Abstract
Background:
Diffusion of new cancer treatments can be both inefficient and incomplete. The uptake of new treatments over time (diffusion) has not been well studied. We analyzed the diffusion of docetaxel in metastatic prostate cancer.
Methods:
We identified metastatic prostate cancer patients diagnosed from 1995 to 2007 using the Surveillance, Epidemiology, and End Results Program (SEER)–Medicare database. Medicare claims through 2008 were analyzed. We assessed cumulative incidence of docetaxel by socioeconomic, demographic, and comorbidity variables, and compared diffusion patterns to landmark events including release of phase III results and FDA approval dates. We compared docetaxel diffusion patterns in prostate cancer to those in metastatic breast, lung, ovarian, and gastric cancers. To model docetaxel use over time, we used the classic “mixed influence” deterministic diffusion model. All statistical tests were two-sided.
Results:
We identified 6561 metastatic prostate cancer patients; 1350 subsequently received chemotherapy. Among patients who received chemotherapy, docetaxel use was 95% by 2008. Docetaxel uptake was statistically significantly slower (P < .01) for patients older than 65 years, blacks, patients in lower income areas, and those who experienced poverty. Eighty percent of docetaxel diffusion occurred prior to the May, 2004 release of phase III results showing superiority of docetaxel over standard-of-care. The maximum increase in the rate of use of docetaxel occurred nearly simultaneously for prostate cancer as for all other cancers combined (in 2000).
Conclusion:
Efforts to increase the diffusion of treatments with proven survival benefits among disadvantaged populations could lead to cancer population survival gains. Docetaxel diffusion mostly preceded phase III evidence for its efficacy in castration-resistant prostate cancer, and appeared to be a cancer-wide—rather than a disease-specific—phenomenon. Diffusion prior to definitive evidence indicates the prevalence of off-label chemotherapy use.
The diffusion of new health care innovations can be inefficient: sometimes treatments with proven benefit permeate slowly through the treatment community, while in other instances, uptake of new drugs occurs prior to definitive evidence (1–3). For such reasons, the study of diffusion has been a major focus of agencies within the National Institutes of Health (NIH) (1). The past several decades have witnessed the introduction of multiple new cancer therapies. The appropriate and rapid adoption of proven new cancer treatments could impact population survival (4,5).
Diffusion is the transmission of a new innovation over time within a social system and is driven by perceptions of the innovation, characteristics of adopters, and contextual factors (6,7). Perceptions of an innovation pertain to (often qualitative) assessments of the risks and benefits of the new innovation. Presentation of efficacy findings for a new drug at a scientific conference or in a journal may influence the perception of new treatment benefits (8). Drugs with clearly positive benefit/risk ratios may be taken up immediately into clinical practice. One question is whether adoption follows definitive evidence of a new treatment in a phase III study. Patient characteristics may also influence patterns of chemotherapy use. For instance, older lymphoma and ovarian cancer patients are less likely to receive chemotherapy (2,9).
Patients with metastatic prostate cancer typically receive androgen deprivation therapy (ADT) (10), with response durations of 18 to 24 months (11,12). For patients with castration-resistant prostate cancer (CRPC), standard therapy was mitoxantrone combined with prednisone following positive clinical trials in the 1990s, showing that mitoxantrone provided palliative relief but no survival benefit (13,14). Docetaxel (Taxotere, Sanofi-Aventis) received US Food and Drug Administration (FDA) approval for treatment of advanced breast and lung cancers in the late 1990s. Thereafter in 2004, docetaxel was shown to provide both pain relief and improved survival in CRPC, reducing the risk of death by about 20%, and, with concurrent FDA approval, became new standard care (15,16). In this analysis, we hypothesized that docetaxel uptake followed definitive evidence of docetaxel efficacy in a phase III trial, and that diffusion was slower for disadvantaged patient populations.
Methods
We used the linked Surveillance, Epidemiology, and End Results Program (SEER)–Medicare database, a vital resource combining national cancer registry data (SEER) with medical claims data (Medicare) (17). The primary analysis included men older than 65 years diagnosed with metastatic prostate cancer from 1995 to 2007 (inclusive). Medicare claims through 2008 were analyzed. To avoid attributing receipt of chemotherapy to another cancer, men must have had no other prior or subsequent cancers. To ensure that patients had a minimum amount of Medicare claims coverage to provide an opportunity to receive treatment, we required patients to have had continuous Medicare Parts A and B, with no HMO participation, for one or more years after diagnosis.
Receipt of chemotherapy was identified at any time after diagnosis using Medicare claims according to ICD-9 Healthcare Common Procedure Coding System (HCPCS) J-codes from hospital outpatient and physician reimbursement records. Hospital inpatient records were used to identify diagnostic and surgical procedures for establishing comorbidity status (18,19). Docetaxel use was defined based on HCPCS J-code J9170 (first implemented on January 1, 1998), and mitoxantrone use was based on HCPCS J-code J9293 (first implemented on January 1, 1990) (20). Other potential chemotherapy types are shown in Supplementary Table 1 (available online). Although docetaxel did not receive a J-code until 1998, we included metastatic prostate cancer patients diagnosed from 1995 onward to allow up to three years of follow-up time, such that a set of patients would already be at risk of becoming castration-resistant (identified through receipt of chemotherapy) beginning in 1998, when the docetaxel J-code was first available, in order to better characterize docetaxel use early in the period. Unspecified J-codes (J8999, J9999) were not used because they may identify unanticipated procedures (21).
Dependent Variable
A challenge for this analysis was that prostate cancer patients who became castration resistant were the candidate population for chemotherapy. However, CRPC cases are not explicitly identifiable using SEER data. Rather, SEER patients are indexed according to the stage of their presenting diagnosis (local, regional, or distant metastatic). Thus the denominator of patients with CRPC was not explicitly identifiable.
