes in their lifetime (N = 39 771: 36 135 with passive exposure, 3636 without). Former smokers (FS) were classified as having smoked ≥100 cigarettes but not smoking at study baseline (N = 31 804). Current smokers (CS) reported smoking at baseline (N = 4729). CS and FS also reported age at smoking initiation, cigarettes/day, years of smoking, and age at quitting smoking (FS only).
We classified women who had only passive smoking exposure (i.e. no history of active smoking) as ‘passive smokers’. Passive smoking data were self-reported in three categories in our analysis: childhood (<18 years), adult home (lived with smoker), and work (worked with smoker). For positive categories, women also reported exposure duration (childhood: <1, 1–4, 5–9, 10–18 years; adult home/work: <1, 1–4, 5–9, 10–19, 20–29, 30–39, ≥40 years).
The multivariable model adjusted for the following confounders (defined a priori, including established and hypothesized risk factors for lung cancer): age at enrollment, BMI, ethnicity, lung cancer history, family history of cancer, education, supplemental/dietary vitamin D, occupation, hormone therapy use, oral contraceptive use, alcohol use, physical activity, and servings/day of fruit, vegetables, and red meat.
classification of cases (follow-up and ascertainment)
Cancer cases were initially self-reported in annual questionnaires administered through 2009, with 93%–96% completion rates. Physicians adjudicated lung cancer diagnoses through medical records review, according to guidelines from Surveillance Epidemiology and End Results (SEER). When available through pathology reports, tumors were histologically classified according to International Classification of Disease for Oncology, second edition. Cases were further classified into non-small-cell lung cancer [NSCLC, subtypes: adenocarcinoma, squamous cell carcinoma (SqCC), large cell, neuroendocrine, other, unspecified], small-cell lung cancer (SCLC), and Other (carcinoid), according to SEER, AJCC Cancer Staging Handbook, and WHO [25].
Over 10.5 average years of follow-up through August 2009, N = 901 lung cancer cases were identified: CS N = 531 (58.9%), FS N = 218 (23.1%), NS with passive exposure N = 136 (15.1%), NS without passive exposure N = 16 (1.8%).
statistical analysis
The primary outcome of interest was time to development of lung cancer. We used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Times were defined from enrollment into the WHI-OS to date of lung cancer diagnosis, death, loss to follow-up, or administrative censoring date (14 August 2009), whichever occurred first. We fit two models, age- and multivariable-adjusted. Participants missing information on any covariates in the model were excluded. For lung cancer subtypes, we used multinomial logistic regression models to calculate incidence because time of diagnosis was not available for subtype data. Kaplan–Meier method was used to graphically present results on lung cancer event-free survival by smoking status.
For the active smoking analysis, we estimated HRs for lung cancer development using the reference group of all NS. A secondary analysis on pack-years used 0–5 pack-years as the reference group and adjusted for age at smoking initiation. For the passive smoking analysis, we included only participants with no history of active smoking. We defined passive smoking categories a priori based on the methodology of Luo et al. [26] which investigated breast cancer incidence and active/passive smoking in the WHI-OS. Their predefined passive exposure categories included dichotomizing childhood exposure (<10 years, ≥10 years), adult home exposure (<20 years, ≥20 years), and work exposure (<10 years, ≥10 years). Due to literature suggesting dose-dependent associations between lung cancer incidence and passive smoking [14, 15, 18, 23], we further expanded the adult home ≥20 years category (20–<30 years, ≥30 years) and work ≥10 years category (10–<20 years, ≥20 years) a priori. We examined lung cancer incidence in relation to these predefined categories, as well as combinations where multiple exposures were summed in an un-weighted manner. Luo et al. defined an ‘extensive exposure’ category within the triple exposure category as childhood ≥10 years + adult home ≥20 years + work ≥10 years, which we further expanded as described above. We calculated HRs and Global Wald tests within passive smoking categories. All statistical analyses were completed using SAS 9.3 (SAS Institute, Cary, NC), and were two-sided at the 0.05 significance level.
results
The baseline characteristics of study participants, stratified by smoking status, are presented in Table 1. Among 76 304 women included in the study cohort, the vast majority had active and/or passive smoking exposure [N = 3636 NS-no passive (4.8%), N = 36 135 NS-passive (47.4%), N = 31 804 FS (41.7%), N = 4729 CS (6.2%)]. Approximately 85% of the participants were Caucasian. CS were more likely to be younger and have less education, lower physical exercise levels, lower BMIs, higher alcohol intake, higher use of oral contraceptives, and lower vitamin D intake. Additional characteristics of the cohort are presented in supplementary Tables S1–S4, available at Annals of Oncology online. There were not enough cases in each ethnic group to formally analyze incidence among NS.
Table 1.
