Abstract
Microglia play an important role in neuronal protection and damage. However, the molecular and cellular relationship between microglia and neurons is unclear. We carried out a prospective study to detect that activation of BV2 microglia induced PC12 cell apoptosis in vitro through the TLR4/adapter protein myeloid differentiation factor 88 (MyD88)/nuclear factor-κB (NF-κB) signaling pathway. BV2 microglia were treated with different concentrations of LPS for 24 h. Western blot was utilized to detect the expression of TLR4 and the downstream signaling pathway. The level of inflammatory mediator was quantified using a specific ELISA kit. The supernatant of 10 μg/ml LPS-treated BV2 cells was used as conditioned medium (CM). PC12 cells were co-culture with CM for 24 h. Cell viability was determined by MTT assay and cell apoptosis was tested by flow cytometry. BV2 microglia were treated with 10, 20, or 30 μg/ml LPS for 24 h. The expression of TLR4, MyD88, and NF-κB significantly increased. When PC12 cells were co-cultured with CM for 24 h, cell viability decreased. CM up-regulated the Bax level and down-regulated the Bcl-2 protein level in PC12 cells. PC12 cells pretreated with interleukin-1 receptor antagonist (IL-1RA) for 30 min, significantly alleviated CM-induced PC12 cell apoptosis. These results suggest that BV2 microglia activated by LPS triggered TLR4/MyD88/NF-κB signaling pathway that induced the release of IL-1β and could participate in the PC12 cells injury.
Keywords: BV2 microglia, TLR4, PC12 cell, Lipopolysaccharide, Interleukin-1β
Introduction
The central nervous system (CNS) diseases remain a prevalent and persistent clinical problem because of our incomplete understanding of their pathogenesis. In the past, the relevant research about CNS diseases was focused on neurons. Over the last decade, a rapidly growing body of evidence indicated that microglia, as the representative of immune cells in CNS, play important roles in neuropathic disease (Schulz 2007; Suter et al. 2007). Activated microglia are involved in various CNS diseases, such as pain (Baron 2006; Inoue and Tsuda 2009; Smith 2010; Tsuda et al. 2012), infection (Lehnardt 2010), neurodegeneration, brain trauma (Mao et al. 2012), stroke, and any real or potential danger to the CNS (Aravalli et al. 2007).
Microglia are double-edged sword and can be either neuroprotective or neurotoxic. As immune cells, microglia trigger the first line of host defense against pathogens. They are responsible for neuronal development and maintenance. When the brain is injured or affected by brain diseases, “resting” microglia morphologically transform into “activated microglia” through a series of cellular and molecular changes (Nimmerjahn et al. 2005; Graeber 2010; Rev 2011). But the chronic activation of microglia can in turn cause neuronal injury through the release of potentially cytotoxic molecules such as proinflammatory cytokines, reactive oxygen intermediates, nitric oxide (NO), nerve growth factor (NGF), and chemotactic cytokines (Hughes 2012). However, the specific molecular and cellular relationship between microglia and neurons is still unclear.
Toll-like receptors (TLRs), which are widely expressed in microglia, could recognize pathogen-associated molecular patterns (PAMP) on the surface of pathogens and initiate intracellular signals that regulate gene expression (Akira et al. 2006; Medzhitov 2001, 2007; Miyake 2007; Rajagopal 2008; Mogensen 2009; Takeuchi and Akira 2010). TLRs are involve in a variety of CNS diseases such as infection, trauma (Arslan et al. 2010; Brea et al. 2010), neurodegenerative diseases (Ding et al. 2011; Kawai and Akira 2006; Panaro et al. 2008; Vollmar et al. 2009), and autoimmunity. TLR1-9 including TLR4 is expressed on the surface of murine microglia (Arumugam et al. 2009; Johnson et al. 2003). TLR4 is necessary for activated microglia to induced neuronal injury in vivo (Lehnardt et al. 2003). In mixed CNS cultures prepared from mice lacking functional TLR4, LPS does not induce neuronal injury. In the model of neuropathic pain, thermal and tactile hypersensitivity in the TLR4 knockout mice is attenuated (Tanga et al. 2005).