Therefore, we first analyzed the five-year cumulative incidence of first docetaxel use from diagnosis of metastatic prostate cancer. Following a closed cohort over time using cumulative incidence accounts for competing events (ie, death) and is useful for assessing factors associated with use of docetaxel (22). However, the estimates represent docetaxel use among all patients diagnosed with metastatic prostate cancer, not those with CRPC.
Secondly, we analyzed the subset of metastatic prostate cancer patients who received chemotherapy from 1998 to 2008. This subset represents CRPC cases, because chemotherapy would typically not be used in prostate cancer prior to castration resistance. Within each yearly interval, among those who received their first chemotherapy, we calculated the rate of docetaxel use. This approach mimics a series of cross-sectional yearly cohorts (23) and has the advantage of assessing docetaxel usage rates over calendar time. First receipt of chemotherapy was used because it is consistent with the cumulative incidence analysis and in the real world represents the chemotherapy of first choice.
The Model
In diffusion analyses, cumulative adoption over time typically adheres to an S-shaped or sigmoid curve, representing a pattern of bounded geometric growth in which adoption occurs infrequently at first, accelerates as more individuals adopt, then slows as adoption reaches a natural ceiling (7,24–26). To model yearly docetaxel use rates, we used the classic “mixed influence” deterministic diffusion model, which describes the instantaneous change in the shape of the diffusion curve by the differential equation:
where F(t) is the cumulative number of adopters at time t, F is the total potential number of adopters, and k 1 and k 2 are coefficients representing a mix of influences both external to the social system (k 1) and internal to the social system (k 2) (24,27). Conceptually, the behavior of this function indicates the influence of social dynamics on diffusion, because, if the magnitude of k 2 is nontrivial, then the instantaneous rate of change of diffusion with respect to time is proportional to the interaction between prior adopters (k 2 *F(t)) and potential adopters (F-F(t)) (28). Thus, the magnitude of k 2 relative to k 1 suggests the extent to which an underlying social process influences diffusion.
Independent Variables
Socioeconomic Status, Demographic, Comorbidity, and Geographic Variables
We analyzed the five-year cumulative incidence of docetaxel by demographic variables including age (split at 75 years) and race (black vs other). Socioeconomic (SES) factors were income and education, based on whether the patient’s Year 2000 Census tract median income and percentage with some college education were, respectively, higher or lower than the study sample median. Poverty status was based on individual-level data reflecting prior Medicaid participation (yes vs no) (29). Differences by baseline comorbidity index within one year prior to diagnosis were analyzed using the Charlson index, modified as per Klabunde (18,19,30). Binary indicator variables were used for consistency across variables and to aid interpretation, with the exception of geographic region, which was analyzed by SEER registry area (East vs Midwest vs West). Univariate associations were tested using Gray’s test (31) and multivariable associations using Cox regression (32). All statistical tests were two-sided.
Landmark Events
We estimated the proportion of total diffusion occurring prior to October, 1999, the period prior to the first early phase (phase I and II) study reports regarding docetaxel efficacy (33–37); the proportion of diffusion occurring between October 1999 and May 2004, the period between the first early phase study reports and phase III reports/FDA approval (which occurred nearly simultaneously) (38–40); and after May, 2004.
Docetaxel Use over Time in Prostate Cancer Compared With Other Cancers
To evaluate whether docetaxel diffusion patterns for prostate cancer were unique among cancers, we compared them with those in advanced breast, gastric, ovarian, and non–small cell lung cancer (NSCLC), which served as controls. For these other cancers, docetaxel is often indicated after failure of initial chemotherapy. Therefore, rather than using first chemotherapy, within each year a patient received chemotherapy, we coded patients as “1” if docetaxel was received, “0” if other chemotherapy was received, and “0.5” if both were received. We compared patterns across all cancers with FDA approval times.
Results
Cohort Characteristics
We identified 6561 patients with metastatic prostate cancer meeting the inclusion criteria (Table 1). The majority of patients (58%) were age 75 years or older, 14% were black, and 7% were Hispanic. Poverty was reported in 21% of patients. Median Census tract income was $42 654, higher than the median US year 2000 income for this age cohort (41). Thirty percent had evidence of prior comorbidity.
Table 1.
Cohort characteristics
| Factor | Subsequently received chemotherapy? | ||
|---|---|---|---|
| Metastatic prostate cancer (n = 6561) | Yes (ie, castration-resistant prostate cancer) (n = 1350) | No (n = 5211) | |
| Age, ≥75 y, % | 58 | 41 | 63* |
| Black, % | 14 | 10 | 16* |
| Asian/Pacific Islander, % | 6 | 5 | 6 |
| Hispanic origin, % | 7 | 8 | 6 |
| Income, % | |||
| ≥$50 000/year, % | 37 | 46* | 35 |
| Poverty, % | 21 | 15 | 23* |
| Median, $ | 42 654 | 47 344* | 41 692 |
| Median proportion with some college, % | 28 | 28 | 28 |
| Site‡ | |||
| East, % | 18 | 20† | 17 |
| Midwest, % | 37 | 35 | 37 |
| West, % | 45 | 44 | 46 |
| Comorbidity index ≥1, % | 30 | 25 | 31* |
* Statistically significantly higher, P < .001.
† Statistically significantly higher, .01 ≤ P ≤ .05.
‡ Surveillance, Epidemiology, and End Results Program registry areas were categorized according to geographic location as follows: “East” included Connecticut and New Jersey; “Midwest” included Atlanta, Detroit, Iowa, Kentucky, Louisiana, Rural Georgia, and Utah; and “West” included California, Hawaii, New Mexico, and Seattle.
We identified 1350 patients who subsequently received chemotherapy. Compared with the 5211 patients without subsequent chemotherapy, chemotherapy patients were younger, less likely to be black, had higher income, and had less comorbidity.