Baseline characteristics of participants in the Women's Health Initiative (WHI) Observational Study (OS) Cohort, stratified by smoking status
| Covariate | Smoking exposure category (number, %) |
Total | P-value (χ2 test) | |||
|---|---|---|---|---|---|---|
| Never, no passive | Never, passive | Former | Current | |||
| Total | 3636 4.77% |
36 135 47.36% |
31 804 41.68% |
4729 6.20% |
76 304 | |
| Person-years of follow-up (per 100 000) | 0.38 | 3.82 | 3.36 | 0.46 | ||
| Age group (at enrollment) | ||||||
| <50–59 | 1059 29.13% |
11 321 31.33% |
10 219 32.13% |
1981 41.89% |
24 580 | <0.0001 |
| 60–69 | 1524 41.91% |
15 679 43.39% |
14 487 45.55% |
2043 43.20% |
33 733 | |
| 70–79+ | 1053 28.96% |
9135 25.28% |
7098 22.32% |
705 14.91% |
17 991 | |
| BMI category | ||||||
| <25 | 1691 46.51% |
15 409 42.64% |
12 899 40.56% |
2226 47.07% |
32 225 | <0.0001 |
| 25–<30 | 1204 33.11% |
12 048 33.34% |
10 905 34.29% |
1525 32.25% |
25 682 | |
| ≥30 | 741 20.38% |
8678 24.02% |
8000 25.15% |
978 20.68% |
18 397 | |
| Ethnicity/race | ||||||
| American Indian or Alaskan Native | 15 0.41% |
140 0.39% |
112 0.35% |
36 0.76% |
303 | <0.0001 |
| Asian or Pacific Islander | 204 5.61% |
1529 4.23% |
508 1.60% |
82 1.73% |
2323 | |
| Black or African-American | 212 5.83% |
2528 7.00% |
2087 6.56% |
593 12.54% |
5420 | |
| Hispanic/Latino | 246 6.77% |
1403 3.88% |
741 2.33% |
186 3.93% |
2576 | |
| White (not of Hispanic origin) | 2905 79.90% |
30 126 83.37% |
28 057 88.22% |
3785 80.04% |
64 873 | |
| Other | 54 1.49% |
409 1.13% |
299 0.94% |
47 0.99% |
809 | |
| Prior history of lung cancer | ||||||
| No | 3630 99.83% |
36 104 99.91% |
31 678 99.60% |
4716 99.73% |
76 128 | <0.0001 |
| Yes | 6 0.17% |
31 0.09% |
126 0.40% |
13 0.27% |
176 | |
| Cancer, male relative | ||||||
| No | 2504 68.87% |
23 136 64.03% |
20 225 63.59% |
3123 66.04% |
48 988 | <0.0001 |
| Yes | 1132 31.13% |
12 999 35.97% |
11 579 36.41% |
1606 33.96% |
27 316 | |
| Cancer, female relative | ||||||
| No | 1982 54.51% |
18 371 50.84% |
15 926 50.08% |
2491 52.67% |
38 770 | <0.0001 |
| Yes | 1654 45.49% |
17 764 49.16% |
15 878 49.92% |
2238 47.33% |
37 534 | |
| Education | ||||||
| Primary | 100 2.75% |
538 1.49% |
252 0.79% |
79 1.67% |
969 | <0.0001 |
| Some high school | 104 2.86% |
1083 3.00% |
899 2.83% |
249 5.27% |
2335 | |
| High school | 455 12.51% |
6416 17.76% |
4534 14.26% |
840 17.76% |
12 245 | |
| Some college | 913 25.11% |
12 928 35.78% |
11 976 37.66% |
2012 42.55% |
27 829 | |
| College | 537 14.77% |
4129 11.43% |
3880 12.20% |
473 10.00% |
9019 | |
| Graduate school | 1527 42.00% |
11 041 30.55% |
10 263 32.27% |
1076 22.75% |
23 907 | |
| Supplemental and dietary vitamin D | ||||||
| <400 IU | 1822 50.11% |
19 047 52.71% |
16 374 51.48% |
2886 61.03% |
40 129 | <0.0001 |
| ≥400 IU | 1814 49.89% |
17 088 47.29% |
15 430 48.52% |
1843 38.97% |
36 175 | |
| Main occupation | ||||||
| Managerial/professional | 1746 48.02% |
15 289 42.31% |
14 652 46.07% |
1829 38.68% |
33 516 | <0.0001 |
| Technical/sales/admin | 638 17.55% |
10 629 29.41% |
9165 28.82% |
1483 31.36% |
21 915 | |
| Service/labor | 604 16.61% |
6298 17.43% |
4964 15.61% |
998 21.10% |
12 864 | |
| Full-time homemaker | 648 17.82% |
3919 10.85% |
3023 9.51% |
419 8.86% |
8009 | |