TLR4 signaling is divided into MyD88-dependent and MyD88-independent (TRIF-dependent) pathways (Lu et al. 2008). Upon LPS stimulation, microglia TLR4 and MyD88 are activated which lead to the degradation of inhibitor κB (IκB) proteins and the subsequent translocation of the transcription nuclear factor κB (NF-κB), which controls the expression of proinflammatory cytokines (such as interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha, interleukin-12, and interferon-beta) (Aravalli et al. 2007; Lehnardt et al. 2002). Proinflammatory cytokines as neurotoxic factors can cause neuronal damage. Our previous studies both in vitro and in vivo verified that TLR4/MyD88/NF-κB signaling pathway was involved in the neuroinflammatory response and innate immune response in microglia under traumatic brain injury and glucose deprivation/ reoxygenation (Mao et al. 2012; Qin et al. 2012).
In the present study, in order to clarify the direct effects and relative mechanism of active microglia on neurons, we used BV2 microglia and PC12 cells to represent microglia and neurons respectively in vitro. BV2 microglia cells were from an immortalized murine microglia line with inflammatory and phagocytic properties following activation. PC12 cells were originally established from a transplantable rat adrenal pheochromocytoma. In many physiological studies for neuronal functions and neurodegenerative diseases, as a cell model, the PC12 cells have been widely used. We hypothesized that TLR4/MyD88/NF-κB signaling pathway may mediate the activation of BV2 microglia by LPS and induce the release of proinflammatory factor IL-1 that could participate in PC12 cell injury.
Materials and methods
Reagents
Lipopolysaccharide (LPS) and polymyxin B sulfate (PMBS) were purchased from Sigma Aldrich (St Louis, MO, USA). The TLR4 (sc-16240), MyD88 (sc-11356,), NF-κB p65 (sc-8008), IκB (sc-371), p-IκB(8404), Bax (sc-526), and Bcl-2 (sc-492) antibodies were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat anti-rabbit IgG (A0208) was obtained from Beyotime Institute of Biotechnology (Jiangsu, China). Primary antibody dilution buffer (P0023A), secondary antibody dilution buffer (P0023D), phenylmethanesulfonyl fluoride (ST506), bicinchoninic acid protein assay kit (P0012), SDS–polyacrylamide gel electrophoresis (PAGE) sample loading buffer (P0015), and BCIP/NBT alkaline phosphatase color development kit (C3206) were purchased from Beyotime Institute of Biotechnology (Jiangsu, China). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] was obtained from Amresco (Solon, OH, USA). The annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) apoptosis kit were obtained from Baosai Company (Beijing, China).
Cell culture
BV2 microglia and PC12 cells were purchased from the Institute of Basic Medical Sciences of the China Science Academy. The cells were maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin. They were maintained in humidified 5 % CO2/95 % air environment at 37 °C.
Extraction of nuclear proteins
Cells were collected and resuspended at 4 °C in buffer A (0.5 M HEPES, 0.5 M EDTA, 1 M DTT, 10 mM aprotinin, 1 M NaF, 1 M KCl, 0.5 M EGTA, 100 mM PMSF, 10 mM leupeptin, and 0.1 M Na3VO4), incubated on ice for 15 min, and then vortexed for 1 min. Samples were centrifuged and the supernatant containing cytosolic proteins were removed. The pellet was resuspended in cold buffer C (0.5 M HEPES, 0.5 M EDTA, 1 M DTT, 10 mM aprotinin, 1 M NaF, 5 M NaCl, 0.5 M EGTA, 100 mM PMSF, 10 mM leupeptin, and 0.1 M Na3VO4) and incubated on ice for 50 min for high salt extraction. Cellular debris was removed by centrifugation for 10 min at 4 °C; the supernatant containing nuclear proteins was collected. Nuclear extracts were aliquoted and stored at −80 °C for Western blotting analysis of NF-κB p65 protein activity.
