Abstract
The hallmark of disease caused by tick- and louse-borne relapsing fever due to Borrelia infection is cyclic febrile episodes, which in humans results in severe malaise and may lead to death. To evaluate the pathogenesis of relapsing fever due to spirochetes in an animal model closely related to humans, disease caused by Borrelia turicatae after tick bite was compared in 2 rhesus macaques in which radiotelemetry devices that recorded body temperatures in 24-hour increments were implanted. The radiotelemetry devices enabled real-time acquisition of core body temperatures and changes in heart rates and electrocardiogram intervals for 28 consecutive days without the need to constantly manipulate the animals. Blood specimens were also collected from all animals for 14 days after tick bite, and spirochete densities were assessed by quantitative polymerase chain reaction. The complexity of disease caused by relapsing-fever spirochetes was demonstrated in the nonhuman primates monitored in real time. The animals experienced prolonged episodes of hyperthermia and hypothermia; disruptions in their diurnal patterns and repolarization of the heart were also observed. This is the first report of the characterizing disease progression with continuous monitoring in an animal model of relapsing fever due to Borrelia infection.
Keywords: relapsing fever, spirochetes, Borrelia, rhesus macaques, Borrelia turicatae
Tick- and louse-borne relapsing-fever spirochetes are blood-borne pathogens and a leading cause of child morbidity and mortality in underdeveloped countries [1–6], with infections associated with >1 × 107 spirochetes/mL of blood [7, 8]. A family of surface lipoproteins responsible for antigenic variation acts as endotoxins [9, 10], inducing a rapid rise in temperature, rigors, tachycardia, headache, shock, and possibly death [11–14]. The murine model has been used to study multiple aspects of relapsing-fever spirochete pathogenesis, including neuroborreliosis, the immune evasion strategies used by the bacteria, and the importance of B1b lymphocytes in pathogen clearance [10, 15, 16]. A potential limitation of the murine model is that rodents are often asymptomatic and afebrile during infection [17–19]. Previous studies with Borrelia turicatae indicated that the spirochetes caused a mild spirochetemia in mice, attaining 1 × 105–1 × 106 spirochetes/mL, and the animals did not appear ill [17, 20, 21]. Thermoregulation also significantly differs between mammalian species, with rodents typically becoming hypothermic in response to spirochete lysates or endotoxin challenge [18, 19, 22, 23].
To test countermeasures to prevent disease, animal models that closely reproduce clinical symptoms observed in humans is important. Early studies demonstrated that disease in nonhuman primates infected with B. turicatae and Borrelia recurrentis closely mimicked that observed in humans [24, 25]. However, given the lack of technology at the time, apart from visualizing bacteria in the blood, determining body temperatures, and assessing histopathologic findings, no other information about pathogenesis was reported. Radiotelemetry has demonstrated the physiological complexity of disease progression in nonhuman primate models of Zaire ebolavirus and chikungunya virus [26, 27], and we used this technology to further investigate the clinical burden of B. turicatae in a susceptible animal model. Two of 4 animals had a radiotelemetry device implanted, and we continuously monitored physiological changes in body temperature, heart rate, and electrocardiogram (ECG) parameters, with results reported as hourly means. With the remaining 2 animals, temperatures were recorded manually at 24-hour intervals. Blood samples were also collected from all animals for 14 consecutive days to quantify spirochete densities. Blood chemistry analyses and complete blood counts were performed for each animal. Cerebrospinal fluid (CSF) specimens were obtained 28 days after infection and at the time of necropsy to detect B. turicatae. Following euthanasia, sections of cerebellum, cerebrum, medulla/brainstem, lung, liver, spleen, and kidney were collected to recover spirochetes from the tissues and evaluate histopathologic findings. Our results demonstrate the development of a susceptible animal model that may be used to test vaccine candidates and evaluate novel treatment options.
METHODS
Ticks
An uninfected colony of Ornithodoros turicata was maintained at 25°C–27°C and 85% relative humidity [28]. To obtain a cohort of infected ticks, O. turicata in the second nymphal stage fed to repletion on a spirochetemic mouse that was needle inoculated with B. turicatae 91E135 [29]. Murine studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Mississippi State University and accorded with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and the National Institutes of Health Office of Laboratory Animal Welfare. Animal husbandry was provided by veterinary staff and technicians.
Nonhuman Primates, Telemetry, and Transmission of B. turicatae
Practices in the housing and care of nonhuman primates conformed to the regulations and standards of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. The Tulane National Primate Research Center (TNPRC) is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care–International. The IACUC at the TNPRC approved all animal-related protocols, including infection of and sample collection from nonhuman primates. All animal procedures were overseen by veterinarians and their staff.
Four Indian rhesus macaques (JB23, JB60, JD03, and IN57) 2.02–2.85 years of age were used in the study. Subcutaneous radiotelemetry transmitters (T34G-8; Konigsberg Instruments [KI]), combined with sensors capable of detecting biopotential signals of heart rate, ECG intervals, and temperature, were surgically implanted in 2 animals (JD03 and IN57). The animals were housed in antenna-mounted cages (TR38-1FG; KI) configured to receive and transmit signals to a KI data acquisition base station. Data were continuously recorded using the Notocord-hem Acquisition System (Notocord) and reported as hourly averages, and baseline data curves were generated for 2 weeks following implantation (Figure 1). QT intervals (defined as the durations of ventricular depolarization and repolarization) were corrected for heart rate, using Fridericia's formula:
in which both the QT interval and the RR interval (defined as the duration between beats) are in seconds [30].