| Physical activity (MET hours per week) | ||||||
| 0–≤1.67 | 592 16.28% |
6917 19.14% |
5442 17.11% |
1408 29.77% |
14 359 | <0.0001 |
| >1.67–≤8.33 | 943 25.94% |
9812 27.15% |
7644 24.03% |
1458 30.83% |
19 857 | |
| >8.33–≤20 | 1130 31.08% |
10 811 29.92% |
9867 31.02% |
1121 23.70% |
22 929 | |
| >20 | 971 26.71% |
8595 23.79% |
8851 27.83% |
742 15.69% |
19 159 | |
| Alcohol intake | ||||||
| Non-drinker | 1026 28.22% |
6344 17.56% |
904 2.84% |
179 3.79% |
8453 | <0.0001 |
| Past drinker | 480 13.20% |
6338 17.54% |
6208 19.52% |
953 20.15% |
13 979 | |
| <1 drink/month | 437 12.02% |
4724 13.07% |
3141 9.88% |
630 13.32% |
8932 | |
| 1 drink/month–<1 drink/week | 686 18.87% |
7658 21.19% |
6177 19.42% |
876 18.52% |
15 397 | |
| 1–<7 drinks/week | 768 21.12% |
8263 22.87% |
9670 30.40% |
1170 24.74% |
19 871 | |
| ≥7 drinks/week | 239 6.57% |
2808 7.77% |
5704 17.93% |
921 19.48% |
9672 | |
| Hormone therapy use (estrogen or progesterone, not as part of WHI study) | ||||||
| Never used | 1590 43.73% |
14 806 40.97% |
11 818 37.16% |
2137 45.19% |
30 351 | <0.0001 |
| Past user | 516 14.19% |
5192 14.37% |
4898 15.40% |
689 14.57% |
11 295 | |
| Current user | 1530 42.08% |
16 137 44.66% |
15 088 47.44% |
1903 40.24% |
34 658 | |
| Oral contraceptives | ||||||
| No | 2400 66.01% |
22 180 61.38% |
17 956 56.46% |
2581 54.58% |
45 117 | <0.0001 |
| Yes | 1236 33.99% |
13 955 38.62% |
13 848 43.54% |
2148 45.42% |
31 187 | |
| Diet: red meat servings per day (avg, SD) | 0.55, 0.50 | 0.60, 0.56 | 0.58, 0.54 | 0.77, 0.70 | 0.60, 0.56 | <0.0001 |
| Diet: fruit medium servings per day (avg, SD) | 2.27, 1.34 | 2.11, 1.30 | 2.01, 1.26 | 1.47, 1.20 | 2.04, 1.29 | <0.0001 |
| Diet: vegetables medium servings per day (avg, SD) | 2.39, 1.40 | 2.27, 1.33 | 2.35, 1.36 | 1.85, 1.22 | 2.28, 1.35 | <0.0001 |
| Total MET hours per week (avg, SD) | 14.63, 14.81 | 13.39, 14.10 | 14.78, 14.60 | 9.74, 12.22 | 13.80, 14.29 | <0.0001 |
| General health construct (SF 36) (avg, SD) | 75.61, 17.71 | 74.23, 17.95 | 74.57, 18.10 | 71.28, 19.18 | 74.26, 18.10 | <0.0001 |
| Lung cancer during follow-up | ||||||
| No | 3620 99.56% |
35 999 99.62% |
31 273 98.33% |
4511 95.39% |
75 403 | <0.0001 |
| Yes | 16 0.44% |
136 0.38% |
531 1.67% |
218 4.61% |
901 | |
| Died during follow-up | ||||||
| No | 3334 91.69% |
33 201 91.88% |
28 368 89.20% |
3901 82.49% |
68 804 | <0.0001 |
| Yes | 302 8.31% |
2934 8.12% |
3436 10.80% |
828 17.51% |
7500 | |
χ2 test between different smoking categories. Significant for all categories.
METs, metabolic equivalent tasks; SD, standard deviation.
The overall annualized lung cancer incidence rate was 112.3/100 000 person-years (CS 472.9, FS 158.1, NS 36.2, Table 2). When compared with NS, both CS (HR 13.44, 95% CI 10.80–16.75, P < 0.0001; multivariable-adjusted) and FS (HR 4.20, 95% CI 3.48–5.08, P < 0.0001) were significantly more likely to develop lung cancer, with CS also having a significantly higher risk than FS. For both CS and FS, the risk of developing lung cancer increased with pack-years (HR 1.58 for each 5-pack-year category, 95% CI 1.50–1.65, P < 0.0001); interaction term between CS and FS for the impact of increasing pack-years on risk was not significant (P = 0.49). The increased risk did not plateau up to ≥35 pack-years.
Table 2.