Western blotting assay
Western blotting analysis was implemented to explore the expressions of TLR4, MyD88, NF-κB p65, IκB, p-IκB, Bcl2, and Bax. BV2 cells were pretreated with LPS (10, 20, and 30 μg/ml) for 24 h. PC12 cells were exposed to the CM. Then, equal amounts of protein were resolved by SDS-PAGE and transferred electrophoretically onto nitrocellulose membrane. The membrane was blocked with 3 % BSA for 2 h at room temperature and then incubated with primary antibodies directed against TLR4, MyD88, NF-κB p65, IκB, p-IκB, Bcl2, and Bax overnight at 4 °C. After rewarming for 30 min at room temperature, the membranes were washed and then incubated with secondary antibody for 2 h. Proteins were visualized by BCIP/NBT Alkaline Phosphatase Color Development Kit.
Cytokine IL-1β production assay
IL-1β in the supernatants of activation of BV2 microglia by 10, 20, and 30 μg/ml LPS was measured by enzyme-linked immunosorbent assay (ELISA). BV2 microglia were cultured at the concentration of 106/well in a 6-well plate. The supernatants of the BV2 cells culture medium which were stimulated with LPS were collected and one portion was compounded with 10 μg/ml PMBS for 30 min. Afterwards, the level of IL-1β in BV2 microglia was determined by ELISA kits. ELISA was carried out according to the manufacturer’s instructions. The results were expressed as picogram per milliliter.
Cytotoxicity assay
PC12 cell viability in response to the supernatants of BV2 cells was determined using MTT (0.5 mg/ml) assay and trypan blue exclusion (TBE). PC12 cells were cultivated at a density of 10,000 cells/well in 96-well plates. Cells were stained with 20 μl MTT stock solution (5 mg/ml) to each well for 4 h. After that, the cells were dissolved with DMSO, and the optical density was determined at 490 nm. Trypsin-detached PC12 cells were harvested and then stained with 0.4 % trypan blue.
Apoptosis assay
PC12 cells were cultured in humidified 5 % CO2/95 % air at 37 °C, and then the cells were exposed to the CM which was combined with PMBS. Cells were washed with PBS, centrifuged and resuspended in 200 μl binding buffer, and then placed at room temperature for 15 min after adding 10 μl V-FITC to the cells suspension. Following that 300 μl binding buffer and 5 μl PI were added to the suspension. The apoptotic cells (the annexin V positive and PI-negative cells) were demonstrated as the percentage of gated cells. The stained cells were analyzed by flow cytometry (Becton Dickinson, San Jose, CA, USA) to measure the fluorescence emission at 530 nm. The proportion of apoptotic BV2 cells was analyzed by the WinMDI 2.9 software.
Inmmunofluorescence
BV2 cells were incubated with 10 μg/ml of LPS in humidified 5 % CO2/95 % air for 24 h at 37 °C. The cells were fixed in 4 % paraformaldehyde for 30 min at room temperature and washed three times with PBS. The cells were treated with 0.1 % Triton X-100 for 15 min at room temperature. Being washed, the cells were incubated with a blocking serum for 1 h and incubated overnight with a 1:100 dilution of primary NF-κB p65 antibody. The cells were then washed and incubated with a 1:2000 dilution of goat anti-rabbit IgG for 2 h in a dark room. For nuclear staining, the cells were incubated with a 1:1000 dilution of DAPI for 5 min. The slide was finally washed and mounted for microscopic examination. Staining with NF-κB p65 antibody exhibited green fluorescence, which was detected by fluorescence microscopy.
Statistical analysis
All data were presented as means ± SD. Statistical analyses were performed using one-way analysis of variance (SPSS 13.0 for Windows). Differences between experimental groups were determined by the Fisher’s Student–Newman–Keuls test. P value <0.05 was considered significant.