Cumulative Incidence by Year of Diagnosis
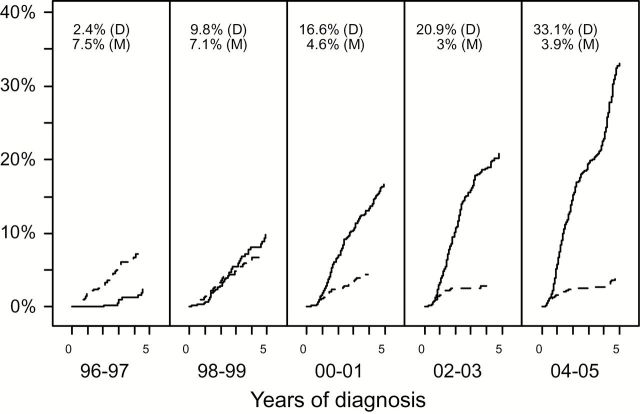
Five-year cumulative incidence of docetaxel use after diagnosis of metastatic prostate cancer increased from 2% for patients diagnosed in 1996 or 1997 to 33% for patients diagnosed in 2004 or 2005 (Figure 1). The use of mitoxantrone decreased in conjunction with the increased use of docetaxel, although five-year cumulative incidence of mitoxantrone use never exceeded 7%.
Figure 1.
Five-year cumulative incidence of docetaxel (D, solid line) and mitoxantrone (M, dashed line) use from diagnosis among patients presenting with metastatic prostate cancer. The figure shows cumulative incidence for each two-year cohort from 1996 through 2005, inclusive. The cohort intervals were constructed such that the latest interval, for patients diagnosed from 2004 through 2005, had up to five years of potential follow-up (given that Medicare claims through 2008 were used). Cumulative incidence for patients diagnosed in 1995, representing only a single year of diagnoses, is not shown. Five-year cumulative incidence estimates are indicated at the top of each panel.
Cumulative Incidence by Factors
Figure 2 shows five-year cumulative incidence of docetaxel use combined over all years of diagnosis (1995–2007) by SES, demographic, comorbidity, and geographic factors. The cumulative incidence was slower for older patients, black patients, patients experiencing poverty, lower-income patients, and patients from Western and Midwestern SEER regions (all P < .01), in both univariate and multivariable settings.
Figure 2.
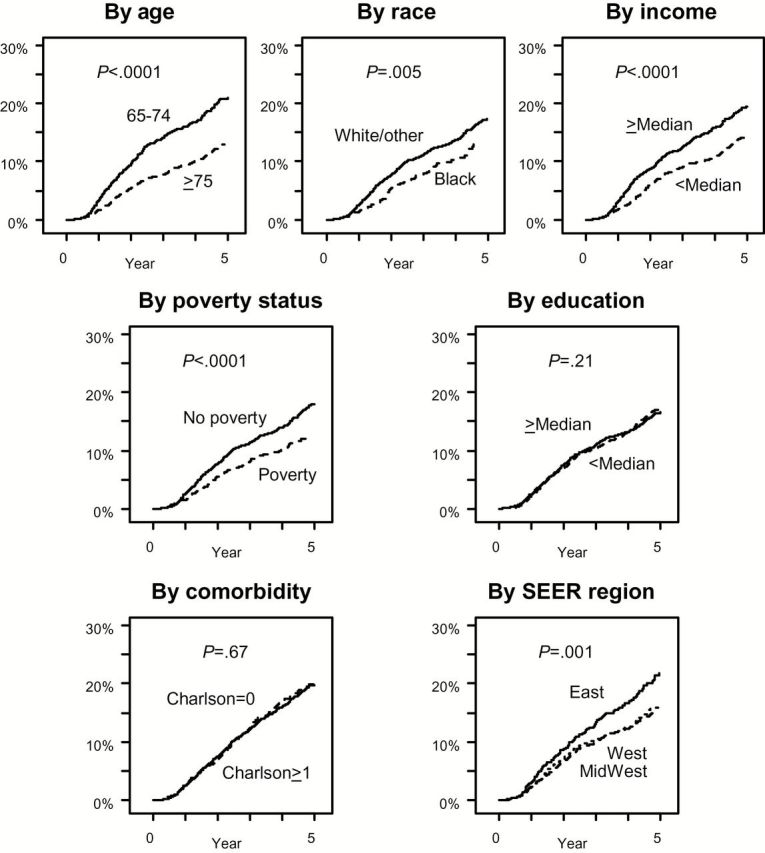
Five-year cumulative incidence of docetaxel use from diagnosis among patients presenting with metastatic prostate cancer by socioeconomic status, demographic, comorbidity, and geographic factors. (Cumulative incidence by ethnicity was not analyzed because of the small subset of Hispanic patients.) Patients for all years of diagnosis were included. Five-year cumulative incidence estimates by factor were: by age: 24.8% (95% confidence interval [CI] = 22.7% to 26.9%) for patients age 65 to 74 years vs 15.4% (95% CI = 13.7% to 17.1%) for patients older than 75 years; by race: 20.3% (95% CI = 18.9% to 21.8%) for white/other patients vs 16.9% (95% CI = 13.5% to 20.6%) for black patients; by income: 22.8% (95% CI = 20.8% to 24.8%) for patients from Census tract regions with incomes greater than the median vs 16.9% (95% CI = 15.2% to 18.8%) for patients from Census tract regions with incomes lower than the median; by poverty status: 21.2% (95% CI = 19.7% to 22.8%) for patients with no evidence of poverty and 14.5% (95% CI = 12.0% to 17.1%) for patients with evidence of poverty; by education: 20.1% (95% CI = 18.2% to 22.0%) for patients from Census tract regions with education over the median vs 19.8% (17.9% to 21.7%) for patients from Census tract regions with education under the median; by comorbidity status: 19.8% (18.2% to 21.3%) for patients with a Charlson score of 0 vs 20.1% (95% CI = 17.4% to 22.9%) for patients with a Charlson score over 1; by Surveillance, Epidemiology, and End Results Program (SEER) region: 25.6% (95% CI = 22.0% to 29.4%) for patients from Eastern regions vs 18.8% (95% CI = 16.9% to 20.8%) for patients from Western regions vs 18.5% (95% CI = 16.4% to 20.7%) for patients from Midwestern regions. All four statistically significant univariate predictors (age, race, income, and SEER region) remained statistically significant predictors (P < .01 for each) in adjusted multivariable regression. All statistical tests were two-sided. SEER = Surveillance, Epidemiology, and End Results Program.
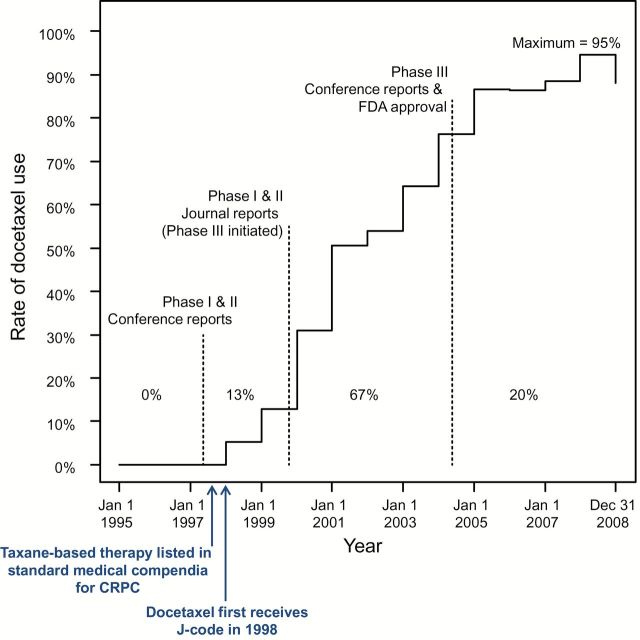
Association of Landmark Events With Docetaxel Use Over Time
As shown in Figure 3, among metastatic prostate cancer patients who received chemotherapy, the observed proportion whose first chemotherapy was docetaxel was 95% by 2008. Docetaxel uptake in this patient population began well before the results for the phase III trials were reported. Thirteen percent of total (maximum) diffusion occurred prior to initial phase I and II journal reports, 67% between phase I and II journal reports and phase III conference reports/FDA approval, and 20% after the phase III reports/FDA approval.
Figure 3.
Proportion using docetaxel over time with landmark events. Thirteen percent of total (ie, maximum) diffusion occurred prior to phase I and II journal reports, 67% between phase I and II journal reports and initial phase III conference reports and US Food and Drug Administration (FDA) approval, and 20% after the phase III reports/FDA approval. Importantly, taxane-based therapy was included in standard medical compendia (ie, National Comprehensive Cancer Network) as a treatment for castration-resistant prostate cancer as early as 1997, with docetaxel in particular listed prior to publication of phase III evidence and FDA approval (76,77). CRPC = castration-resistant prostate cancer; FDA = US Food and Drug Administration.
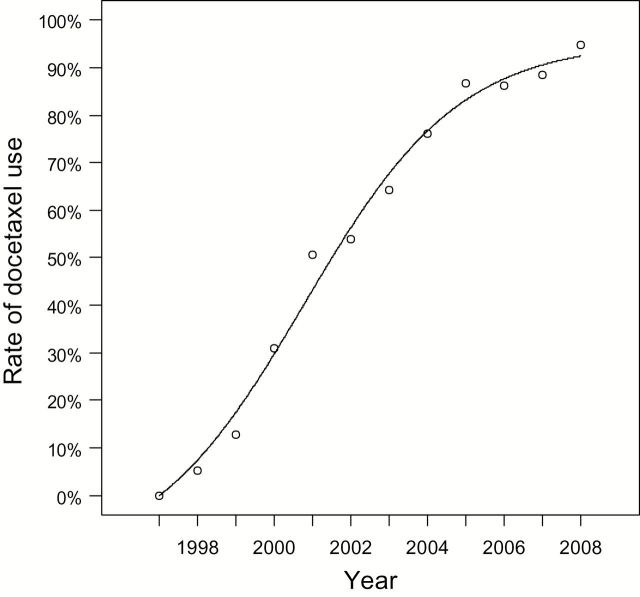
The diffusion model fitted to the rates in Figure 3 showed an S-shaped trajectory (Figure 4). The model explained 99.2% of total variation. Importantly, the regression coefficient for the internal influence factor (k 2) was about 7.2x greater than the coefficient for the external influence factor (k 1), consistent with the notion that social dynamics within the prostate cancer treatment community contributed to diffusion.
Figure 4.
Mixed influence diffusion model fit of rate of docetaxel use over time by year. The model explained 99.2% of total observed variation. The coefficient for k 2 (= 0.462), the measure of internal influence, was 7.2x the magnitude of the coefficient for k 1 (= 0.064), consistent with the hypothesis that social dynamics played an important role in the diffusion of docetaxel.
Use of Docetaxel for Multiple Cancers in Relation to FDA Drug Approval
Figure 5A compares yearly rates of docetaxel use in metastatic prostate cancer to those in metastatic breast, NSCLC, gastric, and ovarian cancers. Uptake of docetaxel began and achieved maximums at similar times for all cancers, regardless of whether FDA approval was received early in the period (breast and NSCLC), late in the period (prostate and gastric), or never (ovarian cancer). Maximum diffusion was notably higher among prostate cancer patients, likely because of fewer effective chemotherapy options.
Figure 5.
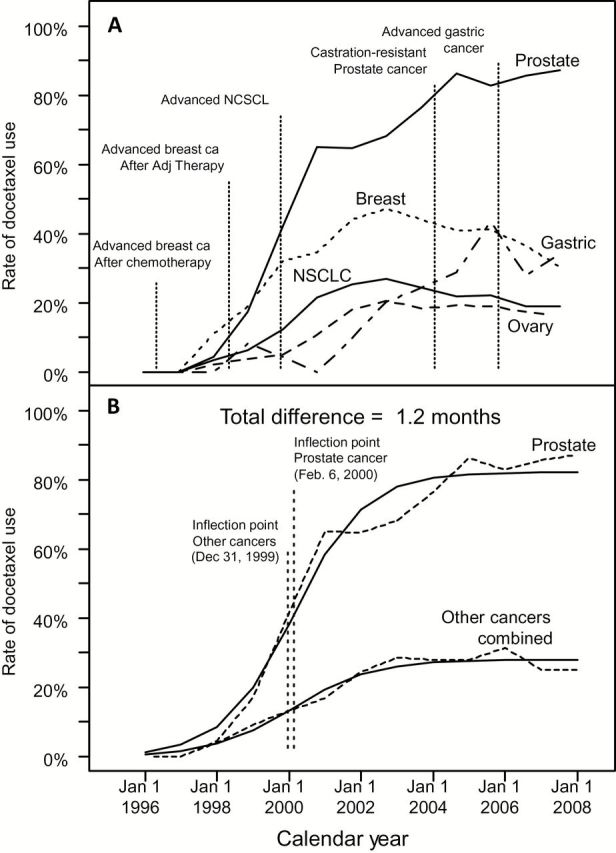
Rate of docetaxel use over time, by cancer type, with landmark events and model-fitted curves. A) The proportion using docetaxel over time for metastatic prostate cancer is compared with rates in advanced breast, lung, gastric, and ovarian cancers. US Food and Drug Administration (FDA) approval times for each cancer are shown. Docetaxel was approved for use in patients with locally advanced or metastatic breast cancer after failure of prior chemotherapy in May, 1996; in patients with metastatic breast cancer who failed adjuvant therapy in June, 1998; in patients with non–small cell lung cancer who failed cisplatin-based treatment in December, 1999; and in patients with advanced gastric cancer (in combination with 5-FU and cisplatin) in March, 2006. Docetaxel has not received an FDA indication for use in ovarian cancer patients, but is included to convey the similarity of diffusion patterns of docetaxel for a cancer in which prescriptions are strictly off-label. B) The observed proportions using docetaxel over time for metastatic prostate cancer are compared with the combined rates from advanced breast, lung, gastric, and ovarian cancers. Fitted model-based estimates are superimposed. The inflection points for the fitted curves indicate the time of maximum increase in the rate of docetaxel use and are approximately the same (1.2 months or 37 days difference) between the two curves. NSCLC = non–small cell lung cancer.
Figure 5B shows the observed diffusion rates for prostate cancer compared with all other cancers combined, with model fitted curves superimposed. The inflection point—representing the time of maximum increase in the use of docetaxel—for prostate cancer and for all other cancers combined occurred within 1.2 months of each other. Therefore, docetaxel diffusion patterns with respect to time were very similar.
Discussion
In this study, we found that docetaxel diffusion largely preceded definitive phase III evidence of efficacy in CRPC. Importantly, docetaxel diffusion was slower for certain subgroups of disadvantaged patients, including blacks and those with lower income. Also, docetaxel diffusion occurred simultaneously across multiple cancers, suggesting that its uptake was independent of clinical evidence for particular cancers.
Studies of cancer treatment use by patient SES, demographic levels, and health status have frequently shown lower usage for disadvantaged patients (8,9,42–54). Differences by geographic region have also been found (55). Diffusion, which tracks patterns of usage over time, has been explicitly studied in some instances. Slower diffusion for older patients, minorities, and patients with lower SES were identified (22,56,57). In this study, docetaxel diffusion was slower for socioeconomically and demographically disadvantaged patients. The absence of differences in cumulative incidence by comorbidity status is surprising, but may be because of sicker patients becoming castration resistant more quickly, hastening receipt of chemotherapy.
The observation that disadvantaged patients have slower diffusion presents opportunities to improve uptake of proven new therapies in subpopulations. For instance, direct-to-consumer advertising (DTCA) has recently been shown to improve the appropriate use of aromatase inhibitors (58). Since oncology patients are frequently aware of DTCA, DTCA could be a useful tool to promote the use of proven new therapies in certain target populations (59). Even if patient out-of-pocket costs for newer treatments are similar, anxiety about how to pay may exist (60–61), in which case improved communication between physicians and patients is crucial for clarifying treatment costs (62).
Evidence of a sigmoid shape for use of docetaxel over time is consistent with prior observations that social dynamics, especially among physicians, accelerate new innovation diffusion (7,25). Thus, enhancing communication channels among physicians, especially between key opinion leaders and their colleagues, may encourage more rapid adoption of treatments. One factor that has been repeatedly identified to increase adoption rates is attendance at scientific symposia (8,63), which serve as forums for disseminating information about new treatments. Unfortunately, the nature of the relationships among physicians was not analyzable in this study because of lack of data on their social links.
The rapid uptake of docetaxel in CRPC prior to definitive evidence from a phase III trial is a concern. Considerations that may have led to early adoption of docetaxel include prior FDA indications in other solid tumors and the fact that conventional treatment, mitoxantrone, provided only palliative relief, whereas early pilot trials for docetaxel showed the additional promise of a survival benefit (64). However, despite the early evidence, the positive result for docetaxel in randomized trials was not a foregone conclusion. Indeed, multiple phase III trials for drugs already in wide use have returned negative results (65–67). In some instances, phase III evidence led to an appropriate diminution in the use of the new drug (3,68), though not in all (48,69).
The evidence in Figure 5 indicates that docetaxel diffusion occurred across different cancers approximately simultaneously, in most cases prior to FDA approval. This suggests that once oncologists begin to use a drug for a given cancer, they may be more likely to do so for other cancers; the mechanism that allows this is off-label drug use. Off-label use is considered appropriate in many instances, with 25% to 50% of cancer drug prescriptions delivered off label (70–73). Reimbursement for off-label drug use is facilitated by inclusion in medical compendia. For instance, Medicare contractors are required by Congress to pay for cancer drug prescriptions if their use is supported by selected standard medical compendia (74,75). Importantly, taxane-based therapy, including docetaxel, was itself included in standard medical compendia for treatment of CRPC prior to publication of phase III evidence (76,77). Reliance on compendia to facilitate treatment reimbursement represents an attempt to balance tradeoffs. On the one hand, the requirement that every variation in target population for a drug require a separate FDA indication would overwhelm available resources. On the other hand, reliance on compendia of potentially questionable quality may lead to inappropriate use. And, in fact, questions about the quality of the medical compendia have been raised. A recent review found that compendia “lack transparency, cite little current evidence, and lack systematic methods to review or update evidence” (78). The evaluation of evidence for docetaxel in particular was found to be problematic (79). The questionable quality of medical compendia is astonishing in light of the role compendia play in determining reimbursement. The American Society of Clinical Oncology has stated that the “system for identifying medically appropriate cancer therapies, including those that involve off-label uses… requires attention” (70).
One limitation in this study is the inability to identify the true denominator of patients who become castration resistant, a key eligibility criterion for receiving docetaxel. Identifying CRPC cases based on receipt of chemotherapy does not capture patients with CRPC who received no chemotherapy. Such patients may be too sick to receive chemotherapy. Thus, diffusion estimates for CRPC are likely biased high. In this context, performance status would be an informative descriptive factor, but it was not available. The necessity of using Medicare claims to identify relapse or recurrence is often problematic (21), but especially when treatment is itself the endpoint, because it raises the question of whether patients not identified as relapse/recurrent by Medicare claims (ie, HMO patients) may be different, limiting generalizability of the findings. Also, the use of Medicare data limits the analysis to patients age 65 years or older. However, prostate cancer occurs primarily in patients age 65 years or older (~70% of cases) (80), and older patients may receive suboptimal care, representing a critical target population (81,82). Physician-level data were not available; therefore, the extent to which physicians were exposed to marketing efforts (which may have reinforced docetaxel uptake) was not identifiable in the data, nor were differences in diffusion according to facility type or degree of provider specialization. Finally, these results may not be generalizable to agents with different trial evidence profiles.
Currently, the FDA is reviewing whether to loosen constraints on the marketing of drugs prescribed off label (83). In contrast, our findings point to the potential risks of off-label use. By enabling the widespread diffusion of a new therapy prior to definitive phase III evidence, the off-label mechanism undermines the assumption that phase III comparative clinical trials necessarily determine which treatments become standard care. In this setting, inappropriate use is inevitable, with potential costs in increased morbidity and mortality if a different drug may have been more appropriate. Inappropriate use also places an unnecessary financial burden on health care payers. As declared by ASCO, medical compendia, which facilitate off-label reimbursement, require greater oversight (70), especially if they serve as the arbiters of reimbursement for Medicare, a federal program. Ultimately, greater levels of investment in clinical research are required to produce the highest levels of evidence—especially phase III trial evidence—for a given indication, in order to reduce the tendency for off-label use.
Funding
Funding for this work was provided to the SWOG cancer research cooperative group, Outcomes and Comparative Effectiveness Committee (Cancer Control and Prevention) by Public Health Service grant CA37429 awarded by the National Cancer Institute (NCI), Division of Cancer Prevention.
Supplementary Material
None of the authors have any conflicts to declare with this manuscript. This study used the linked Surveillance, Epidemiology, and End Results Program (SEER)–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program of the NCI, the Office of Research, Development and Information of the Centers for Medicare and Medicaid Services, Information Management Services (IMS), Inc., and the SEER Program tumor registries in the creation of the SEER–Medicare database.
Prior Presentations: The results of this study were previously presented at the Scientific Session of the SWOG Outcomes and Comparative Effectiveness Committee meeting on May 2, 2013. The results have not been previously published.
References
- 1. Agency for Healthcare Research and Quality. Translating Research into Practice. http://grants.nih.gov/grants/guide/pa-files/PA-02-066.html. Accessed July 22, 2014.
- 2. Grann VR, Hershman D, Jacobson JS, et al. Outcomes and diffusion of doxorubicin-based chemotherapy among elderly patients with aggressive non-Hodgkin lymphoma. Cancer. 2006;107(7):1530–1541. [DOI] [PubMed] [Google Scholar]
- 3. Haas JS, Kaplan CP, Gerstenberger EP, et al. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–188. [DOI] [PubMed] [Google Scholar]
- 4. Booth CM, Shepherd FA, Peng Y, et al. Adoption of adjuvant chemotherapy for non-small-cell lung cancer: a population-based outcomes study. J Clin Oncol. 2010;28(21):3472–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Unger JM, LeBlanc M, Crowley JJ, et al. Estimating the impact of new clinical trial proven cancer therapy and cancer chemoprevention on population mortality: the Karnofsky Memorial lecture. J Clin Oncol. 2003;21(23 Suppl):246s–252s. [DOI] [PubMed] [Google Scholar]
- 6. Berwick DM. Disseminating innovations in health care. JAMA. 2003;289(15):1969–1975. [DOI] [PubMed] [Google Scholar]
- 7. Rogers EM. Diffusion of Innovations. 5th ed. New York, NY: Free Press; 2003. [Google Scholar]
- 8. Giordano SH, Duan Z, Kuo YF, et al. Impact of a scientific presentation on community treatment patterns for primary breast cancer. J Natl Cancer Inst. 2006;98(6):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sundararajan V, Hershman D, Grann VR, et al. Variations in the use of chemotherapy for elderly patients with advanced ovarian cancer: a population-based study. J Clin Oncol. 2002;20(1):173–178. [DOI] [PubMed] [Google Scholar]
- 10. Perlmutter MA, Lepor H. Androgen Deprivation Therapy in the Treatment of Advanced Prostate Cancer. Rev Urol. 2007;9(Suppl 1): S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 11. Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321(7):419–424 [Erratum, N Engl J Med. 1989;321:1420.] [DOI] [PubMed] [Google Scholar]
- 12. Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339(15):1036–1042. [DOI] [PubMed] [Google Scholar]
- 13. Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–1764. [DOI] [PubMed] [Google Scholar]
- 14. Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999;17(8):2506–2513. [DOI] [PubMed] [Google Scholar]
- 15. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. [DOI] [PubMed] [Google Scholar]
- 16. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. [DOI] [PubMed] [Google Scholar]
- 17. Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–748. [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 19. Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. [DOI] [PubMed] [Google Scholar]
- 20. ICD9Data.com. http://www.icd9data.com/HCPCS/2011/J/J9170. htm. Accessed July 22, 2014.
- 21. Earle CC, Nattinger AB, Potosky AL, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40(Suppl 8):S75–S81. [DOI] [PubMed] [Google Scholar]
- 22. Sima CS, Panageas KS, Heller G, et al. Analytical strategies for characterizing chemotherapy diffusion with patient-level population-based data. Appl Health Econ Health Policy. 2010;8(1):37–51. [DOI] [PubMed] [Google Scholar]
- 23. Cronin KA, Yu B, Binbing Y, Krapcho M, et al. Modeling the dissemination of mammography in the United States. Cancer Causes Control. 2005;16(6):701–712. [DOI] [PubMed] [Google Scholar]
- 24. Bass FM. A new-product growth model for consumer durables. Management Science. 1969;15(5):215–227. [Google Scholar]
- 25. Coleman J, Katz E, Menzel H. The diffusion of an innovation among physicians. Sociometry. 1957;20(4):253–270. [Google Scholar]
- 26. Mansfield E. Technical change and the rate of imitation. Econometrica. 1961;29(4):741–766. [Google Scholar]
- 27. Sharif MN, Ramanathan K. Binomial innovation diffusion models with dynamic potential adopter population. Technological Forecasting and Social Change. 1981;20(1):63–87. [Google Scholar]
- 28. Mahajan V, Peterson RA. “Models for innovation diffusion.” Sage University Paper Series on Quanitative Applications in the Social Sciences, CA: Sage; 1985. [Google Scholar]
- 29. Bach PB, Guadagnoli E, Schrag D, et al. Patient demographic and socioeconomic characteristics in the SEER-Medicare database: Applications and Limitations. Med Care. 2002;40(8 Suppl):IV-19–25. [DOI] [PubMed] [Google Scholar]
- 30. National Cancer Institute website. SEER-Medicare: Calculation of comorbidity weights. http://healthservices.cancer.gov/seermedicare/program/comorbidity.html. Accessed July 22, 2014.
- 31. Gray R. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals Statis. 1998;16(3):1141–1154. [Google Scholar]
- 32. Cox DR. Regression models and life tables (with discussion). J Roy Stat Soc Serv B. 1972;34(2):187–220. [Google Scholar]
- 33. Beer TM, Pierce WC, Lowe BA, et al. Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Ann Oncol. 2001;12(9):1273–1279. [DOI] [PubMed] [Google Scholar]
- 34. Berry W, Dakhil S, Gregurich MA, et al. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;28(4 Suppl 15):8–15. [DOI] [PubMed] [Google Scholar]
- 35. Friedland D, Cohen J, Miller R, Jr, et al. A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: correlation of antitumor effect to phosphorylation of Bcl-2. Semin Oncol. 1999;26(5 Suppl 17):19–23. [PubMed] [Google Scholar]
- 36. Picus J, Schultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin Oncol. 1999;26(5 Suppl 17):14–18. [PubMed] [Google Scholar]
- 37. Savarese D, Taplin ME, Halabi S, et al. A phase II study of docetaxel (Taxotere), estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: preliminary results of cancer and leukemia group B Trial 9780. Semin Oncol. 1999;26(5 Suppl 17):39–44. [PubMed] [Google Scholar]
- 38. Dagher R, Li N, Abraham S, et al. Approval summary: Docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10(24):8147–8151. [DOI] [PubMed] [Google Scholar]
- 39. Eisenberger MA, De Wit R, Berry W, et al. A multicenter phase III comparison of docetaxel (D) + prednisone (P) and mitoxantrone (MTZ) + P in patients with hormone-refractory prostate cancer (HRPC). ASCO. 2004;22(14 Suppl 4). [Google Scholar]
- 40. Petrylak DP, Tangen C, Hussain M, et al. Randomized phase III trial of docetaxel (D)/estramustine (E) vs mitoxantrone(M)/prednisone(p) in men with androgen-independent prostate cancer (AIPCA). ASCO. 2004;22(14 Suppl 3). [Google Scholar]
- 41. U.S. Census Bureau, Current Population Reports, P60-213, Money Income in the United States: 2000. Washington, DC: U.S. Government Printing Office; 2001. [Google Scholar]
- 42. Giordano SH, Lin YL, Kuo YF, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hershman DL, Buono D, Jacobson JS, et al. Surgeon characteristics and use of breast conservation surgery in women with early stage breast cancer. Ann Surg. 2009;249(5):828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat. 2012;136(2):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matushansky I, Dela Cruz F, Insel BJ, et al. Chemotherapy use in elderly patients with soft tissue sarcoma: A population-based study. Cancer Invest. 2013;31(2):83–91. [DOI] [PubMed] [Google Scholar]
- 46. Neugut AI, Hillyer GC, Kushi LH, et al. Noninitiation of adjuvant chemotherapy in women with localized breast cancer: the breast cancer quality of care study. J Clin Oncol. 2012;30(31):3800–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neugut AI, Becker DJ, Insel BJ, et al. Uptake of oxaliplatin and bevacizumab for treatment of node-positive and metastatic colon cancer. J Oncol Pract. 2012;8(3):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005. [DOI] [PubMed] [Google Scholar]
- 49. Spencer BA, Insel BJ, Hershman DL, Benson MC, Neugut AI. Racial disparities in the use of palliative therapy for ureteral obstruction among elderly patients with advanced prostate cancer. Support Care Cancer. 2013;21(5):1303–1311. [DOI] [PubMed] [Google Scholar]
- 50. Strauss J, Hershman DL, Buono D, et al. Use of adjuvant 5-fluorouracil and radiation therapy after gastric cancer resection among the elderly and impact on survival. Int J Radiat Oncol Biol Phys. 2010;76(5):1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sundararajan V, Grann VR, Jacobson JS, et al. Variations in the use of adjuvant chemotherapy for node-positive colon cancer in the elderly: a population-based study. Cancer J. 2001;7(3):213–218. [PubMed] [Google Scholar]
- 52. Temple LK, Hsieh L, Wong WD, et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22(17):3475–3484. [DOI] [PubMed] [Google Scholar]
- 53. Wright JD, Burke WM, Wilde ET, et al. Comparative effectiveness of robotic vs laparoscopic hysterectomy for endometrial cancer. J Clin Oncol. 2012;30(8):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zafar SY, Malin JL, Grambow SC, et al. Early dissemination of bevacizumab for advanced colorectal cancer: A prospective cohort study. BMC Cancer. 2011:11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nattinger AB, Gottlieb MS, Veum J, et al. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326(17):1102–1107. [DOI] [PubMed] [Google Scholar]
- 56. Chen AY, Halpern MT, Schrag NM, et al. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998–2005). J Natl Cancer Inst. 2008;100(7):462–474. [DOI] [PubMed] [Google Scholar]
- 57. Flowers CR, Fedewa SA, Chen AY, et al. Disparities in the early adoption of chemoimmunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abel GA, Chen K, Taback N, et al. Impact of oncology-related direct-to-consumer advertising: Association with appropriate and inappropriate prescriptions. Cancer. 2013;119(5):1065–1072. [DOI] [PubMed] [Google Scholar]
- 59. Abel GA, Burstein HJ, Hevelone ND, et al. Cancer related direct-to-consumer advertising: Awareness, perceptions, and reported impact among patients undergoing active cancer treatment. J Clin Oncol. 2009;27(25):4182–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim P. Cost of cancer care: the patient perspective. J Clin Oncol. 2007;25(2):228–232. [DOI] [PubMed] [Google Scholar]
- 61. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schrag D, Hanger M. Medical oncologists’ views on communicating with patients about chemotherapy costs: A pilot survey. J Clin Oncol. 2007;25(2):233–237. [DOI] [PubMed] [Google Scholar]
- 63. Buban GM, Link BK, Doucette WR. Influences on oncologists’ adoption of new agents in adjuvant chemotherapy of breast cancer. J Clin Oncol. 2001;19(4):954–959. [DOI] [PubMed] [Google Scholar]
- 64. Petrylak DP. Chemotherapy for advanced hormone refractory prostate cancer. Urology. 1999;54(6A Suppl):30–35. [DOI] [PubMed] [Google Scholar]
- 65. Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10): 971–977. [DOI] [PubMed] [Google Scholar]
- 66. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 67. Stadtmauer EA, O’Neill A, Goldstein LJ, et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. Philadelphia Bone Marrow Transplant Group. N Engl J Med. 2000;342(15):1069–1076. [DOI] [PubMed] [Google Scholar]
- 68. Bekelman JE, Rosenzweig KE, Bach PB, et al. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):492–499. [DOI] [PubMed] [Google Scholar]
- 69. Soulos PR, Yu JP, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30(14):1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. American Society of Clinical Oncology. Reimbursement for cancer treatment: coverage of off-label drug indications. J Clin Oncol. 2006;24(19):3206–3208. [DOI] [PubMed] [Google Scholar]
- 71. Conti RM, Bernstein AC, Villaflor VM, et al. Prevalence of Off-Label Use and Spending in 2010 Among Patent-Protected Chemotherapies in a Population-Based Cohort of Medical Oncologists. J Clin Oncol. 2013;31(9):1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. United States General Accounting Office: Off-Label Drugs: Reimbursement Policies Constrain Physicians in Their Choice of Cancer Therapies. September 1991: GAO/PEMD-91-14. http://archive.gao.gov/d18t9/144933.pdf. Accessed July 22, 2014.
- 73. Van Allen EM, Miyake T, Gunn N, et al. Off-label use of rituximab in a multipayer insurance system. J Oncol Pract. 2011;7(2):76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Omnibus Budget Reconciliation Act of 1993. www.gpo.gov/fdsys/pkg/BILLS-103hr2264enr/pdf/BILLS-103hr2264enr.pdf. Accessed July 22, 2014.
- 75. Center for Medicare and Medicaid Services regulations and guidance transmittal. Subject: “Compendia and Authoritative Sources for use in the Determination of a ‘Medically Accepted Indication’ of Drugs and Biologicals Used Off-Label in an Anti-Cancer Chemotherapeutic Regimen.” October 24, 2008. http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r96bp.pdf. Accessed July 22, 2014.
- 76. Millikan R, Logothetis C. Update of the NCCN guidelines for treatment of prostate cancer. Oncology. 1997;11:180–193. [PubMed] [Google Scholar]
- 77. Scherr D, Swindle PW, Scardino PT. National Comprehensive Cancer Network. National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology. 2003;61(2 Suppl 1):14–24. [DOI] [PubMed] [Google Scholar]
- 78. Abernethy AP, Raman G, Balk EM, et al. Systematic review: reliability of compendia methods for off-label oncology indications. Ann Intern Med. 2009;150(5):336–343. [DOI] [PubMed] [Google Scholar]
- 79. Agency for Healthcare Research and Quality Technology Assessment Program. Compendia for coverage of off-label uses of drugs and biologics in an anticancer chemotherapeutic regiment. Final report. May 7, 2007. http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id46ta.pdf. Accessed July 22, 2014. [PubMed]
- 80. Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24(1):141–144. [DOI] [PubMed] [Google Scholar]
- 81. Bennett CL, Greenfield S, Aronow H, et al. Patterns of care related to age of men with prostate cancer. Cancer. 1991;67(10):2633–2641. [DOI] [PubMed] [Google Scholar]
- 82. Greenfield S, Blanco DM, Elashoff RM, et al. Patterns of care related to age of breast cancer patients. JAMA. 1987;257(20):2766–2770. [PubMed] [Google Scholar]
- 83. Dennis B. FDA has free-speech, safety issues to weigh in review of ‘off-label’ drug marketing rules. Washington Post. July 9, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.