Cox proportional hazards models for time to lung cancer incidence in the WHI-OS cohort: current/former and never smokers
| Models for time to development of lung cancer | Cases/ non-cases | Annualized incidence rates (cases per 100 000 person-years) | Age-adjusted model hazard ratio (95% CI) | Multivariable- adjusted model hazard ratio (95% CI) |
|---|---|---|---|---|
| Current/former smokers | ||||
| Smoking status for the entire cohort (N = 76 304) | 901/75 403 | 112.3 | P < 0.001 | P < 0.001 |
| Never smoker | 152/39 619 | 36.2 | Ref | Ref |
| Former smoker | 531/31 273 | 158.1 | 4.48 (3.75, 5.38) | 4.20 (3.48, 5.08) |
| Current smoker | 218/4511 | 472.9 | 15.26 (12.39, 18.79) | 13.44 (10.80, 16.74) |
| Pack-years smoked among current and former smokers (N = 36 484) | ||||
| Pack-years | P < 0.0001 | P < 0.0001 | ||
| 0–<5 (reference) | 52/11 286 | 42.9 | Ref | Ref |
| 5–<10 | 37/4132 | 83.0 | 1.81 (1.19, 2.75) | 1.80 (1.18, 2.75) |
| 10–<15 | 53/4887 | 100.6 | 2.26 (1.54, 3.32) | 2.26 (1.54, 3.31) |
| 15–<25 | 143/6231 | 216.0 | 3.86 (2.79, 5.32) | 3.86 (2.80, 5.35) |
| 25–<35 | 82/2792 | 273.3 | 5.59 (3.94, 7.94) | 5.68 (3.98, 8.06) |
| ≥35 | 382/6456 | 567.6 | 9.62 (7.14, 12.99) | 9.80 (7.25, 13.33) |
| Interaction of pack-years with smoking status (former or current) | P = 0.4282 | P = 0.4910 | ||
| Pack-years trend | P < 0.0001 | P < 0.0001 | ||
| Increase in pack-year category | 1.57 (1.49, 1.64) | 1.58 (1.50, 1.65) | ||
| Never smokers | ||||
| Any passive smoking exposure among never smokers only (N = 39 771) | P = 0.6044a | P = 0.8449a | ||
| No passive exposure | 16/3620 | 42.0 | Refb | Refb |
| Passive exposure | 136/35 999 | 35.6 | 0.87 (0.52, 1.45) | 0.88 (0.52, 1.49) |
| Passive exposure categories among never smokers only (N = 39 771) | ||||
| Live with smoker as a child | P = 0.8830 | P = 0.8240 | ||
| No | 65/16 766 | 36.9 | Refc | Refc |
| Yes | 87/22 853 | 35.7 | 1.03 (0.73, 1.43) | 1.04 (0.74, 1.46) |
| Live with smoker as adult (adult home) | P = 0.3785 | P = 0.3012 | ||
| No | 51/15 170 | 31.4 | Refd | Refd |
| Yes | 101/24 449 | 39.2 | 1.17 (0.83, 1.66) | 1.21 (0.85, 1.72) |
| Work with a smoker (work)e | P = 0.9544 | P = 0.8437 | ||
| No | 48/12 749 | 35.6 | Reff | Reff |
| Yes | 104/26 870 | 36.5 | 1.01 (0.72, 1.43) | 1.04 (0.73, 1.47) |
| Interaction of childhood and adult home exposure | P = 0.6445 | P = 0.5868 | ||
| Interaction of childhood and work exposure | P = 0.9870 | P = 0.9906 | ||
| Interaction of adult home and work exposure | P = 0.0792 | P = 0.0643 | ||
| Interaction of childhood, adult home, and work exposure | P = 0.7872 | P = 0.7843 | ||
| Passive exposure durations/categories among never smokers only (N = 39 771) | ||||
| Childhood exposure category | P = 0.9928 | P = 0.9783 | ||
| No childhood exposure | 65/16 766 | 36.9 | Refc | Refc |
| <10 years | 13/3552 | 34.6 | 1.02 (0.56, 1.85) | 1.03 (0.57, 1.88) |
| 10–18 years | 74/19 301 | 35.9 | 1.02 (0.72, 1.45) | 1.04 (0.73, 1.47) |
| Adult home exposure category | P = 0.3406 | P = 0.2448 | ||
| No adult home exposure | 51/15 170 | 31.4 | Refd | Refd |
| <20 years | 52/14 211 | 34.1 | 1.09 (0.74, 1.63) | 1.11 (0.74, 1.65) |
| 20–<30 years | 17/4560 | 35.8 | 1.07 (0.61, 1.88) | 1.11 (0.63, 1.96) |
| ≥30 years | 32/5678 | 55.0 | 1.52 (0.95, 2.42) | 1.61 (1.00, 2.58) |
| Work exposure category | P = 0.4017 | P = 0.4349 | ||
| No work exposure | 48/12 749 | 35.6 | Reff | Reff |
| <10 years | 59/14 281 | 38.5 | 1.14 (0.78, 1.67) | 1.16 (0.79, 1.70) |
| 10–<20 years | 18/6385 | 26.5 | 0.72 (0.42, 1.24) | 0.74 (0.43, 1.29) |
| ≥20 years | 27/6204 | 42.0 | 1.02 (0.63, 1.64) | 1.05 (0.64, 1.72) |
| Passive exposure combinations among never smokers only (N = 39 771) | ||||
| Category of exposure | P = 0.4438 | P = 0.2938 | ||
| No passive exposure (childhood, adult home, work) | 16/3620 | 42.0 | Refb | Refb |
| Adult home + work + no childhood | 49/13 146 | 35.5 | 0.81 (0.46, 1.43) | 0.83 (0.47, 1.46) |
| Any childhood + no adult home or work | 9/2338 | 35.4 | 0.97 (0.43, 2.20) | 0.96 (0.42, 2.17) |
| Childhood <10 + any adult (work or home) | 13/3043 | 40.5 | 1.01 (0.49, 2.11) | 1.04 (0.50, 2.18) |
| Childhood ≥ 10 + adult <20 + work <10 years | 21/7467 | 25.8 | 0.70 (0.36, 1.34) | 0.70 (0.36, 1.36) |
| Childhood ≥ 10 + adult <20 + work ≥ 10 years | 5/2186 | 21.1 | 0.56 (0.20, 1.53) | 0.57 (0.21, 1.58) |
| Childhood ≥ 10 + adult ≥ 20 + work <10 years | 5/1425 | 33.6 | 0.83 (0.30, 2.25) | 0.81 (0.30, 2.24) |
| Childhood ≥ 10 + adult ≥ 20 + work ≥ 10 years | 2/535 | 35.7 | 0.87 (0.20, 3.79) | 0.96 (0.22, 4.22) |
| Childhood ≥ 10 + adult < 30 + work ≥ 20 years | 8/2381 | 32.0 | 0.76 (0.33, 1.78) | 0.80 (0.34, 1.89) |
| Childhood ≥ 10 + adult ≥ 30 + work < 20 years | 15/2390 | 60.7 | 1.35 (0.67, 2.73) | 1.48 (0.72, 3.04) |
| Childhood ≥ 10 + adult ≥ 30 + work ≥ 20 years | 9/1088 | 81.1 | 1.76 (0.78, 3.98) | 1.96 (0.85, 4.55) |
Multivariable-adjusted model is adjusted for age group, BMI category, ethnicity, prior history of lung cancer, family history of cancer in female and male relatives, education, vitamin D category, main occupation, hormone therapy use, oral contraceptive use, fruit servings per day, vegetable servings per day, red meat servings per day, alcohol use and physical activity.
Models of pack-years among current and former smokers further adjust for age started smoking.
All passive exposure categories defined a priori.
aAll P-values in this table represent Global Wald t-tests.
bNo passive smoking exposure during childhood, adult home, or work. Note: Reference groups in this table differ depending on category of passive smoking tested.
cNo passive smoking exposure during childhood only.
dNo passive smoking exposure during adult home only.
eWork passive smoking exposure is likely to be during adulthood, but not explicitly defined as such.
fNo passive smoking exposure during work only.
Among NS, lung cancer incidence did not differ between NS with passive exposure compared with NS without passive exposure (HR 0.88, 95% CI 0.52-1.49; Table 2), nor did it differ among predefined passive smoking subcategories (childhood, adult home, work, or combinations or durations of these passive exposures) compared with reference groups (NS without passive exposure, either overall or in a specific setting). However, borderline significant increased lung cancer risk was seen in NS with adult home exposure ≥30 years when compared with women with no adult home exposure (HR 1.61, 95% CI 1.00-2.58). In models exploring duration of childhood exposure, adult exposure, and exposure at work (Table 2), no significant interactions were seen among passive smoking categories, though the interaction between adult home and work approached significance (multivariable-adjusted P = 0.06). Global Wald P-values showed no significant differences in hazard within passive exposure categories.
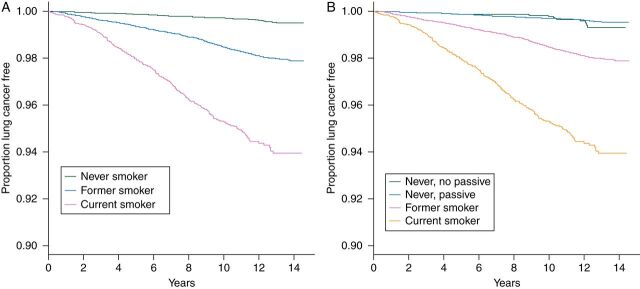
The event-free survival for different smoking categories is displayed in a Kaplan–Meier plot in Figure 1A and B. CS had lower event-free survival rates than both FS and NS, while FS had lower event-free survival rates when compared with NS, log-rank test of equality over smoking categories P < 0.001. The event-free survival for NS with and without passive exposure did not appear to differ (Figure 1B).
Figure 1.
Event-free survival estimates with (A) number of subjects at risk, stratified by smoking status; and (B) number of subjects at risk, with further stratification of never smokers (passive/no passive exposure). Kaplan–Meier event-free survival plots are presented stratified by smoking status (current, former, never–no passive, and never-passive). Log-rank test of equality over smoking categories has P < 0.0001. When never smokers are further segmented, the hazards for never, no passive exposure and never, passive exposure cross over each other at several points and do not seem to be different from each other.
NSCLC incidence was 97.9 per 100 000 person-years, and SCLC incidence was 9.9 (Table 3). Excluding unspecified cases, adenocarcinoma was the most common NSCLC subtype (incidence 55.0), followed by SqCC (14.8). CS were significantly more likely to develop NSCLC (OR 12.05, 95% CI 9.48–15.32) and particularly SCLC (OR 100.84, 95% CI 30.13–337.45) than NS (P < 0.0001); the same was true for FS, with lower ORs than CS. CS and FS had a higher rate of developing all NSCLC subtypes, when compared with NS (P < 0.0001), with the highest risk seen for SqCC and the lowest risk seen for adenocarcinoma.
Table 3.