Results
Effect of LPS on morphological changes of BV2 microglia
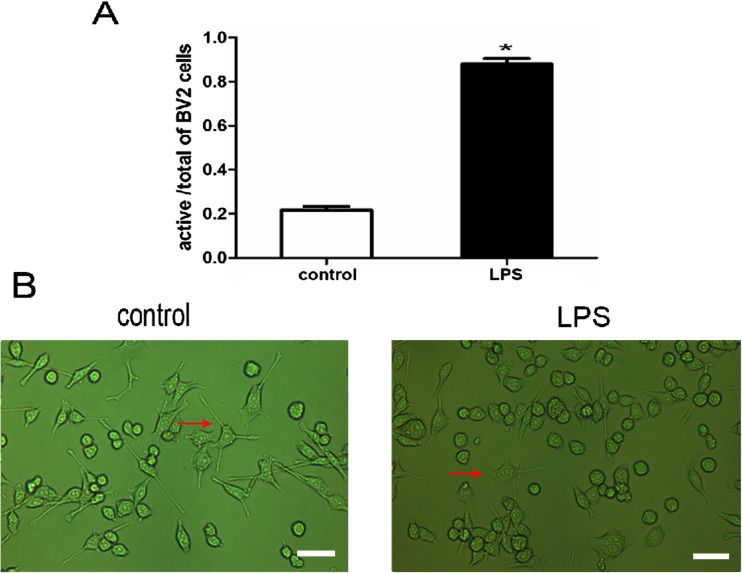
Firstly, we observed the morphology of control and LPS groups. Ten microgram per milliliters LPS was added to normal BV2 microglia culture medium for 24 h. The morphology of control BV2 microglia showed small soma with distal arborization, characteristic of “ramified” microglia. LPS-treated BV2 microglia had fewer branches that were shorter and or appeared to be resorbed into the cell body (Fig. 1).
Fig. 1.
The effect of LPS on morphological changes of BV2 microglia. a Percentage of activated cells calculated by counting the cells in ten microscopic fields after exposure to LPS for 24 h. *P < 0.05, as compared with control group. b The normal morphology of BV2 microglial cells cultured in normal medium for 24 h (left). Morphological changes of BV2 microglia were observed under 10 μg/ml LPS stimulation for 24 h (right). Scale bar = 200 μm. Arrows indicate typical control and LPS-treated BV2 microglia
Effects of LPS on TLR4 and MyD88 protein expressions of BV2 microglia
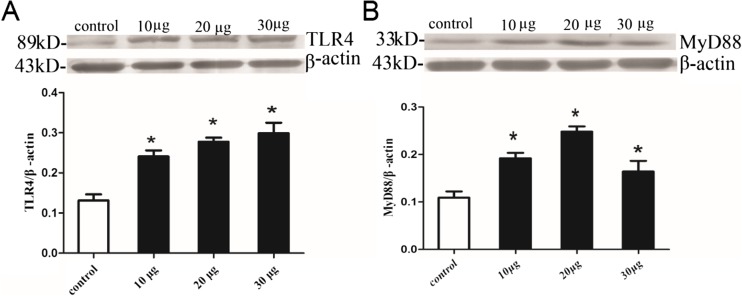
Subsequently, to clarify whether LPS initiated the activation of TLR4/MyD88 signaling pathway in BV2 microglia, we explored the effects of different concentrations of LPS (10, 20, and 30 μg/ml) on BV2 microglia for 24 h by Western blotting. Our results indicated that TLR4 and MyD88 protein levels of LPS-treated BV2 microglia were increased significantly compared with the control BV2 microglia (P < 0.05; Fig. 2).
Fig. 2.
