Abstract
For the first time, we obtained direct intra-neural measurements of muscle sympathetic nerve activity (MSNA) in relapsing-remitting multiple sclerosis (MS) patients to test the hypothesis that spontaneous resting MSNA is reduced in MS patients compared to age, sex-matched healthy controls. Spontaneous MSNA (microneurography; peroneal nerve), plasma norepinephrine, arterial blood pressure (finger photoplethysmography), and heart rate were measured at rest in three groups: 1) relapsing remitting MS patients on disease modifying therapy only (MS-DT; n=6); 2) relapsing-remitting MS patients on disease modifying therapy and medications for MS-related symptoms that are known to effect the central nervous system (MS-DT/ST; n=5), and 3) healthy age and sex-matched controls (CON; n=6). Compared to the CON group, MSNA burst frequency (bursts/min) was significantly lower in both MS-DT (P=0.027) and MS-DT/ST groups (P=0.003). Similarly, MSNA burst incidence (bursts/100 heart beats) was significantly reduced in both MS-DT (P=0.049) and MS-DT/ST groups (P=0.004) compared to the CON group. Burst frequency and burst incidence were not different between MS-DT and MS-DT/ST groups. Resting plasma norepinephrine was also significantly lower in both MS-DT (P=0.039) and MS-DT/ST groups (P=0.021) compared to the CON group. Reduced MSNA may signify an important dysfunction in autonomic control of cardiovascular function in patients with MS.
Keywords: muscle sympathetic nerve activity, autonomic dysfunction, microneurography, blood pressure, plasma norepinephrine, peripheral vasculature
1. INTRODUCTION
Multiple sclerosis (MS) has been shown to impair autonomic control of cardiovascular function (Acevedo et al., 2000; Frontoni et al., 1996; Nasseri et al., 1998; Pentland and Ewing, 1987; Sanya et al., 2005) and this dysfunction may increase with disease progression and increased clinical disability (Flachenecker et al., 2001; Nasseri et al., 1998). Studies suggest that upwards of 50% of individuals with MS may experience symptoms of orthostatic dizziness (Flachenecker et al., 1999; Vita et al., 1993). Although impaired sympathetically-mediated vasomotor control has been suggested to be responsible for the symptoms of orthostatic dizziness observed in MS patients (Flachenecker et al., 1999; Sanya et al., 2005) this has not been directly tested. Due to the obvious health-related concerns of autonomic dysfunction in individuals with MS, characterization of resting sympathetic outflow would provide a novel therapeutic target to alleviate symptoms associated with MS (dizziness, light headedness, thermal sensitivity, etc.) Sympathetic outflow to vasculature supplying skeletal muscle, or muscle sympathetic nerve activity (MSNA), can be recorded using microneurography (Vallbo et al., 1979). However, to date, no direct measurements of resting sympathetic neural function have been reported in individuals with MS. The goal of this investigation was to obtain direct intra-neural measurements of MSNA in MS patients and to test the hypothesis that spontaneous resting MSNA is reduced in MS patients compared to healthy control subjects.
2. METHODS
2.1 Human Subjects
Participants from the following three groups were investigated: 1) individuals with clinically definite relapsing remitting MS currently treated with disease modifying therapy only [MS-DT; n=6 (4 females, 2 males); age=38±7 yrs; height=173±14 cm; weight=70±14 kg, duration from MS diagnosis=7±4 yrs]; 2) individuals with clinically definite relapsing-remitting MS currently on disease modifying therapy and medications for MS-related symptoms that are known to effect the central nervous system (i.e., anti-depressants, psychostimulants, anticonvulsants and anti-spasmatics) [MS-DT/ST; n=5 (4 females, 1 male) age=38±7 yrs; height=166±8 cm; weight=59±11 kg, duration from MS diagnosis=8±4 yrs], and 3) healthy age and sex-matched controls [CON; n=6 (4 females, 2 males); age=36±7 yrs; height=169±10 cm; weight=65±18 kg,]. Participants were normotensive (supine blood pressures <140/90 mmHg) and had no known cardiovascular disease. Subjects refrained from caffeine, alcohol and intensive exercise 24 h before the study. This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. Participants provided informed written consent prior to testing.