Multinomial logistic regression models NSCLC and SCLC incidence by smoking status in the WHI-OS cohort
| Lung cancer histology | Cases/non-cases | Annualized incidence rates (cases per 100 000 person-years) | Odds ratios by smoking status (95% CI) |
P-value | ||
|---|---|---|---|---|---|---|
| Never smoker | Former smoker (95% CI) | Current smoker (95% CI) | ||||
| NSCLC/SCLC (N = 76 267)a | ||||||
| Age-adjusted model | ||||||
| NSCLC | 785/75 403 | 97.9 | Ref | 4.66 (3.84, 5.66) | 13.81 (10.99, 17.35) | <0.0001 |
| SCLC | 79/75 403 | 9.9 | Ref | 17.95 (5.57, 57.91) | 113.29 (34.73, 369.53) | |
| Multivariable-adjusted model | ||||||
| NSCLC | 785/75 403 | 97.9 | Ref | 4.22 (3.45, 5.16) | 12.05 (9.48, 15.32) | <0.0001 |
| SCLC | 79/75 403 | 9.9 | Ref | 16.76 (5.12, 54.86) | 100.84 (30.13, 337.45) | |
| Further lung cancer histology breakdown (N = 76 267)a | ||||||
| Age-adjusted model | ||||||
| NSCLC: adenocarcinoma | 441/75 403 | 55.0 | Ref | 3.62 (2.87, 4.57) | 7.28 (5.35, 9.91) | <0.0001 |
| NSCLC: squamous cell | 119/75 403 | 14.8 | Ref | 21.09 (7.69, 57.83) | 120.10 (43.29, 333.23) | |
| NSCLC: large cell/neuroendocrine/other | 56/75 403 | 7.0 | Ref | 5.23 (2.52, 10.86) | 12.52 (5.16, 30.36) | |
| NSCLC: unspecified | 169/75 403 | 21.1 | Ref | 6.10 (3.80, 9.77) | 24.43 (14.62, 40.82) | |
| SCLC | 79/75 403 | 9.9 | Ref | 17.95 (5.57, 57.91) | 113.29 (34.73, 369.53) | |
| Multivariable-adjusted model | ||||||
| NSCLC: adenocarcinoma | 441/75 403 | 55.0 | Ref | 3.27 (2.56, 4.16) | 6.75 (4.88, 9.32) | <0.0001 |
| NSCLC: squamous cell | 119/75 403 | 14.8 | Ref | 18.55 (6.69, 51.47) | 86.80 (30.66, 245.72) | |
| NSCLC: large cell/neuroendocrine/other | 56/75 403 | 7.0 | Ref | 5.21 (2.46, 11.06) | 12.87 (5.10, 32.43) | |
| NSCLC: unspecified | 169/75 403 | 21.1 | Ref | 5.37 (3.31, 8.72) | 19.42 (11.36, 33.21) | |
| SCLC | 79/75 403 | 9.9 | Ref | 16.76 (5.12, 54.86) | 100.84 (30.13, 337.45) | |
aSample size is reduced as participants for whom NSCLC/SCLC histology was not assigned were excluded from the subtype analysis (32 ‘other’ cases and 5 missing cases—this results in a total of N = 864 cases with known histology rather than N = 901 total cases, and N = 76 267 total sample size rather than N = 76 304 total sample size).
discussion
Few prospective cohort studies contain data on passive smoking. To our knowledge, this is the first study to investigate the relationship between both active and passive smoking with lung cancer risk in a complete prospective cohort of US women. In this cohort of 76 304 postmenopausal women, we found a significant association between active smoking and lung cancer incidence, which was dose-dependent for both CS and FS. CS were over 13 times more likely to develop lung cancer compared with NS; FS were over 4 times more likely. Among NS, we did not find a significant association between any passive smoking and lung cancer incidence; however, adult home passive exposure ≥30 years was of borderline significance. Smoking increased risk of all lung cancer subtypes (particularly SCLC and SqCC), and smoking cessation decreased lung cancer risk.
comparison with other studies
Studies have estimated that active smokers have 5- to 30-fold increase in lung cancer incidence compared with NS [3, 4]. Our study confirms these findings in a prospective cohort of postmenopausal women, whereas most studies have focused on this relationship in men [7] or in case–control studies [3, 13, 14, 19–21, 27]. FS had lower lung cancer risk than CS, which also corroborates prior findings [3, 5, 6]. This analysis also confirms a dose-dependent relationship for active smoking and lung cancer development [4, 5]. For both CS and FS, lung cancer risk increased with 5-year pack-year categories up to ≥35 pack-years, suggesting that the dose-dependent relationship of smoking and lung cancer development continues at high cumulative smoking levels, without plateauing.
Our findings on active smoking and lung cancer subtypes are also consistent with literature [3, 6, 23, 27]. Smoking had the strongest relationship with SCLC and SqCC incidence, and the smallest with adenocarcinoma. Quitting smoking decreases risk of developing all lung cancer subtypes. We found large HRs and CIs for SCLC and SqCC among CS, which may have been due to small number of reference cases. We were unable to examine passive smoking and lung cancer subtypes due to sample size.
Among NS, we found that passive smokers (ever-exposed, as well as predefined categories including childhood, adult home, and work) were not at significantly increased lung cancer risk; however, several passive exposure categories, particularly adult home ≥30 years, had elevated point estimates and approached significance. Literature on passive smoking has been inconsistent with considerable heterogeneity of findings and many case–control studies, which are more susceptible to recall bias than cohort studies. While some publications have reported positive associations [11–14, 17, 27], including dose-dependent relationships [13, 20, 27], other studies have not found significant associations between lung cancer incidence and childhood passive smoking exposure [6, 19, 23, 28], adult spouse/residential passive smoking [11, 28], and workplace exposure [11, 18]. Additionally, some studies have found these associations only at extensive levels (40 or 80 pack-years in some cases) or exposure combinations [18–22, 28].
There are several possible explanations as to why we did not find a clear association between overall passive smoking and lung cancer risk. There may be inaccuracies in self-report of passive exposures, which is likely most pronounced for childhood exposures. However, as exposure information was collected at baseline before lung cancer diagnosis, true recall bias is unlikely. The WHI-OS measured passive smoking exposure in ‘years’ rather than the more precise ‘pack-years’; consequently, varying exposure levels may be combined into a single category. However, prospective studies containing passive smoking data are extremely rare, and self-report of passive smoking pack-years may be impractical and inaccurate. The relatively small overall reference group (NS, no passive exposure) also resulted in wide CIs for some passive smoking categories, and sample size prevented further passive smoking segmentation.
We must also consider that passive smoking may have a weaker than expected association with lung cancer development for postmenopausal women, which some previous prospective cohort studies have suggested (see supplementary Table S5, available at Annals of Oncology online, for comparison to prior prospective studies). Two large Japanese prospective studies with ∼38 000 total participants found excess but insignificant lung cancer risk from overall spousal passive smoking, which is similar to our result [18, 29]; another large Japanese cohort study found significantly increased risk for wives of heavy smokers only [22]. The Nurses' Health Study also found insignificant associations for passive adult smoking exposure with lung cancer in US women, though few cases among NS were reported in the cohort [30]. Additionally, the American Cancer Society CPS-I/II cohort studies did not find a significant relationship between passive smoking and lung cancer mortality (both sexes) [31]. These results from prospective cohort studies are more conservative than many reviews and case–control studies [10, 13–17, 23, 27]. Though our results were not statistically significant, our findings suggest that high levels of passive smoking exposure may increase lung cancer risk, with adult home exposure possibly the greatest contributor. Further passive smoking research is warranted, particularly in a prospective cohort setting with pack-years measurement.
strengths and limitations of the study
Strengths of our study include the prospective cohort design, large size and geographical distribution, high number and pathological confirmation of lung cancer cases/subtypes, and detailed information on active/ passive smoking exposure variables in multiple settings and confounders. Limitations include collection of passive smoking exposure as ‘years’ rather than ‘pack-years’, and potential inaccuracies in self-reported data. Our analytic cohort also had a relatively small overall reference group (NS without passive exposure). We used baseline values for smoking status, as data were collected at study entry. However, as there were relatively few CS, exposure misclassification was likely minimal. Yearly WHI reassessments indicated that 99% of NS abstained from smoking, and ∼60% of CS continued smoking for 6 follow-up years. Lastly, the cohort was primarily Caucasian.
conclusions and policy recommendations
In conclusion, for a prospective cohort of US postmenopausal women, our study confirms literature findings that smoking increases the risk of all lung cancer subtypes. This relationship is dose-dependent with no plateau up to 35 pack-years. Smoking cessation decreases lung cancer risk. Our study did not find a significant relationship between overall passive smoking exposure and lung cancer among NS; however, adult home exposure ≥30 years was associated with borderline significant elevations in risk, suggesting that high levels of passive smoking may contribute to lung cancer risk. These passive smoking findings are intriguing and add to the controversy on this subject; more precise pack-years quantification of passive smoking in a prospective cohort setting is warranted. This study focused only on smoking and lung cancer; public policy must also consider that active and passive smoking have been established as strong contributors to morbidity and mortality associated with many health conditions, including cardiopulmonary disease, other cancers, and pregnancy complications and asthma in children [32].
As lung cancer is the leading cause of US cancer deaths, our prospective study underscores the need for development and implementation of smoking prevention and cessation interventions for all ages, and for women as well as men. Additionally, given the high incidence and mortality of lung cancer with at least 10%–15% cases occurring NS in the United States [8, 9], our results suggest that more research is needed on nonsmoking-related lung cancer risk factors, including but not limited to genetic, behavioral, hormonal, dietary, and environmental factors.
funding
This work was supported by the National Institutes of Health and Stanford University School of Medicine. The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. AW was funded by a Medical Scholars research fellowship from Stanford University School of Medicine. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
disclosure
The authors have declared no conflicts of interest.
ethical approval
This study was approved by the ethics committees at the Women's Health Initiative Coordinating Center, Fred Hutchinson Cancer Research Center, and all 40 clinical centers.
Supplementary Material
acknowledgements
We acknowledge the dedicated efforts of investigators and staff at the Women's Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung and Blood program office (listing available at http://www.whi.org). We also recognize the WHI participants for their extraordinary commitment to the WHI program.
references
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control (U.S.), Centers for Disease Control and Prevention (U.S.) . Morbidity and mortality weekly report: MMWR : HEW Publication, Atlanta, GA. U.S. Dept. of Health, Education, and Welfare, Public Health Service v, 19 March 1976–2 May 1980.
- 3.Agudo A, Ahrens W, Benhamou E, et al. Lung cancer and cigarette smoking in women: a multicenter case-control study in Europe. Int J Cancer. 2000;88:820–827. doi: 10.1002/1097-0215(20001201)88:5<820::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123:21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 5.Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svensson C, Pershagen G, Klominek J. Smoking and passive smoking in relation to lung cancer in women. Acta Oncol. 1989;28:623–629. doi: 10.3109/02841868909092282. [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization & International Agency for Research on Cancer. Lyon: France: 2004. Tobacco Smoke and Involuntary Smoking: IARC Working Group in Lyon. 11–18 June 2002. [Google Scholar]
- 11.Janerich DT, Thompson WD, Varela LR, et al. Lung cancer and exposure to tobacco smoke in the household. N Engl J Med. 1990;323:632–636. doi: 10.1056/NEJM199009063231003. [DOI] [PubMed] [Google Scholar]
- 12.Rachtan J. Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer. 2002;35:129–136. doi: 10.1016/s0169-5002(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 13.Lam TH, Kung IT, Wong CM, et al. Smoking, passive smoking and histological types in lung cancer in Hong Kong Chinese women. Br J Cancer. 1987;56:673–678. doi: 10.1038/bjc.1987.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trichopoulos D, Kalandidi A, Sparros L, MacMahon B. Lung cancer and passive smoking. Int J Cancer. 1981;27:1–4. doi: 10.1002/ijc.2910270102. [DOI] [PubMed] [Google Scholar]
- 15.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315:980–988. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stayner L, Bena J, Sasco AJ, et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am J Public Health. 2007;97:545–551. doi: 10.2105/AJPH.2004.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells AJ. Lung cancer from passive smoking at work. Am J Public Health. 1998;88:1025–1029. doi: 10.2105/ajph.88.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurahashi N, Inoue M, Liu Y, et al. Passive smoking and lung cancer in Japanese non-smoking women: a prospective study. Int J Cancer. 2008;122:653–657. doi: 10.1002/ijc.23116. [DOI] [PubMed] [Google Scholar]
- 19.Brownson RC, Alavanja MC, Hock ET, Loy TS. Passive smoking and lung cancer in nonsmoking women. Am J Public Health. 1992;82:1525–1530. doi: 10.2105/ajph.82.11.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkland LR. Environmental tobacco smoke and lung cancer in nonsmoking women. JAMA. 1995;273:519–520. doi: 10.1001/jama.1995.03520310011006. [DOI] [PubMed] [Google Scholar]
- 21.Correa P, Pickle LW, Fontham E, et al. Passive smoking and lung cancer. Lancet. 1983;2:595–597. doi: 10.1016/s0140-6736(83)90680-3. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. Br Med J (Clin Res Ed) 1981;282:183–185. doi: 10.1136/bmj.282.6259.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boffetta P, Tredaniel J, Greco A. Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: a meta-analysis. Environ Health Perspect. 2000;108:73–82. doi: 10.1289/ehp.0010873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer RD, White E, Lewis CE, et al. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 25.Edge SB American Joint Committee on Cancer . . AJCC Cancer Staging Manual. New York: Springer; 2010. pp. 253–271. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Margolis KL, Wactawski-Wende J, et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ. 2011;342:d1016. doi: 10.1136/bmj.d1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan P, Buffler PA, Reynolds P, et al. Secondhand smoke exposure in adulthood and risk of lung cancer among never smokers: a pooled analysis of two large studies. Int J Cancer. 2004;109:125–131. doi: 10.1002/ijc.11682. [DOI] [PubMed] [Google Scholar]
- 28.Kreuzer M, Krauss M, Kreienbrock L, et al. Environmental tobacco smoke and lung cancer: a case-control study in Germany. Am J Epidemiol. 2000;151:241–250. doi: 10.1093/oxfordjournals.aje.a010199. [DOI] [PubMed] [Google Scholar]
- 29.Nishino Y, Tsubono Y, Tsuji I, et al. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control. 2001;12:797–802. doi: 10.1023/a:1012273806199. [DOI] [PubMed] [Google Scholar]
- 30.Speizer FE, Colditz GA, Hunter DJ, et al. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA) Cancer Causes Control. 1999;10:475–482. doi: 10.1023/a:1008931526525. [DOI] [PubMed] [Google Scholar]
- 31.Cardenas VM, Thun MJ, Austin H, et al. Environmental tobacco smoke and lung cancer mortality in the American Cancer Society's Cancer Prevention Study. II. Cancer Causes Control. 1997;8:57–64. doi: 10.1023/a:1018483121625. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AE, Johnson DC, Kazemi H. Environmental Tobacco-Smoke and ardiovascular-Disease—a Position Paper from the Council on Cardiopulmonary and Critical Care, American-Heart-Association. Circulation. 1992;86:699–702. doi: 10.1161/01.cir.86.2.699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.