Effects of LPS on TLR4 and MyD88 protein levels of BV2 microglia. a Cell lysates were immunoblotted with TLR4 antibody and subsequently reprobed with beta-actin. b Cell lysates were immunoblotted with MyD88 antibody and subsequently reprobed with beta-actin. The differences of the protein expressions between the groups were analyzed with Image J. *P < 0.05, as compared with control group
Effects of LPS on nuclear NF-κB and IκB protein levels in BV2 microglia
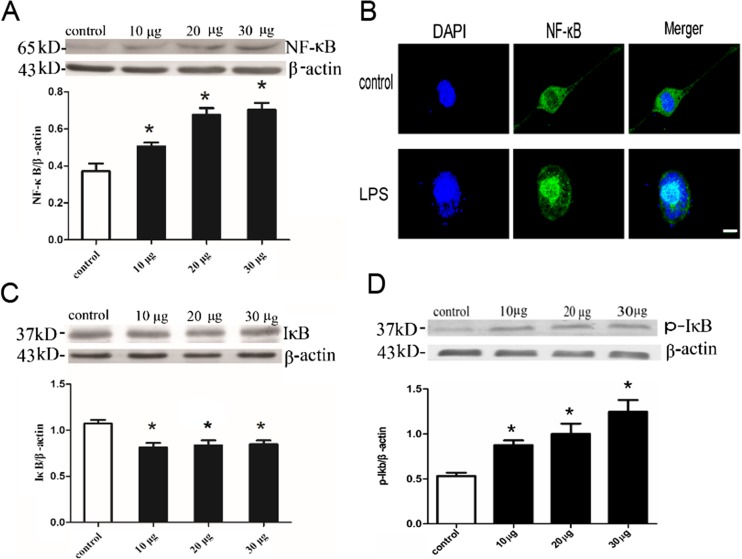
To further determine the downstream pathway of TLR4-mediated signal transduction, we next tested the level of IκB degradation and p-IκB up-regulation in BV2 cells subjected to 10, 20, and 30 μg/ml LPS by Western blotting. As shown in Fig. 3, following LPS stimulation, the level of IκB in BV2 microglia decreased significantly, the level of p-IκB in BV2 microglia increased significantly, and the abundance NF-κB p65 in the nucleus increased significantly (P < 0.05). There was almost complete translocation of NF-κB p65 from the cytoplasm to the nucleus following LPS stimulation (Fig. 3B).
Fig. 3.
Effects of LPS on NF-κB p65 in nucleus and IκB protein levels of BV2 microglia. a Cell nucleus lysates were immunoblotted with NF-κB p65 antibody and subsequently reprobed with beta-actin. b Representative confocal immunofluorescence images showing the distribution of NF-κB p65 following LPS stimulation. Scale bar = 10 μm. c Cell lysates were immunoblotted with IκB antibody and subsequently reprobed with beta-actin. d Cell lysates were immunoblotted with p-IκB antibody and subsequently reprobed with beta-actin. *P < 0.05, as compared with control group
Effect of LPS on release of IL-1β from activated BV2 microglia
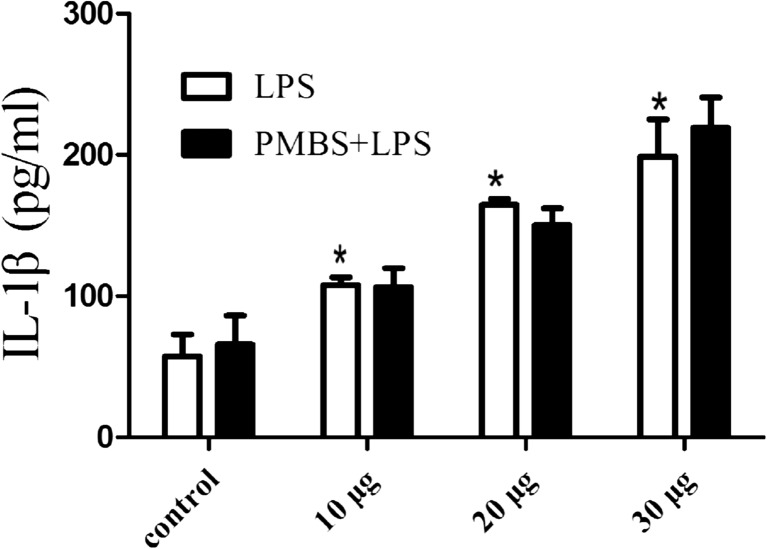
In order to confirm that activation of BV2 microglia triggered the release of proinflammatory cytokine IL-1β by TLR4/MyD88/NF-κB signaling pathways, the supernatant from 24 h LPS-treated BV2 microglia (10, 20, and 30 μg/ml) was collected. Each supernatant was divided into two portions. One portion was added 10 μg/ml polymyxin B sulfate (PMBS) which is a specific antagonist of LPS, then incubated for 30 min. ELISA was used to determine the release of IL-1β from activation of microglia. The results showed that LPS could promote IL-1β secretion (P < 0.05; Fig. 4). Compared with LPS group, LPS + PMBS group have no effect on the level of IL-1β (Fig. 4). PMBS had no significant effect on the release of IL-1β.
Fig. 4.
Effect of LPS on the release of IL-1β from activated BV2 microglia. ELISA was used to determine the release of IL-1β from microglia which were treated with LPS (10, 20, and 30 μg/ml) respectively for 24 h. Data are shown as means ± SD (n = 4). *P < 0.05, as compared with control group
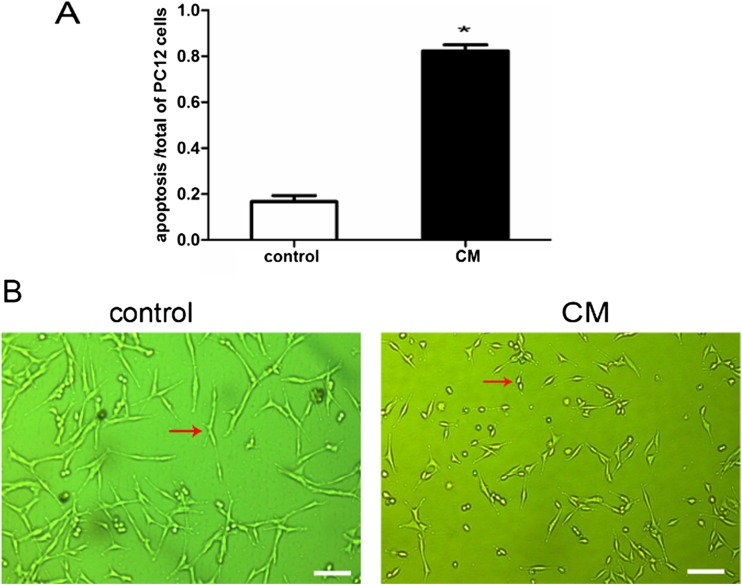
Effect of CM on PC12 cell morphology and viability
The supernatant of 10 μg/ml LPS-treated BV2 cells for 24 h was defined as the CM. As seen in the Fig. 5, PC12 cells in CM had round bodies and short arborizations when compared with the control group.
Fig. 5.
Effect of CM on PC12 cell morphology. a Percentage of apoptosis cells calculated by counting the cells in ten microscopic fields after exposure to CM for 24 h. *P < 0.05, as compared with control group. b The normal morphology of PC12 cells cultured in normal medium for 24 h (left). Morphological changes of PC12 cells were observed after exposure to CM for 24 h (right). *P < 0.05, as compared with control group. Scale bar = 200 μm. Arrows indicate typical control and CM-treated PC12 cells
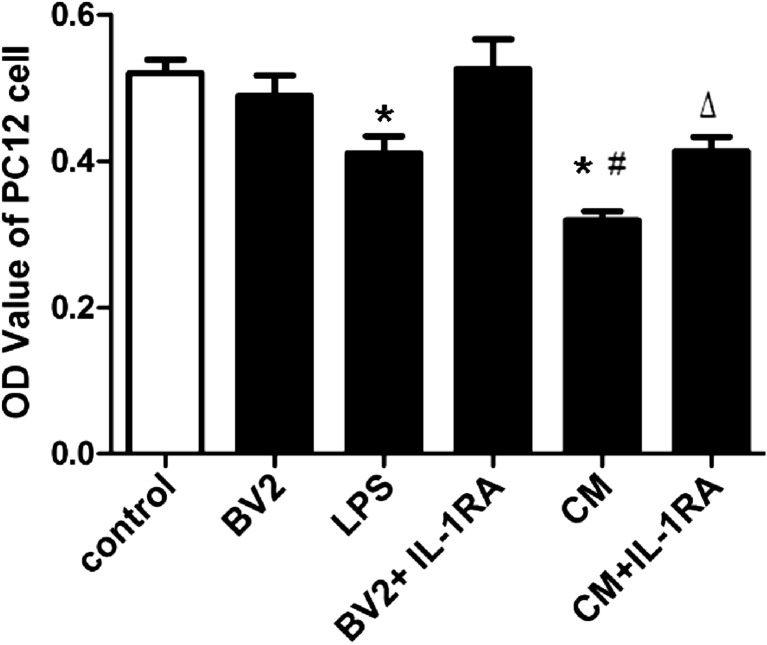
Next, we observed the effect of CM on PC12 cell viability with MTT assay. BV2 group (normal BV2 microglia supernatant pretreated PC12 cells) had no apparent effect on PC12 cell viability. Compared with the control group, the LPS group (10 μg/ml LPS added to control PC12 cells for 24 h) had significantly decreased viability (P < 0.05; Fig. 6). Compared with LPS group, CM group exacerbated PC12 cell injury (P < 0.05; Fig. 6). Pretreated PC12 cells with 100 ng/ml interleukin-1 receptor antagonist (IL-1RA), CM + IL-1RA group alleviated PC12 cells injury compared with CM group (P < 0.05; Fig. 6).
Fig. 6.
Effect of CM on PC12 cell viability. The results are expressed as OD value. Each value indicates the means ± SD. *P < 0.05, as compared with control group; # P < 0.05, as compared with LPS group; △ P < 0.05, as compared with CM group
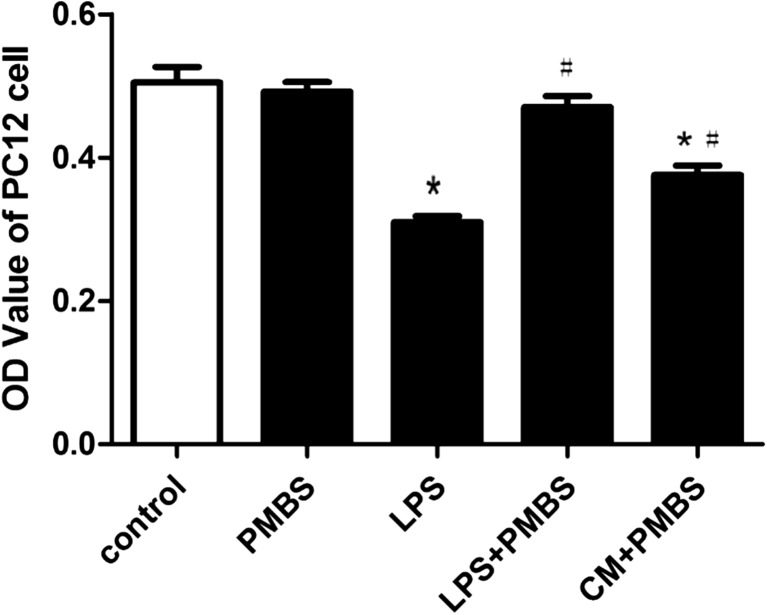
Effect of PMBS on LPS-induced neurotoxicity in PC12 cells
In order to exclude the interference of LPS, antibiotic PMBS (LPS-specific antagonist) was added to CM. We had confirmed that LPS alone could decrease PC12 cell viability (Fig. 6). When 10 μg/ml PMBS was added into control PC12 cells, cell viability did not change significantly compared with control group. Compared with LPS group, LPS + PMBS group could remove the toxic effect of LPS on PC12 cells (P < 0.05; Fig. 7).
Fig. 7.
Effect of PMBS on LPS-induced neurotoxicity in PC12 cells. