Abstract
Icariin is a prenylated flavonol glycoside derived from the Chinese herb Epimedium sagittatum that exerts a variety of pharmacological activities and shows promise in the treatment and prevention of Alzheimer's disease. In this study, we investigated the neuroprotective effects of icariin against amyloid beta protein fragment 25–35 (Aβ 25–35) induced neurotoxicity in cultured rat pheochromocytoma PC12 cells and explored potential underlying mechanisms. Our results showed that icariin dose-dependently increased cell viability and decreased Aβ 25–35-induced apoptosis, as assessed by MTT assay and Annexin V/propidium iodide staining, respectively. Results of western blot analysis revealed that the selective phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 suppressed icariin-induced Akt phosphorylation, suggesting that the protective effects of icariin are associated with activation of the PI3K/Akt signaling pathway. LY294002 also blocked the icariin-induced downregulation of proapoptotic factors Bax and caspase-3 and upregulation of antiapoptotic factor Bcl-2 in Aβ 25–35-treated PC12 cells. These findings provide further evidence for the clinical efficacy of icariin in the treatment of Alzheimer's disease.
1. Introduction
Alzheimer's disease (AD), the leading cause of dementia, is characterized by the progressive loss of memory and other cognitive functions [1]. Despite considerable progress towards understanding the complex molecular mechanisms underlying AD, effective treatments able to prevent, halt, or reverse the pathobiology of AD are still lacking [2]. As the global population ages, the economic impact of dementia is expected to exceed that of cancer, heart disease, and stroke combined [3].
According to the amyloid hypothesis, neurodegeneration and dementia in AD are caused by extensive accumulation of amyloid beta peptide (Aβ) in the cerebrum [4, 5]. Extracellular deposits of the Aβ peptide form diffuse and neuritic plaques, and the microtubule assembly protein tau becomes hyperphosphorylated, accumulating intracellularly as neurofibrillary tangles [1]. In addition, widespread loss of neurons and synapses occurs [6]. Numerous studies have demonstrated that apoptosis is the primary mechanism underlying Aβ-induced neuronal death [7–9]; therefore, drugs blocking apoptosis may be useful to prevent neuronal cell death and treat AD [8, 10, 11]. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which functions as a critical regulator of cell apoptosis, is thought to play an important role in neurological diseases such as AD [12, 13].
Icariin, which is a prenylated flavonol glycoside derived from the Chinese herb Epimedium sagittatum, exerts a variety of pharmacological activities including antioxidant activity [14], immunoregulation [15], antitumor activity [16, 17], and estrogen-like activities [18, 19]. Icariin appears to be able to cross the blood-brain barrier and provide neuroprotective effects by modulating the cholinergic system and inhibiting Aβ neurotoxicity [20]. Studies in mouse models of AD and age-related cognitive decline have demonstrated that icariin improves cognitive ability and inhibits memory impairment [21, 22]. In addition, Zeng et al. demonstrated that icariin reduces neurotoxicity by inhibiting tau protein hyperphosphorylation [23]; however, the mechanism underlying this effect is unknown and the effect of icariin on Aβ-induced apoptosis, a hallmark of AD, remains unclear. A recent study suggested that icariin inhibits apoptosis via the PI3K/Akt pathway [24]. Therefore, in this study we evaluated the role of PI3K/Akt signaling in the protective effects of icariin against Aβ-induced apoptosis in cultured rat pheochromocytoma PC12 cells. Our results provide further evidence for the clinical efficacy of icariin in the treatment of AD.
2. Materials and Methods
2.1. Reagents
Icariin (purity > 98%) was purchased from the Hunan Institute for the Control of Pharmaceutical and Biological Products (Changsha, China). The selective PI3K inhibitor LY294002 was purchased from Sigma-Aldrich (St. Louis, MO, USA), and the Annexin-V-FLUOS staining kit was purchased from Roche (Penzberg, Germany). Dulbecco's modified Eagle medium (DMEM), penicillin, and streptomycin were purchased from Solarbio (Shanghai, China), and fetal bovine serum was purchased from Invitrogen/Gibco (Carlsbad, CA, USA). Antibodies against Akt, p-Akt (Ser473), Bax, Bcl-2, caspase-3, and β-actin were obtained from Abzoom (Dallas, TX, USA). Anti-mouse horseradish peroxide- (HRP-) conjugated IgG and anti-rabbit HRP-conjugated IgG secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Peptide Preparation
Aβ 25–35 was dissolved in deionized distilled water at a concentration of 1 mM and incubated at 37°C for 72 h to induce aggregation [23].
2.3. Cell Culture and Drug Treatments
PC12 cells were maintained in DMEM containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a 5% CO2 incubator; the medium was changed every other day. The cells were cultured in serum-free medium for 12 h prior to treatment with icariin, and Aβ 25–35 was added 1 h later. In experiments involving the inhibition of PI3K/Akt signaling, LY294002 (50 μM) was added to the medium 1 h prior to icariin treatment.
2.4. Cell Viability Assay
PC12 cells were seeded into 96-well plates (0.5 × 104 cells/well) and assessed for response to Aβ 25–35 and icariin using the MTT assay. Briefly, the cells were pretreated with icariin (2.5, 5.0, 10.0, or 20.0 μM) or vehicle only for 1 h and then exposed to 20 μM preaggregated Aβ 25–35 for 24 h in the continued presence of icariin or vehicle. To determine viability, the cells were then treated with 5 mg/mL MTT for 4 h at 37°C and then the medium was carefully removed. The resulting formazan crystals were dissolved in 150 μL dimethyl sulfoxide (DMSO), and absorbance at 570 nm was determined using a plate reader.
2.5. Apoptosis Assay
Apoptosis was detected using the Annexin-V-FLUOS staining kit (Roche). Briefly, 5 × 105 cells were collected, resuspended in phosphate buffered saline, and incubated with Annexin V/PI labeling solution for 20 min at room temperature in the dark. The cells were then analyzed by flow cytometry (Becton Dickinson, USA). A minimum of 10,000 events were counted per sample.
2.6. Western Blot Analysis
Cells were collected after drug treatment, and whole cell lysates were prepared by incubation in RIPA buffer (Cell Signaling Technology, Boston, MA, USA) supplemented with a protease inhibitor cocktail (Roche), according to the manufacturer's instructions. The lysates (20 μg protein) were separated by SDS-PAGE (6%–12%) and transferred to polyvinylidene fluoride membranes. After blocking the membranes with 5% nonfat dry milk, primary antibodies (1 : 500 dilution) were added, and the membranes were incubated for 2 h at room temperature, followed by incubation with the corresponding anti-rabbit IgG, HRP-linked secondary antibodies at room temperature for 1 h. Extensive washes were performed between each step. Bound antibodies were visualized using the ECL Advance western blotting detection kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK), and images were obtained using the LAS-4000 imaging system (Fuji Film, Tokyo, Japan).
2.7. Statistical Analysis
The data are expressed as mean ± standard deviation (S.D.). Groups were compared by Student's t-test and analysis of variance; P < 0.05 was considered significant.
3. Results
3.1. Icariin Increased Viability of PC12 Cells Treated with Aβ 25–35
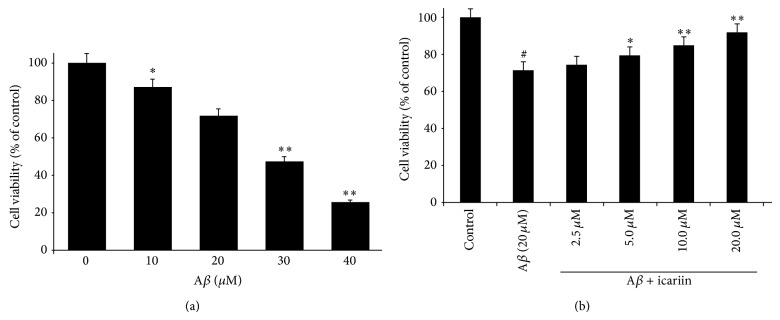
Because Aβ has been shown to induce neuronal apoptosis in the pathogenesis of AD, we evaluated the effect of Aβ 25–35 on PC12 cell viability. Cultures were treated with 10, 20, 30, or 40 μm Aβ 25–35, and cell viability was determined 24 h later using the MTT assay. As shown in Figure 1, 20 μm Aβ 25–35 significantly decreased PC12 cell viability, and treatment with 40 μm Aβ 25–35 resulted in the minimal survival of 25.5% compared with vehicle-treated controls, indicating a dose-dependent effect.
Figure 1.
Icariin protects against Aβ 25–35-induced cytotoxicity in PC12 cells. (a) Effects of Aβ 25–35 on cell viability. PC12 cells were treated with 10–40 μM Aβ 25–35, and cell viability was assessed by the MTT assay. (b) Protective effect of icariin against Aβ 25–35-induced cytotoxicity in PC12 cells. After 1 h pretreatment with icariin (2.5, 5, 10, or 20 μM), PC12 cells were treated with 20 μM Aβ 25–35 for 24 h. Results are presented as mean ± S.D. of five independent experiments. * P < 0.05; ** P < 0.01 versus Aβ 25–35 treatment; # P < 0.01 versus untreated control.
To evaluate the effects of icariin on Aβ 25–35-induced cytotoxicity, we pretreated PC12 cells with 2.5, 5.0, 10.0, or 20.0 μm icariin 1 h prior to 24 h treatment with 20 μm Aβ 25–35. Our results show that, at doses ≥5.0 μm, icariin protected cells against the toxic effects of Aβ 25–35 (Figure 1). Thus, icariin attenuates the effects of Aβ 25–35 on cell viability in a dose-dependent manner.
3.2. Icariin Decreased Aβ 25–35-Induced Apoptosis in PC12 Cells
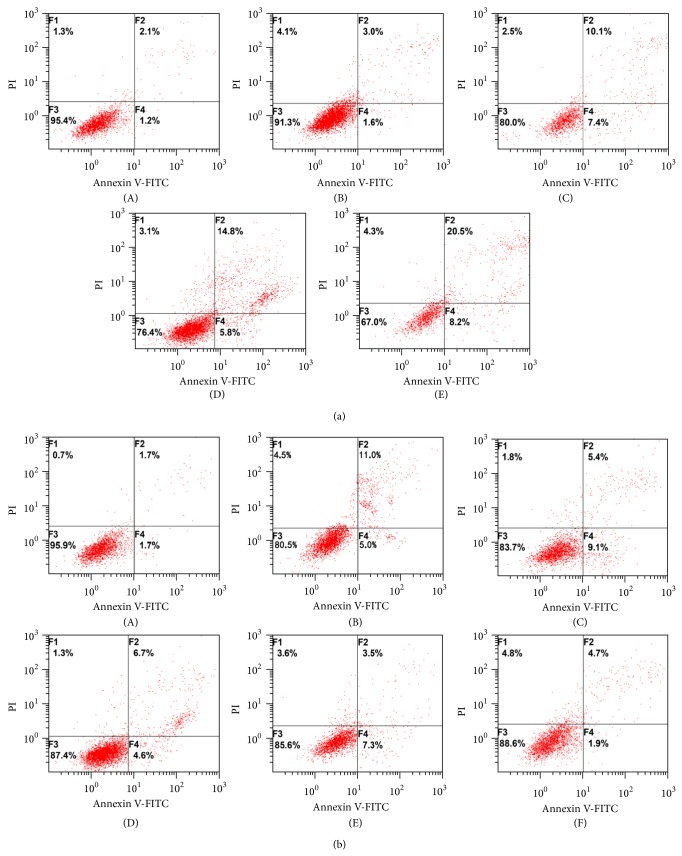
The proapoptotic effects of Aβ 25–35 were then assessed by Annexin V/PI double staining. Our data showed that 10 μm Aβ 25–35 was sufficient to induce PC12 cell apoptosis (Figure 2). Consistent with results of the MTT assay, Aβ 25–35 increased apoptosis in a dose-dependent manner.
Figure 2.
Protective effects of icariin on Aβ 25–35-induced apoptosis in PC12 cells. (a) Proapoptotic effect of Aβ 25–35. After treatment with the following concentrations of Aβ 25–35, cell apoptosis was evaluated by Annexin V/PI staining: (A) 0 μM, (B) 10 μM, (C) 20 μM, (D) 30 μM, or (E) 40 μM. (b) Protective effect of icariin against Aβ 25–35-induced apoptosis. After 1 h pretreatment with icariin (0, 2.5, 5, 10, or 20 μM), PC12 cells were treated with 20 μM Aβ 25–35 for 24 h. Apoptosis was evaluated by Annexin V/PI staining. (A) Untreated control, (B) 20 μM Aβ 25–35 only, (C) 2.5 μM icariin, (D) 5 μM icariin, (E) 10 μM icariin, and (F) 20 μM icariin. Apoptosis rates are shown in Supplementary Figure 1 (available online at http://dx.doi.org/10.1155/2015/235265).
Results of Annexin V/PI staining revealed that icariin is able to prevent Aβ 25–35-induced apoptosis in PC12 cells. As shown in Figure 2, 20 μm Aβ 25–35 increased both early and late apoptosis in PC12 cells, with a total apoptosis rate of 15.8% by 24 h. However, pretreatment with icariin for 1 h prior to Aβ 25–35 exposure decreased the apoptosis rate to 15.0% (2.5 μm icariin), 11.0% (5.0 μm), 10.2% (10 μm), and 6.6% (20 μm), respectively. The apoptosis rate of cells treated with 2.5 μM icariin did not differ significantly from that of controls treated with Aβ 25–35 only. These results indicate that icariin dose-dependently suppresses Aβ 25–35-induced apoptosis.
3.3. Role of PI3K/Akt Signaling in the Protective Effect of Icariin against Aβ 25–35-Induced Apoptosis
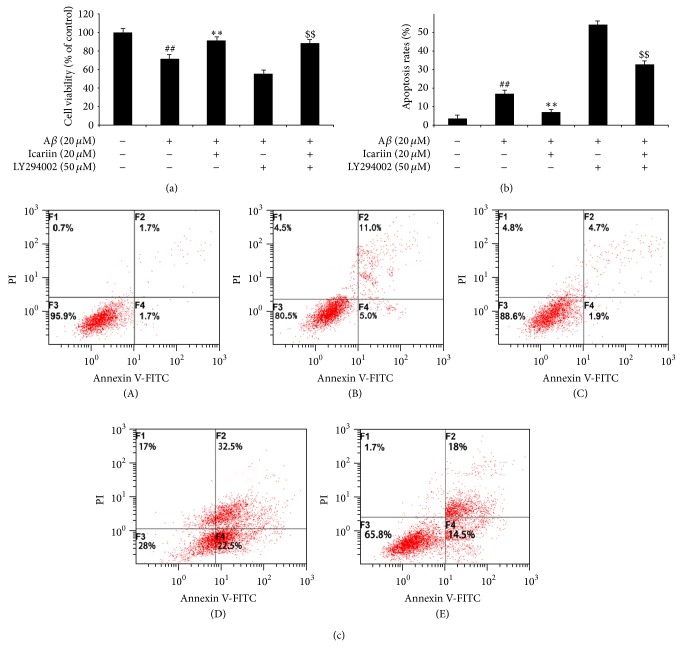
PI3K/Akt signaling plays a pivotal role in cell survival, and its activation may have antiapoptotic effects. We therefore treated PC12 cells with PI3K inhibitor LY294002 (50 μm) and found the LY294002 increased apoptosis and decreased viability in PC12 cells treated with Aβ 25–35 (Figure 3). This result suggests that the PI3K/Akt signaling pathway is involved in Aβ 25–35-induced apoptosis. To confirm the role of PI3K/Akt signaling in the neuroprotective effects of icariin, PC12 cells were pretreated with 50 μmL Y294002 for 30 min, followed by 30 min treatment with icariin (20 μm) and then 24 h treatment with 20 μm Aβ 25–35. Our results show that LY294002 diminished the effects of icariin on Aβ 25–35-induced apoptosis.
Figure 3.
Involvement of PI3K/Akt signaling in the protective effects of icariin against Aβ 25–35-induced cytotoxicity in PC12 cells. After 1 h pretreatment with the PI3K inhibitor LY294002 (50 μM), PC12 cells were treated with 20 μM icariin for 1 h, followed by Aβ 25–35 treatment for 24 h. Cell viability and apoptosis were determined by MTT assay and Annexin V/PI double staining, respectively. (a) LY294002 inhibited the icariin-mediated increase in viability in Aβ 25–35-treated cells. (b) LY294002 inhibited the icariin-mediated decrease in apoptosis in Aβ 25–35-treated cells. (c) (A) Untreated control, (B) Aβ 25–35 treatment, (C) icariin treatment, (D) LY294002 treatment, and (E) icariin + LY294002. Results are presented as mean ± S.D. of triplicate independent experiments. ## P < 0.01 versus untreated control; ** P < 0.01 versus Aβ 25–35 treatment; $$ P < 0.01 versus icariin treatment.
3.4. Icariin Activates the PI3K/Akt Signaling Pathway through Akt Activation
To further investigate the role of PI3K/Akt signaling in the protective effects of icariin against Aβ 25–35-induced apoptosis, we evaluated Akt activation by western blot analysis using phosphorylation state-specific antibodies. A previous study reported that icariin suppresses apoptosis by activating the PI3K/Akt pathway through the phosphorylation of Akt (Ser473) [23]. Our results show that Aβ 25–35 significantly inhibited Akt phosphorylation, but pretreatment with icariin attenuated the effects of Aβ 25–35, indicating that icariin activates the PI3K/Akt pathway (Figure 4). LY294002 significantly inhibited the effects of icariin treatment, providing additional evidence for the role of PI3K/Akt signaling in the protective effects of icariin.
Figure 4.
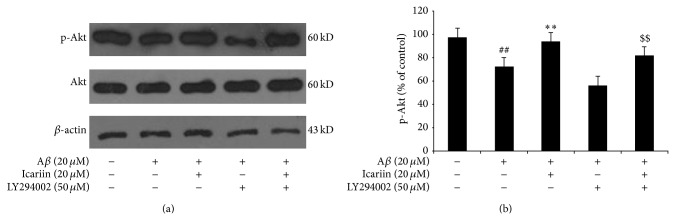
Icariin inhibits Aβ 25–35-induced cytotoxicity through the activation of PI3K/Akt signaling. After 1 h pretreatment with the PI3K inhibitor LY294002 (50 μM), PC12 cells were treated with 20 μM icariin for 1 h, followed by Aβ 25–35 treatment for 24 h. Phosphorylation of Akt was assessed by western blot. Densitometry values represent the mean ± S.D. of triplicate independent experiments. ## P < 0.01 versus untreated control; ** P < 0.01 versus Aβ 25–35 treatment; $$ P < 0.01 versus icariin treatment.
3.5. Icariin Attenuates Bax and Caspase-3 Expression through PI3K/Akt Signaling
The major executioners of apoptosis are the proteases caspase-3 and Bax. Activation of PI3K/Akt directly and indirectly induces the phosphorylation of Bax and caspase-3, promoting their interactions with other proteins and inhibiting apoptotic activity. As shown in Figures 5 and 6, protein levels of caspase-3 and Bax were significantly increased by Aβ 25–35 treatment, compared with untreated controls. However, results of western blotting show that icariin decreased levels of caspase-3 and Bax in Aβ 25–35-treated cells. PI3K inhibitor LY294002 attenuated the effects of icariin, indicating that icariin inhibits expression of Bax and caspase-3 through the activation of PI3K/Akt signaling.
Figure 5.
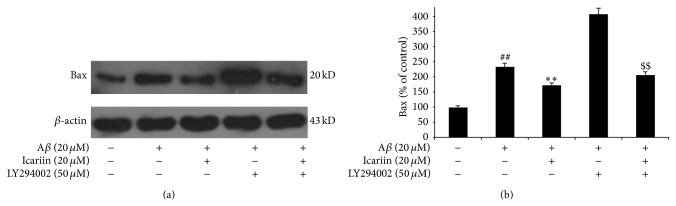
Icariin decreased Bax expression in PC12 cells after exposure to Aβ 25–35. After pretreatment with 50 μM LY294002 for 1 h, PC12 cells were treated with 20 μM icariin for 1 h, followed by Aβ 25–35 treatment for 24 h. Densitometry values are presented as the mean ± S.D. of triplicate independent experiments. ## P < 0.01 versus untreated control; ** P < 0.01 versus Aβ 25–35 treatment; $$ P < 0.01 versus icariin treatment.
Figure 6.
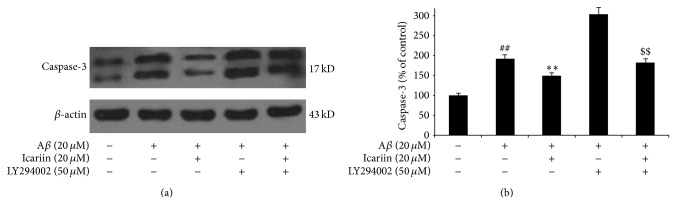
Icariin decreased caspase-3 expression in PC12 cells after exposure to Aβ 25–35. After pretreatment with 50 μM LY294002 for 1 h, PC12 cells were treated with 20 μM icariin for 1 h, followed by Aβ 25–35 treatment for 24 h. Densitometry values are presented as the mean ± S.D. of triplicate independent experiments. ## P < 0.01 versus control; ** P < 0.01 versus Aβ 25–35 treatment; $$ P < 0.01 versus icariin treatment.
3.6. Icariin Stimulates Bcl-2 Expression via the PI3K/Akt Pathway
Because activation of the PI3K/Akt pathway is known to upregulate Bcl-2 and prevent apoptosis, we next evaluated the effects of icariin on Bcl-2 expression. As shown in Figure 7, icariin attenuated the Aβ 25–35-induced decrease in Bcl-2 protein levels, but the effect of icariin on Bcl-2 was diminished by LY294002. This result provides further evidence for the role of PI3K/Akt signaling in the antiapoptotic effects of icariin.
Figure 7.
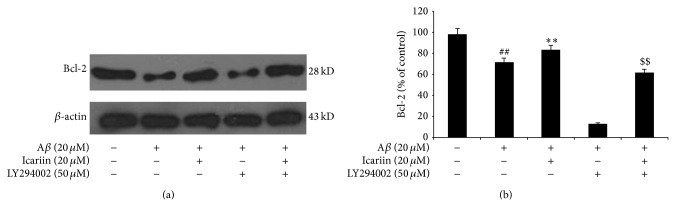
Icariin increased Bcl-2 protein levels in PC12 cells exposed to Aβ 25–35. After pretreatment with 50 μM LY294002 for 1 h, PC12 cells were treated with 20 μM icariin for 1 h, followed by 24 h Aβ 25–35 treatment. Densitometry values are presented as mean ± S.D. of triplicate independent experiments. ## P < 0.01 versus control; ** P < 0.01 versus Aβ 25–35 treatment; $$ P < 0.01 versus icariin treatment.
4. Discussion
Extensive cell death is one of the most important pathological manifestations of AD [25–28]. Although the causal events remain unclear, preventing neuronal cell death is a promising approach to treating AD [29]. The purpose of this study was to better understand the neuroprotective effects of icariin against Aβ neurotoxicity and identify the signaling pathways involved. Because activation of the PI3K/Akt pathway promotes cell survival [12], we assessed the role of PI3K/Akt signaling in the protective effects of icariin. First, we demonstrated that Aβ 25–35 decreased PC12 cell viability and increased apoptosis in a dose-dependent manner. Pretreatment with icariin dose-dependently increased viability and decreased apoptosis in Aβ 25–35-treated cells. These findings are consistent with a previous study reporting that icariin prevents cell death associated with Aβ toxicity [23].
The PI3K/Akt signaling pathway plays a crucial role in cell survival by inhibiting apoptosis [30, 31]. Recent studies suggest that activation of PI3K/Akt signaling attenuates Aβ-induced apoptosis through the inhibition of glycogen synthase kinase-3 beta, which suppresses tau protein hyperphosphorylation and the formation of neurofibrillary tangles [32, 33]. Recent studies have also reported that icariin inhibits corticosterone-induced apoptosis [24] and lipopolysaccharide-induced inflammation through the PI3K/Akt pathway [34]. Cai et al. suggested that the protective effects of icariin against Aβ neurotoxicity depend on the insulin/insulin-like growth factor 1 pathway [35], which is upstream of the PI3K/Akt pathway [36]. Our results demonstrating that icariin increased viability and decreased apoptosis in Aβ-treated PC12 cells and that these effects were attenuated by a PI3K inhibitor strongly support a role for this signaling pathway in icariin-mediated neuroprotection.
Because icariin has been reported to activate PI3K/Akt signaling through the phosphorylation of Akt (Ser473) [23], we evaluated Akt phosphorylation in icariin-treated PC12 cells. The results of western blot analysis confirmed that icariin significantly increased phosphorylation of Akt at Ser473, and that this effect was suppressed by LY294002, providing further evidence that icariin suppresses apoptosis through activation of PI3K/Akt signaling.
PI3K/Akt signaling promotes cell survival by direct or indirect interaction with proteins associated with apoptosis [12], such as Bcl-2, which promotes cell survival [37], and Bax, which promotes mitochondrial permeability leading to apoptosis [13]. Activated Akt directly or indirectly suppresses the apoptotic activity of Bax by serine phosphorylation [13]. Caspase-3 is activated by exposure to Aβ peptides, contributing to the pathophysiology of AD through cell death-independent mechanisms, as well as apoptosis [38, 39]. We found that icariin attenuated the effects of Aβ 25–35 in PC12 cells, increasing protein levels of prosurvival factor Bcl-2 and decreasing protein levels of proapoptotic factors Bax and caspase-3. PI3K inhibitor LY294002 suppressed these effects of icariin.
The extracellular signal-regulated protein kinase (ERK) pathway may also play an important role in the antiapoptotic effects of icariin. ERK, which is a converging point of multiple signal transduction pathways involved in apoptosis [40], has been shown to decrease bcl-2 gene expression through the tumor suppressor p53 [40]. In addition, Akt may act synergistically with p90 ribosomal S6 kinase to inhibit the proapoptotic activity of Bax through phosphorylation [13]. Although several studies have reported that icariin promotes cell proliferation by activating ERK [41, 42], another study suggested that icariin inhibits corticosterone-induced apoptosis in primary cultured rat hippocampal neurons by blocking p38 mitogen-activated protein kinase but not ERK [43]. Therefore, further research is needed to clarify the role of ERK in the neuroprotective effects of icariin.
In conclusion, this study provides evidence that icariin is effective in suppressing Aβ 25–35-induced apoptosis in PC12 cells, which likely occurs through the activation of PI3K/Akt signaling. Our results also indicate that icariin upregulates Bcl-2 and downregulates Bax, two critical downstream effectors in PI3K/Akt signaling. Further studies are needed to better understand the cellular mechanisms underlying the neuroprotective effects of icariin, which may ultimately lead to the development of a novel therapy for the treatment of AD.
Supplementary Material
Supplementary figure 1: Protective effects of icariin on Aß25–35 induced apoptosis in PC12 cells.(I) Pro-apoptotic effect of Aß25–35. After treatment with the following concentrations of Aß25–35,cell apoptosis was evaluated by AnnexinV/PI staining. (II) Protective effect of icariin against Aß25-35-induced apoptosis. After 1h pretreatment with icariin (0, 2.5, 5, 10,or 20 µM), PC12 cells were treated with 20 µM Aß25–35 for 24h. Apoptosis was evaluated by AnnexinV/PI staining. Results are presented as the mean ±S.D. of five independent experiments.∗∗p <0.01 versus Aß25–35 treatment; # p <0.05 versus untreated control.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 8137370); Natural Science Foundation of Hunan Province (no. 13JJ3030); Special Project of High-Tech Industrial Development of Hunan Province (no. 2012SK3212); and Traditional Chinese Medicine Science Research Foundation of the Health Department of Hunan Province (no. 2013146).
Conflict of Interests
The authors certify that they do not have a direct financial relationship with the companies mentioned in their paper and have no other conflict of interests in connection with the submitted paper.
References
- 1.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nature Reviews Neurology. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. Alzheimer's disease. The Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 4.Karran E., Mercken M., Strooper B. D. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nature Reviews Drug Discovery. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J., Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1) doi: 10.1101/cshperspect.a006189.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behl C. Apoptosis and Alzheimer's disease. Journal of Neural Transmission. 2000;107(11):1325–1344. doi: 10.1007/s007020070021. [DOI] [PubMed] [Google Scholar]
- 8.Ankarcrona M., Winblad B. Biomarkers for apoptosis in Alzheimer's disease. International Journal of Geriatric Psychiatry. 2005;20(2):101–105. doi: 10.1002/gps.1260. [DOI] [PubMed] [Google Scholar]
- 9.Calissano P., Matrone C., Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Communicative and Integrative Biology. 2009;2(2):163–169. doi: 10.4161/cib.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camins A., Pallàs M., Silvestre J. S. Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods and Findings in Experimental and Clinical Pharmacology. 2008;30(1):43–65. doi: 10.1358/mf.2008.30.1.1090962. [DOI] [PubMed] [Google Scholar]
- 11.Vila M., Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nature Reviews Neuroscience. 2003;4(5):365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 12.Song G., Ouyang G., Bao S. The activation of Akt/PKB signaling pathway and cell survival. Journal of Cellular and Molecular Medicine. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J., Yankner B. A. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 14.Wing Sze S. C., Tong Y., Ng T. B., Yin Cheng C. L., Cheung H. P. Herba Epimedii: anti-oxidative properties and its medical implications. Molecules. 2010;15(11):7861–7870. doi: 10.3390/molecules15117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J., Li F., Wu Q., Gong Q., Lu Y., Shi J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine. 2010;17(12):950–955. doi: 10.1016/j.phymed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Li S., Dong P., Wang J., et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Letters. 2010;298(2):222–230. doi: 10.1016/j.canlet.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J., Wu J., Chen X., et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. International Immunopharmacology. 2011;11(7):890–898. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Zhang X., Wang H., Qi L., Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience. 2007;145(3):911–922. doi: 10.1016/j.neuroscience.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 19.Yang L., Lu D., Guo J., Meng X., Zhang G., Wang F. Icariin from Epimedium brevicornum Maxim promotes the biosynthesis of estrogen by aromatase (CYP19) Journal of Ethnopharmacology. 2013;145(3):715–721. doi: 10.1016/j.jep.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Nie J., Luo Y., Huang X.-N., Gong Q.-H., Wu Q., Shi J.-S. Icariin inhibits beta-amyloid peptide segment 25–35 induced expression of beta-secretase in rat hippocampus. European Journal of Pharmacology. 2010;626(2-3):213–218. doi: 10.1016/j.ejphar.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Urano T., Tohda C. Icariin improves memory impairment in Alzheimer's disease model mice (5xFAD) and attenuates amyloid β-induced neurite atrophy. Phytotherapy Research. 2010;24(11):1658–1663. doi: 10.1002/ptr.3183. [DOI] [PubMed] [Google Scholar]
- 22.He X.-L., Zhou W.-Q., Bi M.-G., Du G.-H. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence-accelerated mouse prone 8 (SAMP8) mice. Brain Research. 2010;1334:73–83. doi: 10.1016/j.brainres.2010.03.084. [DOI] [PubMed] [Google Scholar]