2.2 Instrumentation and Protocol
All experiments were performed at a constant ambient room temperature (23–24 °C) with the subject in the supine position. Heart rate was monitored using ECG interfaced with a cardiotachometer (CWE, Ardmore, PA, USA). Beat-by-beat blood pressure was measured by continuous finger cuff photoplethysmography (Finometer, FMS, Amsterdam, The Netherlands) with resting values verified by brachial artery auscultation (SunTech, Medical Instruments Raleigh, NC, USA). Multiunit recordings of postganglionic MSNA were obtained by inserting unipolar tungsten microelectrodes percutaneously through the intact, un-anaesthetized skin and positioned into muscle nerve fascicles of the peroneal nerve near the fibular head (Vallbo et al., 1979). The nerve signal was processed by a pre-amplifier and amplifier (Department of Bioengineering, University of Iowa, Iowa City, IA, USA), band pass filtered (700–2000 Hz), rectified and integrated (time constant, 0.1s) to obtain a mean voltage neurogram. The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (Vallbo et al., 1979). For the MS groups, due to their inherently low MSNA, more frequent repositioning of the recording microelectrode and more frequent use of sympatho-excitatory maneuvers (i.e., end expiratory breath holds) were utilized during instrumentation prior to obtaining recordings to ensure an optimal recording site.
Resting blood samples were drawn from a venous catheter placed in the forearm for the measurements of plasma norepinephrine, a general index of global sympathethic outflow. Blood samples were obtained from 4 patients in the MS-DT group, 4 patients in the MS-DT/ST group, and 5 patients in the CON group. Plasma norepinephrine concentrations (pg/ml) were measured using high-precision liquid chromatography by an independent laboratory using standardized procedures.
2.3 Data Analysis
Data were sampled at 200 Hz through a commercial data-acquisition system (Biopac System, Santa Barbara, CA, USA). MSNA bursts were identified and evaluated over a 5-min period via computer software that identified bursts based on fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (Cui et al., 2002) and verified by an experienced microneurographer. As a result of greater instrumentation time and MS symptomology (i.e., bladder issues and spasticity), time associated with data collection varied between subjects. However, a minimum of a 5-minute continuous segment of resting MSNA data was collected in all subjects and used for analyses. MSNA was expressed as both the number of bursts per minute (burst frequency) and number of bursts per 100 heart beats (burst incidence), a HR-independent measure of sympathetic discharge.
Burst frequency, burst incidence, and plasma norepinephrine between groups were compared via one-way ANOVA. Multiple comparison procedures were performed using the Holm-Sidak method. All values are reported as means ± SD. P values < 0.05 were considered statistically significant.
3. RESULTS
Resting heart rate (MS-DT=60±7 bpm, MS-DT/ST=70±6, CON=69±11, P=0.17) and mean arterial pressure (MAP, MS-DT=85±7 mmHg, MS-DT/ST=81±10, CON=82±6, P=0.67) were not different between groups.
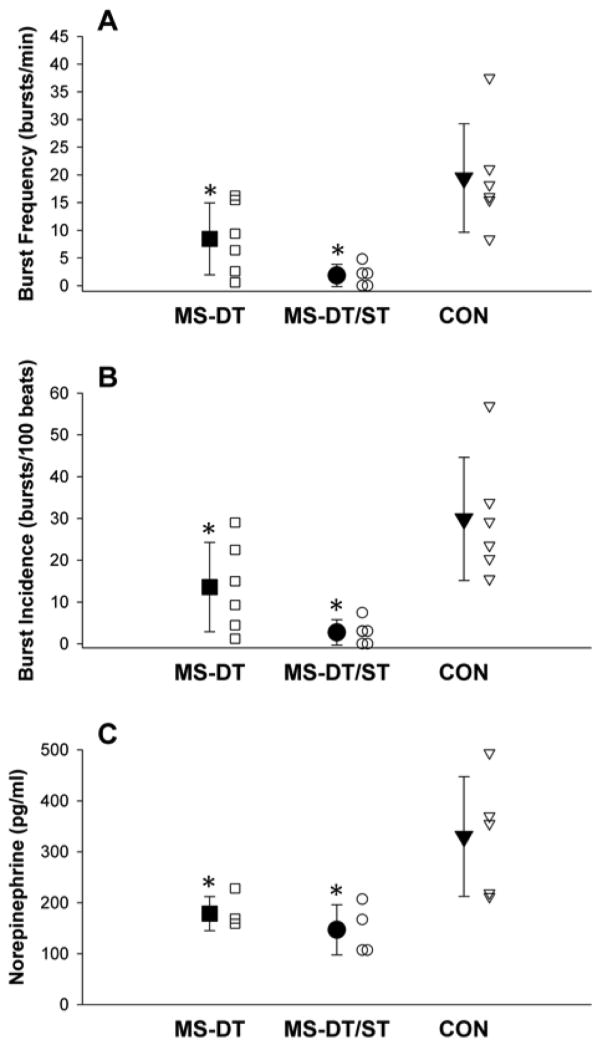
Original tracings of MSNA and arterial blood pressure (finger photoplethysmography) for a patient in the MS-DT group and the MS-DT/ST group and their age and sex-matched controls are presented in Figures 1 and 2, respectively. MSNA burst frequency was lower in the MS-DT (8.4±6.5 bursts/min; P=0.027) and MS-DT/ST (1.9±2.0 bursts/min; P=0.003) groups compared to the CON group (20.4±10.3 bursts/min; Figure 3A). Similarly, MSNA burst incidence was significantly reduced in the MS-DT (13.6±10.7 bursts/100 heart beats; P=0.049) and MS-DT/ST (2.7±3.0 bursts/100 heart beats; P=0.004) groups compared to the CON group (29.5±14.6 bursts/100 heart beats; Figure 3B). Although trending, burst frequency (P=0.16) and burst incidence (P=0.12) were not statistically different between the MS-DT and MS-DT/ST groups. Plasma norepinephrine was significantly reduced in the MS-DT (P=0.039) and the MS-DT/ST (P=0.021) groups compared to the CON group (Figure 3C).
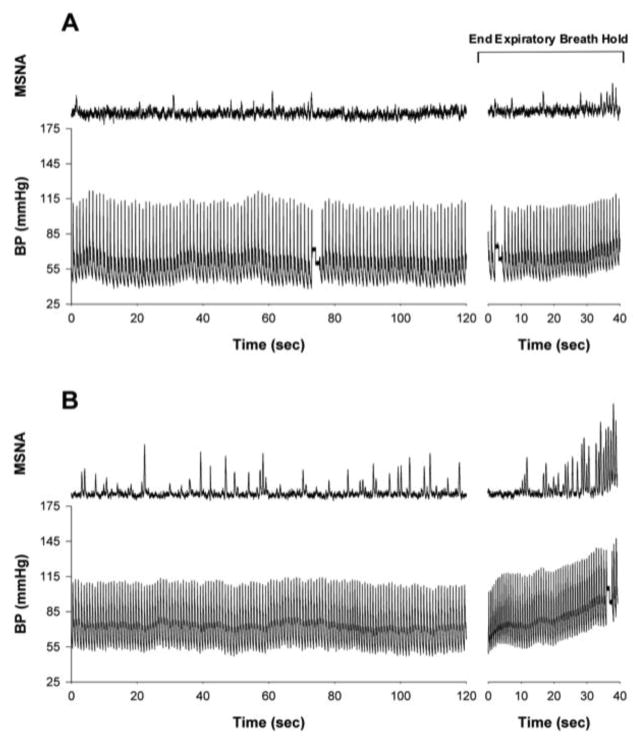
Figure 1.
Original recordings of muscle sympathetic nerve activity (MSNA) and arterial blood pressure (BP; Finometer) during 2 min of supine rest and in response to an end expiratory breath hold in an individual with clinically diagnosed relapsing remitting MS currently on disease modifying therapy only (MS-DT; panel A) and a healthy age and sex-matched control (CON; panel B). An end expiratory breath hold is a common sympatho-excitatory maneuver used during microneurographic instrumentation to determine successful acquisition of an adequate MSNA recording site.
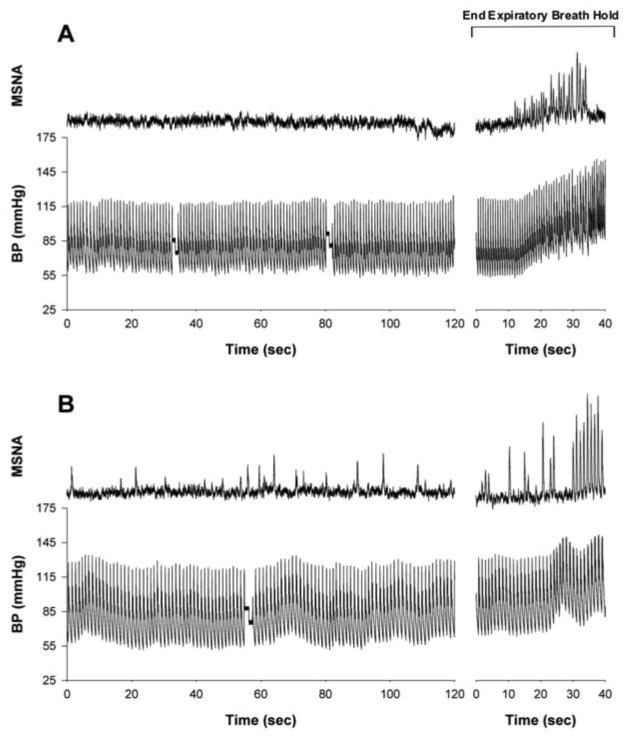
Figure 2.
Original recordings of muscle sympathetic nerve activity (MSNA) and arterial blood pressure (BP; Finometer) during 2 min of supine rest and in response to an end expiratory breath hold in an individual with clinically diagnosed relapsing-remitting MS currently on disease modifying therapy and medications for MS-related symptoms that are known to modify central nervous system function (MS-DT/ST; panel A); and a healthy age and sex-matched control (CON; panel B). An end expiratory breath hold is a common sympatho-excitatory maneuver used during microneurographic instrumentation to determine successful acquisition of an adequate MSNA recording site.
Figure 3.
Mean (±SD; closed symbols) and individual (open symbols) burst frequency (bursts/minute; panel A), burst incidence (bursts/100 heartbeats; panel B), and plasma norepinephrine (pg/ml; Panel C) in individuals with clinically diagnosed relapsing-remitting MS currently on disease modifying therapy only (MS-DT; ■), individuals with clinically diagnosed relapsing-remitting MS currently on disease modifying therapy and medications for MS-related symptoms that are known to modify central nervous system function (MS-DT/ST; ●), and healthy age and sex-matched controls (CON; ▼). *Significant difference from CON, P < 0.05.
4. DISCUSSION
The present study is the first to report direct measures of spontaneous, resting MSNA in individuals with MS. The primary novel finding was that resting MSNA was significantly reduced in relapsing-remitting MS patients compared to age and sex-matched healthy individuals. Moreover, reduced plasma concentrations of norepinephrine mirrored the findings of reduced MSNA in MS. Overall, reduced sympathetic outflow may signify an important derangement in the autonomic control of cardiovascular function in patients with MS.
Dysfunction within the autonomic nervous system is commonly observed in MS and affects a myriad of organ system functions (bladder, bowel, sexual function, sweating, etc.) (Haensch and Jorg, 2006). Impairment in the autonomic control of cardiovascular function has been previously reported (Acevedo et al., 2000; Frontoni et al., 1996; Nasseri et al., 1998; Pentland and Ewing, 1987; Sanya et al., 2005) and is underscored by the altered regulation of blood pressure in up to 50% of individuals with MS, where orthostatic dizziness is a cardinal manifestation (Flachenecker et al., 1999; Vita et al., 1993). Sympathetic innervation of the peripheral vasculature plays a critical role in the regulation of blood pressure (Joyner et al., 2010; Wallin and Charkoudian, 2007). The direct measurements of MSNA obtained in this study constitute evidence of altered sympathetic control of the skeletal muscle circulation. Further, given MSNA involvement in the regulation of blood pressure via arterial baroreceptors, the observed reductions in MSNA in MS patients corroborates the contention that dysautnomia with this central nervous system disorder impairs blood pressure control (Wallin and Charkoudian, 2007).
The particularly low MSNA observed in the MS groups increased the degree of difficulty associated with microneurography, while also demonstrating a clear deficit in this population. Indeed, three of the subjects in the MS-DT group had burst frequencies between 1 and 6 bursts/min. All of the MS-DT/ST subjects had burst frequencies less than 5 bursts/min. In fact, two patients in the MS-DT/ST group had no discernable evidence of spontaneous resting nerve activity despite strong MSNA responses during sympatho-excitatory maneuvers (e.g., end expiratory breath hold; Figure 2B). In light of these observations, studies examining the responses to sympatho-excitatory stimuli (e.g., end expiratory breath hold, gravitational challenge, cold pressor, etc.) in the MS population are warranted.
Symptoms associated with MS are often managed with medications that can have direct effects on the central nervous system (i.e., anti-depressants, psychostimulants, anti-convulsants, anti-spasmatics). Anti-depressants, in particular, have been shown to dramatically inhibit central sympathetic outflow and reduce MSNA (Esler et al., 1991; Scalco et al., 2009). The reduction in MSNA, while not statistically significant, appeared to be even greater with the combination of MS disease modifying therapy and these medications compared to disease modifying therapy alone (Figure 3). These findings may have important clinical implications in that physicians and health care providers need to understand that symptomatic treatment of MS patients with drugs that have direct affects on the central nervous system may have profound consequences on the autonomic control of cardiovascular function.
5. CONCLUSION
In conclusion, spontaneous MSNA is reduced at rest in individuals with MS when compared to age and sex-matched healthy controls. In addition, medications used in the treatment of individuals with MS may further reduce MSNA. This reduction may augment an impaired autonomic control of cardiovascular function in MS patients.
Highlights.
First direct measures of muscle sympathetic nerve activity (MSNA) in MS patients.
Resting MSNA was reduced in MS patients compared to healthy individuals.
Plasma norepinephrine concentrations mirrored the findings of reduced MSNA in MS.
Reduced MSNA may signify impaired autonomic control of cardiovascular function in MS.
Acknowledgments
The considerable time and effort of the participants are greatly appreciated.
GRANTS: This study was supported by National Heart, Lung, and Blood Institute grant R15HL117224 (S. L. Davis) and National Multiple Sclerosis Society grants RG4696A3/2 (S. L. Davis) and PP1440 (S. L. Davis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acevedo AR, Nava C, Arriada N, Violante A, Corona T. Cardiovascular dysfunction in multiple sclerosis. Acta Neurologica Scandinavica. 2000;101(2):85–8. doi: 10.1034/j.1600-0404.2000.101002085.x. [DOI] [PubMed] [Google Scholar]
- 2.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. American Journal of Physiology Heart and Circulatory Physiology. 2002;282(5):H1717–23. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- 3.Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, et al. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. American Journal of Physiology. 1991;260(4 Pt 2):R817–23. doi: 10.1152/ajpregu.1991.260.4.R817. [DOI] [PubMed] [Google Scholar]
- 4.Flachenecker P, Reiners K, Krauser M, Wolf A, Toyka KV. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Multiple Sclerosis. 2001;7(5):327–34. doi: 10.1177/135245850100700509. [DOI] [PubMed] [Google Scholar]
- 5.Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. Journal of Neurology. 1999;246(7):578–86. doi: 10.1007/s004150050407. [DOI] [PubMed] [Google Scholar]
- 6.Frontoni M, Fiorini M, Strano S, Cerutti S, Giubilei F, Urani C, et al. Power spectrum analysis contribution to the detection of cardiovascular dysautonomia in multiple sclerosis. Acta Neurologica Scandinavica. 1996;93(4):241–5. doi: 10.1111/j.1600-0404.1996.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 7.Haensch CA, Jorg J. Autonomic dysfunction in multiple sclerosis. Journal of Neurology. 2006;253 (Suppl 1):I3–9. doi: 10.1007/s00415-006-1102-2. [DOI] [PubMed] [Google Scholar]
- 8.Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56(1):10–6. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasseri K, TenVoorde BJ, Ader HJ, Uitdehaag BM, Polman CH. Longitudinal follow-up of cardiovascular reflex tests in multiple sclerosis. Journal of the Neurological Sciences. 1998;155(1):50–4. doi: 10.1016/s0022-510x(97)00273-6. [DOI] [PubMed] [Google Scholar]
- 10.Pentland B, Ewing DJ. Cardiovascular reflexes in multiple sclerosis. European Neurology. 1987;26(1):46–50. doi: 10.1159/000116311. [DOI] [PubMed] [Google Scholar]
- 11.Sanya EO, Tutaj M, Brown CM, Goel N, Neundorfer B, Hilz MJ. Abnormal heart rate and blood pressure responses to baroreflex stimulation in multiple sclerosis patients. Clinical Autonomic Research. 2005;15(3):213–8. doi: 10.1007/s10286-005-0274-7. [DOI] [PubMed] [Google Scholar]
- 12.Scalco AZ, Rondon MU, Trombetta IC, Laterza MC, Azul JB, Pullenayegum EM, et al. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. Journal of Hypertension. 2009;27(12):2429–36. doi: 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- 13.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59(4):919–57. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 14.Vita G, Fazio MC, Milone S, Blandino A, Salvi L, Messina C. Cardiovascular autonomic dysfunction in multiple sclerosis is likely related to brainstem lesions. Journal of the Neurological Sciences. 1993;120(1):82–6. doi: 10.1016/0022-510x(93)90029-x. [DOI] [PubMed] [Google Scholar]
- 15.Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle and Nerve. 2007;36(5):595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]