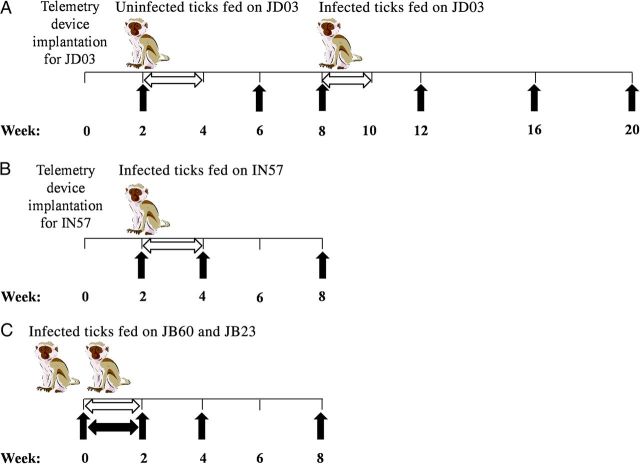
Figure 1.
Experimental design for infecting rhesus macaques with Borrelia turicatae. A and B, JD03 (A) and IN57 (B) were implanted with radiotelemetry devices 2 weeks before ticks fed on the animals. C, After the feeding, body temperatures of the 2 animals were recorded manually (black horizontal arrow) in 24-hour intervals. Horizontal white arrows indicate the time line for collecting blood specimens by ear or tail prick, and black vertical arrows indicate time points for serum specimen collection and performing complete blood count and blood chemistry analyses.
Before infecting the animals with B. turicatae, the potential effects of O. turicata saliva on temperature regulation was evaluated by feeding 10 uninfected ticks to repletion on JD03 and monitoring the animal for 42 days by telemetry. For each animal, sedation was performed by administering 5–8 mg/kg tiletamine/zolazepam by intramuscular injection, hair from a region of the lower abdomen was clipped using an electric trimmer, and 10 third-stage nymphal ticks infected with B. turicatae were allowed to feed. Ticks were placed on an area caudal to the umbilicus with a paintbrush and covered with the lid of a 50-mL conical tube to prevent migration. The ticks fed to repletion within 20–30 minutes. For JD03 and IN57, preexposure results were aligned by the time of day over a 24-hour period and averaged to establish detection thresholds. Significant events were defined to be >1.5 times the maximum standard deviation of the control averages. Individual postexposure results were then aligned by time and compared against preexposure values for each animal. An assessment of fever (hyperthermia) duration in hours, hypothermia duration in hours, and hyperthermia intensity was also performed for each animal. Body temperatures for JB23 and JB60 were manually recorded at 24-hour intervals for 14 consecutive days.
Molecular Detection of B. turicatae
To quantify spirochete densities in the blood of nonhuman primates, 2.5-µL blood specimens were collected by ear or tail prick at the time body temperatures were obtained and were placed into 47.5 µL of Sidestep Lysis and Stabilization Buffer (Agilent Technologies) to achieve a 1:20 dilution. Sampling commenced before the transmission-associated blood meal and continued for 14 consecutive days. Quantitative polymerase chain reaction (qPCR) was performed as previously described, using primers and a probe specific to B. turicatae flaB [20]. A standard curve was developed from cultivated spirochetes grown in mBSK medium (a modified version of Barbour-Stoenner-Kelly II medium containing 12% rabbit serum) [31, 32] at concentrations of 1 × 108–1 × 103 bacteria/mL and spiked with uninfected nonhuman primate blood at a 1:20 dilution.
To detect B. turicatae DNA in nonhuman primate tissues, primers were designed against the B. turicatae gene encoding glycerophosphodiester phosphodiesterase (glpQ), and real-time PCR was performed using total DNA extracted from the kidney, heart, bladder, skin, lung, liver, brain, and spleen, using the DNeasy Blood and Tissue Kit (Qiagen). To estimate the limit of detection for B. turicatae, 5-fold serial dilutions were performed with 0.6–0.0096 ng of spirochete DNA isolated from bacteria grown in vitro and spiked into 100 ng of brain or heart DNA from an uninfected nonhuman primate. Real-time PCR was performed in triplicate, using 2× Power SYBR Green master mix (Life Technologies), with 200 nM forward (5′-GGATGAAGCACCAACAGACTA-3′) and reverse (5′-GGGTATCCAAGGTCCAATTCC-3′) primers at a final volume of 20 µL. PCR conditions were 1 cycle at 95°C for 10 minutes, 55 cycles at 95°C for 15 seconds, followed by 1 cycle at 60°C for 1 minute.
Clinical Diagnostic Tests
Complete blood counts and serum chemistry panels measuring 12 analytes were performed before the transmission-associated blood meal, 28 days after infection, and at necropsy, 60–65 days after infection Manual cell counts were performed for the first 2 weeks after infection by a clinical pathologist (B. J. G.) for each monkey. Overall cellularity of erythrocytes and leukocytes was determined at 10 times the original magnification, while differential leukocyte counts were made using an oil immersion objective at 100 times the original magnification. Blood counts from uninfected animals were used as a control to determine increases or decreases in leukocyte counts. Red blood cell and total leukocyte numbers were semiquantitatively scaled as follows: 0, marked decrease; 1, moderate decrease; 2, slight decrease; 3, adequate; 4, slight increase; 5, moderate increase; and 6, marked increase.
Spirochete concentrations were scored as 0 (none), 1 (low; defined as 1 spirochete per 100 microscopic fields), 2 (moderate; defined as 1–3 spirochetes per field), and 3 (high; defined as >3 spirochetes per field). CSF specimens were also collected 28 days after infection and at necropsy and cellularity, and differential blood counts were performed.
Pathologic Analyses and B. turicatae Cultivation From Tissues
Following euthanasia, a complete necropsy was performed on each animal. Tissues were collected and stored in Z-fix fixative for histologic analysis and were frozen in optimal-cutting-temperature medium, using methanol and dry ice baths. CSF specimens and 2-mm3 specimens of cerebellum, cerebrum, medulla/brainstem, lung, heart, liver, spleen, and kidney were also collected, homogenized, added to 5–10 mL of mBSK-H medium, and incubated at 34°C in a microaerophilic tri-gas incubator to culture B. turicatae. Cultures were inspected for spirochete growth every week for 9 weeks.
RESULTS
Transmission of B. turicatae by Tick Bite
Reports of fever and anaphylaxis caused by the saliva of hematophagous ectoparasites [33] indicated the necessity of determining the effects of O. turicata saliva on temperature regulation in nonhuman primates. Ten uninfected ticks fed on JD03, and the animal was monitored for 56 consecutive days, with no indication that the saliva affected temperature regulation. Subsequently, 10 infected nymphs were allowed to feed on JB23, JB60, JD03, and IN57, causing hemorrhagic lesions that spread and coalesced when observed the following day (Figure 2A–C).
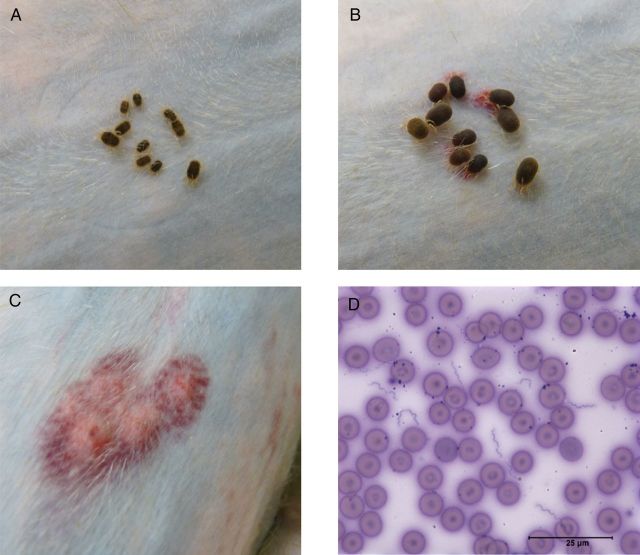
Figure 2.
A and B, Ornithodoros turicata ticks feeding on JD03. The duration between tick placement (A) and repletion (B) was between 20–30 minutes. C, Subdermal hemorrhage on JD03 was seen at the site where ticks fed. D, Spirochetes were visualized in the blood within 6 days after the transmission-associated blood meal (D), and a 25-µm scale is shown. These results from JD03 were representative of those for the 3 other animals.
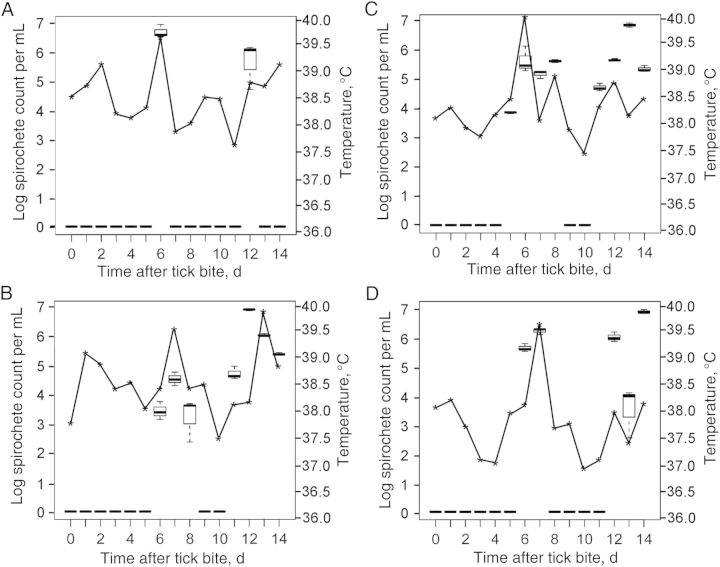
Spirochetes were visualized by microscopy in all animals by the sixth day after tick bite (Figure 2D), and quantification of bacterial loads in the blood by qPCR indicated that B. turicatae densities ranged from undetectable levels to 1 × 103–1 × 107 spirochetes/mL (Figure 3A–D). Furthermore, JB23 experienced 2 spirochetemic episodes, while JB60, JD03, and IN57 remained spirochetemic for several days, followed by a 2–4-day period in which B. turicatae was undetectable by qPCR. By the twelfth day after tick bite, spirochetes repopulated the blood of all animals (Figure 3A–D). Body temperatures recorded manually at the time of blood collection (JB23 and JB60) and by telemetry (JD03 and IN57) indicated that elevated temperatures were associated with spirochete densities in the blood (Figure 3A–D). Interestingly, periods when B. turicatae was detected in blood of animals by qPCR without accompanying hyperthermia were also observed (Figure 3B–D).
Figure 3.
A–D, Comparisons of Borrelia turicatae densities in the blood with daily temperature readings for JB23 (A), JB60 (B), JD03 (C), and IN57 (D). Box and whisker plots indicate the lower and upper quartiles, median, and lower and upper extremes of spirochete densities, while the asterisks represent the temperature at the time of blood specimen collection.
Radiotelemetry Findings
Changes in core body temperature, heart rate, and ECG intervals in response to B. turicatae infection were characterized in detail for JD03 and IN57, using radiotelemetry (Figure 4A–C). JD03 experienced normal diurnal patterns in all measured physiologic parameters after exposure to feeding by uninfected ticks (Figure 4A–C). The animal's body temperature ranged between 36.6°C in the evening and 38.3°C during the day, with a diurnal change of ±0.85°C before infection by tick bite. Upon challenge, dramatic physiological changes occurred. Throughout the infection duration, there was a continuous interruption in the normal diurnal pattern of temperature. The animals experienced hypothermic temperatures at night for several days before onset an initial febrile episode, characterized by an abrupt onset of clinical signs with a rapid spike in body temperature, 5–6 days after infection (Figure 4A). The duration of the initial febrile episode varied greatly in both animals, lasting approximately 9 and 33 hours for IN57 and JD03, respectively. The animals also experienced 3–4 significant febrile episodes in the following weeks after infection, with peak increases in temperature of 2.83°C and 3.81°C for IN57 and JD03, respectively (Figure 4A). While the intensities of hypothermic and hyperthermic episodes were greater in JD03 (Figure 4A), the pattern and timing of febrile relapses and concomitant cardiac responses were similar in both animals.
Figure 4.
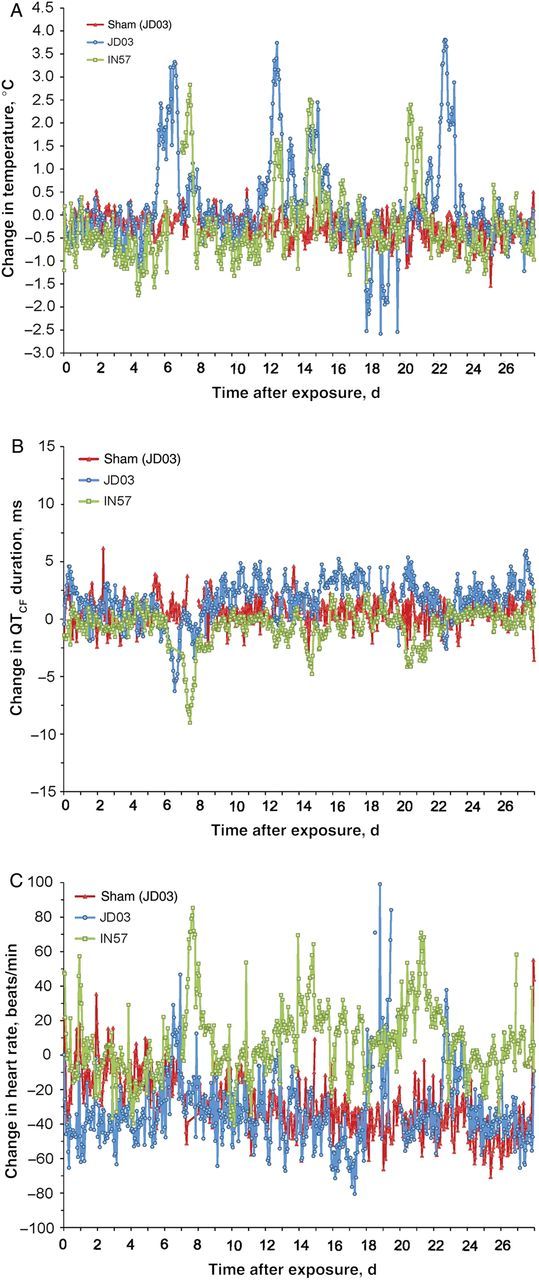
Real-time measured physiological responses of JD03 and IN57. JD03 was fed upon by uninfected ticks (red data points) as a sham infection. A–C, After Borrelia turicatae challenge by tick bite, changes in body temperature (A), corrected QT interval (defined as the duration of ventricular depolarization and repolarization; B), and heart rate (C) are displayed as hourly means for JD03 (blue) and IN57 (green). The period when temperature data were not collected for IN57, on day 6, was a result in temporary unavailability of the hard drive of the data-acquisition unit.
There was a pathological repolarization effect on the heart, as indicated by significant changes in QTCF that coincided with febrile episodes (Figure 4A and 4B). Interestingly, both animals experienced a dramatic decrease in QTCF duration, which began with the onset of the initial febrile episode occurring 5–7 days after infection and lasted throughout the fever and during the recovery period into the following day. Although there was an increased heart rate at this time in both animals (Figure 4C), they were within reasonable ranges to account for using the correction formula, suggesting that the cardiac responses are significant and pathological in nature.
The initial recovery period for JD03 following the first febrile episode was characterized by a sustained increase in the QTCF duration (Figure 4B) that began approximately 9 days after infection, with intermittent responses lasting for several weeks. Both animals experienced a second febrile episode (first relapse) 11–12 days after infection (Figure 4A). At this time, the diurnal pattern for JD03 was interrupted and lasted approximately 2 days before recovery, whereas IN57 experienced a slight disturbance for approximately 6 hours. The third febrile response (second relapse) was experienced by both animals within 12–24 hours of the first relapse and was followed by a recovery period within 24 hours (Figure 4A). Animal IN57 exhibited a decreased QTCF duration concurrent with this febrile episode that lasted until recovery, as well as an increased heart rate, with a significant sustained tachycardia, that lasted for several weeks (Figure 4B and 4C). An acute yet transient tachycardia was also observed for JD03 on days 18–19 and 22–23 after infection (Figure 4B and 4C). The recovery period following the third febrile episode for JD03 was characterized by hypothermic temperatures during the day and a loss of diurnal patterns for approximately 3 days.
JD03 and IN57 experienced a fourth febrile episode characterized by an increased spike in temperature occurring approximately 20–21 days after infection (Figure 4A). JD03 experienced a more robust temperature response that lasted approximately 2 days before recovery, with increases in QTCF occurring just before and after the fever episode (Figure 4A and 4B). IN57 experienced a slight temperature response lasting approximately 1 day, with significant decreases in QTCF occurring throughout the febrile episode before final recovery. Although the number of animals was too small for statistical analysis, cardiac disturbances were concurrent with the febrile episodes caused by B. turicatae infection.
Results of Clinical Laboratory Analyses
Manual counts of blood cells were performed for the first 14 days of infection, and leukocyte counts during spirochetemic episodes were compared with those between spirochetemic episodes (Table 1). Only the values for JD03 are shown, because the control infection values were used for comparison. The scale generated reflected the semiquantitative nature of the results, and there were some samples for which blood smears could not be evaluated, owing to insufficient volume or severe clumping.
Table 1.
Manual Differential Blood Counts for JD03 14 Days After Feeding by Uninfected Ornithodoros turicata and Spirochete-Infected Ticks
| Variable, by Tick Infection Status | Day |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Uninfected | |||||||||||||||
| RBC counta | 3 | 3.5 | 3 | 2.5 | 2.5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| WBC counta | 3.5 | 4 | 3 | 3 | 3 | 3 | 3 | 3.5 | 1 | 3 | 3 | 3 | 3 | 3 | 3 |
| PMNs, % | 52 | 48 | 49 | 37 | 55 | 41 | 63 | 50 | 62 | 52 | 41 | 47 | 33 | 47 | 41 |
| Lymphocytes, % | 35 | 39 | 40 | 49 | 35 | 46 | 33 | 44 | 32 | 33 | 54 | 40 | 53 | 43 | 48 |
| Monocytes, % | 3 | 6 | 8 | 8 | 5 | 10 | 2 | 4 | 0 | 8 | 0 | 8 | 4 | 4 | 6 |
| Eosinophils, % | 6 | 5 | 3 | 5 | 5 | 2 | 2 | 2 | 6 | 5 | 4 | 5 | 10 | 5 | 4 |
| Basophils, % | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 |
| Spirochetes, no. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infected | |||||||||||||||
| RBC counta | 3 | 3 | ND | 3 | 2 | 2 | 3 | 3 | 2.5 | 2.5 | 2 | 1 | ND | 1 | 1 |
| WBC counta | 3 | 1 | ND | 3 | 0 | 2 | 3.5 | 0 | 2 | 3.5 | 3.5 | 0 | ND | 0 | 1 |
| PMNs, % | 35 | 34 | ND | 14 | 28 | 56 | 89 | 60 | 51 | 53 | 15 | 48 | ND | 96b | 60 |
| Lymphocytes, % | 50 | 58 | ND | 85 | 64 | 42 | 8 | 40 | 31 | 49 | 71 | 28 | ND | 0 | 20 |
| Monocytes, % | 6 | 2 | ND | 1 | 4 | 1 | 3 | 0 | 15 | 15 | 11 | 16 | ND | 0 | 16 |
| Eosinophils, % | 9 | 6 | ND | 0 | 4 | 1 | 0 | 0 | 3 | 2 | 2 | 4 | ND | 4 | 4 |
| Basophils, % | 0 | 0 | ND | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | ND | 0 | 0 |
| Spirochetes, no. | 0 | 0 | ND | 0 | 0 | 1 | 2 | 3 | 1 | 0 | 0 | 0 | ND | 3 | 2 |
Bold values emphasize substantial changes.
Abbreviations: PMN, polymorphonuclear leukocyte; RBC, red blood cell; WBC, white blood cell.
a Semiquantitatively scaled as follows: 0, marked decrease; 1, moderate decrease; 2, slight decrease; 3, adequate; 4, slight increase; 5, moderate increase; and 6, marked increase.
b On the basis of the low WBC count and lymphocyte and monocyte percentages, the increased percentage of neutrophils likely represents an absolute decrease in lymphocytes and monocytes, rather than an absolute increase in neutrophils.
There was a striking decrease in total leukocyte counts at several intervals, with recovery to baseline values between these decreases (Table 1). Notable changes in the differential counts included an increase in the neutrophil percentage several days following leukopenia and mild increases in monocyte percentages several days after the initial leukopenic episode. These abnormalities were noted to some degree in all of the animals, with the neutrophilia coinciding with spirochetemia. An increase in the lymphocyte percentage was observed prior to the detection of spirochetes in the blood, and this was followed by a decline during spirochetemia. This decrease in lymphocyte percentage may reflect the migration of the cells to lymph nodes.
Serum chemistry panels (data not shown) and complete blood counts were performed by the clinical laboratory at the TNPRC before infection and on days 28 and days 64 after infection (Table 2). The absolute lymphocyte concentrations in the blood were increased for all animals at day 28, compared with values before infection. These numbers returned to normal levels by day 64 for all but one animal (IN57). Results of serum chemistry analyses were also within the reference intervals for all parameters throughout the study.
Table 2.
Comprehensive Blood Counts for Nonhuman Primates Before and After Inoculation With Borrelia turicatae
| Animal, Day | Percentage or No. of White Blood Cells |
|||||
|---|---|---|---|---|---|---|
| Neutrophils, % | Lymphocytes, % | Monocytes, % | Neutrophils, No. | Lymphocytes, No. | Monocytes, No. | |
| JB60 | ||||||
| 0a | 62.8 | 33.8 | 2.7 | 6.03 | 3.25 | 0.26 |
| 28 | 55.1 | 42.1 | 2 | 9.52 | 7.27 | 0.34 |
| 64 | 37.3 | 60.2 | 1.3 | 2.99 | 4.84 | 0.11 |
| JB23 | ||||||
| 0a | 71.4 | 25.8 | 2.1 | 5.34 | 1.93 | 0.16 |
| 28 | 66.3 | 30.5 | 2.5 | 13.23 | 6.07 | 0.49 |
| 64 | 72.9 | 24.5 | 2.1 | 8.8 | 2.96 | 0.25 |
| IN57 | ||||||
| 0a | 71 | 25.1 | 2.9 | 4.03 | 1.43 | 0.16 |
| 28 | 46.5 | 49.9 | 2.1 | 4.23 | 4.54 | 0.19 |
| 64 | 27.5 | 65.2 | 3.9 | 1.7 | 4.03 | 0.19 |
| JD03 | ||||||
| 0a | 78.8 | 17.8 | 2.7 | 9.63 | 2.17 | 0.33 |
| 28b | 73.6 | 20 | 3.9 | 10.14 | 2.75 | 0.53 |
| 28 | 15.1 | 78.5 | 2.8 | 1.81 | 9.41 | 0.34 |
| 64 | 60.4 | 35.5 | 2.6 | 9.26 | 5.44 | 0.4 |
Data were from analyses of specimens obtained from infected primates, unless otherwise indicated.
a Uninfected; data were from analyses of specimens obtained before exposure to tick feeding.
b Uninfected; data were from analyses of specimens obtained 28 days after feeding uninfected ticks.
Tests for Detection of Spirochetes in Tissues and Pathologic Analyses
We were unable to cultivate B. turicatae in mBSK medium (data not shown) or to detect spirochete DNA from the CSF, kidney, heart, bladder, skin, lung, liver cerebrum, or spleen, suggesting that the bacteria did not colonize the tissues. In control assays, B. turicatae was detected by real-time PCR after nonhuman primate DNA was spiked with 0.6–0.0096 ng of spirochete DNA, indicating that the limit of detection was <0.0096 ng (Supplementary Figure 1).
Evidence of inflammation or degeneration in the animals was evaluated by a board-certified veterinary pathologist (T. W. M.). Changes in the neuropile or meninges of the cerebrum, cerebellum, and brain stems were absent. Similarly, evidence of pathological effects caused by B. turicatae were absent in the synovium of the diarthrodial joints, liver, spleen, and heart (data not shown).
DISCUSSION
Productive infection with B. turicatae by tick bite in 4 nonhuman primates was achieved in this study, and the febrile episodes and spirochete densities in the blood were comparable to what is observed in humans [8, 34]. Real-time data acquisition enabled the ability to monitor disease progression without having to manipulate the animals daily and aided in the identification of specific intricacies of infection not previously reported. For example, disruption of temperature homeostasis, episodes of hypothermia, and cardiac abnormalities in the form of changes in QT intervals in association with febrile episodes were observed.
In mammals, the range of normal body temperatures is small [35, 36], with prolonged hypothermic and hyperthermic conditions fluctuating by 2°C beyond normal values associated with tissue damage and impaired fetal development [35, 37, 38]. Interestingly, mammalian species have evolved unique thermoregulatory mechanisms in defense against pathogens. Rodents typically remain afebrile or decrease body temperatures in response to bacterial challenge and endotoxin administration [19, 22, 23, 39, 40]. In contrast, the variable membrane proteins (Vmps) of relapsing-fever spirochetes are implicated in inducing a toxic shock-like syndrome in humans, with acute febrile episodes and cardiac distress [9, 11, 12].
The susceptibility of rhesus macaques to B. turicatae challenge was first described by Dr Edward Francis, in 1938 [24], and subsequent primate models were developed with Borrelia recurrentis–infected grivet monkeys, Cercopithecus aethiops [25]. These studies suggested that the emergence of hyperthermia and hypothermia during infection with B. recurrentis can lead to terminal illness. In addition to the severity of temperature changes, we observed a disruption of QT intervals and tachycardia in the animals. Surprisingly, the physiological change in JD03 during the QT interval differed from that in IN57; specifically, the time the heart muscle repolarized following contraction was altered. For JD03, the QT interval increased significantly following the first febrile period and continued to increase for the duration of the study. This phenomenon has been observed in patients with Chagas disease cardiomyopathy and human immunodeficiency virus infection [41–43]. In contrast, IN57 showed a decreased QT interval during and after the febrile episodes, which became apparent after the duration was corrected for increased heart rate. A shortened QT interval has not been associated with infections but, rather, is considered a heritable condition [44]. Long-term studies with rhesus macaques and humans exposed to relapsing-fever spirochetes will be necessary to determine whether infections result in life-threatening cardiomyopathy.
Upon necropsy, pathological effects of infection were not significant, and we were unable to recover B. turicatae or detect spirochete DNA in brain tissue, heart, lung, liver, spleen, or kidney specimens. Similar pathological findings were observed in C. aethiops that had recovered after needle inoculation with B. recurrentis [45]. Judge et al reported that the histopathologic findings observed in a cohort of animals necropsied 3–4 days after infection were absent in the cohort of nonhuman primates evaluated during remission [45]. Furthermore, our inability to culture B. turicatae or detect bacterial DNA by real-time PCR suggests that the animals cleared the infection or that the spirochetes persisted at undetectable levels.
Species of relapsing fever Borrelia display various degrees of neurotropism. Borrelia hermsii inconsistently infects murine brains, while Borrelia duttonii, B. recurrentis, Borrelia crocidurae, and B. turicatae invade the central nervous system [21, 46]. While B. duttonii has been recovered by in vitro cultivation from brain tissues 270 days after infection [21], B. recurrentis, B. crocidurae, and B. turicatae were detected during acute infection [21, 46]. Furthermore, the kinetics of spirochete infection and vmp genes expressed by B. turicatae are involved with brain infection [21, 46]. In mice, production of VmpA was associated with the neurotropism of B. turicatae, while spirochetes producing VmpB were detected in the joints and heart [46–48]. Although we were unable to detect B. turicatae in the brain 64 days after transmission, we hypothesize that spirochetes would be detected during acute infection.
The administration of antibiotics can resolve infections caused by relapsing-fever spirochetes, yet they also induce the Jarisch-Herxheimer reaction, which is associated with a sharp rise in body temperature and cardiorespiratory distress and may be fatal to both mother and fetus [11, 49]. Prolonged periods of hyperthermia have also been linked to adverse outcomes in fetal development [37, 50]. Future studies will expand on the B. turicatae model to evaluate immunopathology during pregnancy, with a long-term goal of testing countermeasures against relapsing-fever spirochetes. The use of a nonhuman primate model and the ability to monitor disease progression in real time will enable the assessment of novel treatment measures and vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Tom Schwan at the Rocky Mountain Laboratories, NIAID, NIH for kindly providing the B. turicatae isolate used in the studies and the ticks necessary for the transmission studies.
Financial support. This work was supported by the Tulane National Primate Research Center.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vial L, Diatta G, Tall A, et al. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet. 2006;368:37–43. doi: 10.1016/S0140-6736(06)68968-X. [DOI] [PubMed] [Google Scholar]
- 2.McConnell J. Tick-borne relapsing fever under-reported. Lancet Infect Dis. 2003;3:604. doi: 10.1016/s1473-3099(03)00787-4. [DOI] [PubMed] [Google Scholar]
- 3.Jongen VH, van Roosmalen J, Tiems J, Van Holten J, Wetsteyn JC. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet Gynecol Scand. 1997;76:834–8. doi: 10.3109/00016349709024361. [DOI] [PubMed] [Google Scholar]
- 4.Melkert PW. Relapsing fever in pregnancy: analysis of high-risk factors. Br J Obstet Gynaecol. 1988;95:1070–2. doi: 10.1111/j.1471-0528.1988.tb06516.x. [DOI] [PubMed] [Google Scholar]
- 5.Melkert PW. Mortality in high risk patients with tick-borne relapsing fever analysed by the Borrelia-index. East Afr Med J. 1991;68:875–9. [PubMed] [Google Scholar]
- 6.Melkert PW, Stel HV. Neonatal Borrelia infections (relapsing fever): report of 5 cases and review of the literature. East Afr Med J. 1991;68:999–1005. [PubMed] [Google Scholar]
- 7.Coffey EM, Eveland WC. Experimental relapsing fever initiated by Borrelia hermsii. II. Sequential appearance of major serotypes in the rat. J Infect Dis. 1967;117:29–34. doi: 10.1093/infdis/117.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin MS, Schwan TG, Anderson DE, Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am. 2008;22:449–68. doi: 10.1016/j.idc.2008.03.006. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal V, Scragg IG, Cutler SJ, et al. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat Med. 1998;4:1416–20. doi: 10.1038/4007. [DOI] [PubMed] [Google Scholar]
- 10.Barbour AG, Dai Q, Restrepo BI, Stoenner HG, Frank SA. Pathogen escape from host immunity by a genome program for antigenic variation. Proc Natl Acad Sci U S A. 2006;103:18290–5. doi: 10.1073/pnas.0605302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryceson AD. Clinical pathology of the Jarisch-Herxheimer reaction. J Infect Dis. 1976;133:696–704. doi: 10.1093/infdis/133.6.696. [DOI] [PubMed] [Google Scholar]
- 12.Bryceson AD, Parry EH, Perine PL, Warrell DA, Vukotich D, Leithead CS. Louse-borne relapsing fever. Q J Med. 1970;39:129–70. [PubMed] [Google Scholar]
- 13.Salih SY, Mustafa D, Abdel Wahab SM, Ahmed MA, Omer A. Louse-borne relapsing fever: I. A clinical and laboratory study of 363 cases in the Sudan. Trans R Soc Trop Med Hyg. 1977;71:43–8. doi: 10.1016/0035-9203(77)90206-1. [DOI] [PubMed] [Google Scholar]
- 14.Warrell DA, Pope HM, Parry EH, Perine PL, Bryceson AD. Cardiorespiratory disturbances associated with infective fever in man: studies of Ethiopian louse-borne relapsing fever. Clin Sci. 1970;39:123–45. doi: 10.1042/cs0390123. [DOI] [PubMed] [Google Scholar]
- 15.Colombo MJ, Alugupalli KR. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol. 2008;180:4858–64. doi: 10.4049/jimmunol.180.7.4858. [DOI] [PubMed] [Google Scholar]
- 16.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–90. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Cadavid D, Thomas DD, Crawley R, Barbour AG. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–42. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright DJ. Reaction following treatment of murine borreliosis and Shwartzman type reacion with borrelial sonicates. Parasite Immunol. 1980;2:201–21. doi: 10.1111/j.1365-3024.1980.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 19.Wright DJ, Woodrow DF. Terminal changes in mice experimentally infected with Borrelia duttoni. J Pathol. 1980;130:83–90. doi: 10.1002/path.1711300204. [DOI] [PubMed] [Google Scholar]
- 20.Lopez JE, Wilder HK, Hargrove R, et al. Development of genetic system to inactivate a Borrelia turicatae surface protein selectively produced within the salivary glands of the arthropod vector. PLoS Negl Trop Dis. 2013 doi: 10.1371/journal.pntd.0002514. 7:e2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson C, Andersson M, Pelkonen J, Guo BP, Nordstrand A, Bergstrom S. Persistent brain infection and disease reactivation in relapsing fever borreliosis. Microbes Infect. 2006;8:2213–9. doi: 10.1016/j.micinf.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Halberg F, Spink WW. The influence of Brucella somatic antigen (endotoxin) upon the temperature rhythm of intact mice. Lab Invest. 1956;5:283–94. [PubMed] [Google Scholar]
- 23.Bennett IL, Jr, Cluff LE. Bacterial pyrogens. Pharmacol Rev. 1957;9:427–79. [PubMed] [Google Scholar]
- 24.Francis E. Longevity of the tick Ornithodoros turicata and of Spirochaeta recurrentis with this tick. Publ Hlth Rep. 1938;53:2220–41. [Google Scholar]
- 25.Judge DM, La Croix JT, Perine PL. Experimental louse-borne relapsing fever in the grivet monkey, Cercopithecus aethiops. I. Clinical course. Am J Trop Med Hyg. 1974;23:957–61. doi: 10.4269/ajtmh.1974.23.957. [DOI] [PubMed] [Google Scholar]
- 26.Kortepeter MG, Lawler JV, Honko A, et al. Real-time monitoring of cardiovascular function in rhesus macaques infected with Zaire ebolavirus. J Infect Dis. 2011;204(suppl 3):S1000–10. doi: 10.1093/infdis/jir337. [DOI] [PubMed] [Google Scholar]
- 27.Roy CJ, Adams AP, Wang E, et al. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014 doi: 10.1093/infdis/jiu014. 209:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:232–7. [Google Scholar]
- 29.Schwan TG, Raffel SJ, Schrumpf ME, et al. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J Clin Microbiol. 2005;43:3851–9. doi: 10.1128/JCM.43.8.3851-3859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valentinuzzi ME. A mathematical model of the electrocardiographic QT-RR relationship. Med Biol Eng. 1971;9:255–61. doi: 10.1007/BF02474080. [DOI] [PubMed] [Google Scholar]
- 31.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Battisti JM, Raffel SJ, Schwan TG. A system for site-specific genetic manipulation of the relapsing fever spirochete Borrelia hermsii. Methods Mol Biol. 2008;431:69–84. doi: 10.1007/978-1-60327-032-8_6. [DOI] [PubMed] [Google Scholar]
- 33.Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis. 2010;50:1629–34. doi: 10.1086/652769. [DOI] [PubMed] [Google Scholar]
- 34.Butler T, Hazen P, Wallace CK, Awoke S, Habte-Michael A. Infection with Borrelia recurrentis: pathogenesis of fever and petechiae. J Infect Dis. 1979;140:665–75. doi: 10.1093/infdis/140.5.665. [DOI] [PubMed] [Google Scholar]
- 35.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann N Y Acad Sci. 1998;856:224–33. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- 36.Roberts NJ., Jr Temperature and host defense. Microbiol Rev. 1979;43:241–59. doi: 10.1128/mr.43.2.241-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards MJ. Review: Hyperthermia and fever during pregnancy. Birth Defects Res A Clin Mol Teratol. 2006;76:507–16. doi: 10.1002/bdra.20277. [DOI] [PubMed] [Google Scholar]
- 38.Tiruvoipati R, Ong K, Gangopadhyay H, Arora S, Carney I, Botha J. Hypothermia predicts mortality in critically ill elderly patients with sepsis. BMC Geriatr. 2010;10:70. doi: 10.1186/1471-2318-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colvin JW, Mills CA. Ease of body heat loss and resistance to infection. Science. 1939;90:275–6. doi: 10.1126/science.90.2334.275-a. [DOI] [PubMed] [Google Scholar]
- 40.Klein MS, Conn CA, Kluger MJ. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol Behav. 1992;52:1133–9. doi: 10.1016/0031-9384(92)90472-e. [DOI] [PubMed] [Google Scholar]
- 41.Alvarado-Tapias E, Miranda-Pacheco R, Rodriguez-Bonfante C, et al. Electrocardiography repolarization abnormalities are characteristic signs of acute chagasic cardiomyopathy. Invest Clin. 2012;53:378–94. [PubMed] [Google Scholar]
- 42.Shimabukuro-Vornhagen A, Rybniker J, Zoghi S, et al. Acquired long QT syndrome and torsade de pointes associated with HIV infection. Case Rep Med. 2010 doi: 10.1155/2010/278427. 2010. Article ID 278427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinsch N, Buhr C, Krings P, et al. Prevalence and risk factors of prolonged QTc interval in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2009;10:261–8. doi: 10.1310/hct1004-261. [DOI] [PubMed] [Google Scholar]
- 44.Tristani-Firouzi M. The long and short of it: insights into the short QT syndrome. J Am Coll Cardiol. 2014;63:1309–10. doi: 10.1016/j.jacc.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Judge DM, La Croix JT, Perine PL. Experimental louse-borne relapsing fever in the grivet monkey, Cercopithecus aethiops. II. Pathology. Am J Trop Med Hyg. 1974;23:962–8. doi: 10.4269/ajtmh.1974.23.962. [DOI] [PubMed] [Google Scholar]
- 46.Cadavid D, Pennington PM, Kerentseva TA, Bergström S, Barbour AG. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–60. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pennington PM, Cadavid D, Barbour AG. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun. 1999;67:4637–45. doi: 10.1128/iai.67.9.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennington PM, Allred CD, West CS, Alvarez R, Barbour AG. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–92. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melkert PW. Fatal-Jarisch Herxheimer reaction in a case of relapsing fever misdiagnosed as lobar pneumonia. Trop Geogr Med. 1987;39:92–3. [PubMed] [Google Scholar]
- 50.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Maternal fever and birth outcome: a prospective study. Teratology. 1998;58:251–7. doi: 10.1002/(SICI)1096-9926(199812)58:6<251::AID-TERA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.