- 23.Zeng K.-W., Ko H., Yang H. O., Wang X.-M. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology. 2010;59(6):542–550. doi: 10.1016/j.neuropharm.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H., Liu B., Wu J., et al. Icariin inhibits corticosterone-induced apoptosis in hypothalamic neurons via the PI3-K/Akt signaling pathway. Molecular Medicine Reports. 2012;6(5):967–972. doi: 10.3892/mmr.2012.1041. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.-H., Cheon Y.-H., Woo R.-S., Song D.-Y., Moon C., Baik T.-K. Evidence of early involvement of apoptosis inducing factor-induced neuronal death in Alzheimer brain. Anatomy & Cell Biology. 2012;45(1):26–37. doi: 10.5115/acb.2012.45.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W., Mechawar N., Krantic S., Quirion R. Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. The American Journal of Pathology. 2010;176(5):2209–2218. doi: 10.2353/ajpath.2010.090496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckert A., Marques C. A., Keil U., Schüssel K., Müller W. E. Increased apoptotic cell death in sporadic and genetic Alzheimer's disease. Annals of the New York Academy of Sciences. 2003;1010:604–609. doi: 10.1196/annals.1299.113. [DOI] [PubMed] [Google Scholar]
- 28.Smale G., Nichols N. R., Brady D. R., Finch C. E., Horton W. E., Jr. Evidence for apoptotic cell death in Alzheimer's disease. Experimental Neurology. 1995;133(2):225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 29.Donev R., Kolev M., Millet B., Thome J. Neuronal death in Alzheimer's disease and therapeutic opportunities. Journal of Cellular and Molecular Medicine. 2009;13(11-12):4329–4348. doi: 10.1111/j.1582-4934.2009.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochemical Journal. 2008;415(3):333–344. doi: 10.1042/bj20081056. [DOI] [PubMed] [Google Scholar]
- 31.Hemmings B. A. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275(5300):628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 32.Lee H.-K., Kumar P., Fu Q., Rosen K. M., Querfurth H. W. The insulin/Akt signaling pathway is targeted by intracellular β-amyloid. Molecular Biology of the Cell. 2009;20(5):1533–1544. doi: 10.1091/mbc.e08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rickle A., Bogdanovic N., Volkman I., Winblad B., Ravid R., Cowburn R. F. Akt activity in Alzheimer's disease and other neurodegenerative disorders. NeuroReport. 2004;15(6):955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- 34.Xu C.-Q., Liu B.-J., Wu J.-F., et al. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NFκB signaling pathway. European Journal of Pharmacology. 2010;642(1–3):146–153. doi: 10.1016/j.ejphar.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Cai W.-J., Huang J.-H., Zhang S.-Q., et al. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans . PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028835.e28835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanhaesebroeck B., Stephens L., Hawkins P. PI3K signalling: the path to discovery and understanding. Nature Reviews Molecular Cell Biology. 2012;13(3):195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 37.Adams J. M., Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 38.Snigdha S., Smith E. D., Prieto G. A., Cotman C. W. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neuroscience Bulletin. 2012;28(1):14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Amelio M., Sheng M., Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends in Neurosciences. 2012;35(11):700–709. doi: 10.1016/j.tins.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Cagnol S., Chambard J. C. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS Journal. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 41.Nan Y., Zhang X., Yang G., et al. Icariin stimulates the proliferation of rat Sertoli cells in an ERK1/2-dependent manner in vitro. Andrologia. 2014;46(1):9–16. doi: 10.1111/and.12035. [DOI] [PubMed] [Google Scholar]
- 42.Chung B. H., Kim J. D., Kim C. K., et al. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochemical and Biophysical Research Communications. 2008;376(2):404–408. doi: 10.1016/j.bbrc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Liu B., Zhang H., Xu C., et al. Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Research. 2011;1375:59–67. doi: 10.1016/j.brainres.2010.12.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Protective effects of icariin on Aß25–35 induced apoptosis in PC12 cells.(I) Pro-apoptotic effect of Aß25–35. After treatment with the following concentrations of Aß25–35,cell apoptosis was evaluated by AnnexinV/PI staining. (II) Protective effect of icariin against Aß25-35-induced apoptosis. After 1h pretreatment with icariin (0, 2.5, 5, 10,or 20 µM), PC12 cells were treated with 20 µM Aß25–35 for 24h. Apoptosis was evaluated by AnnexinV/PI staining. Results are presented as the mean ±S.D. of five independent experiments.∗∗p <0.01 versus Aß25–35 treatment; # p <0.05 versus untreated control.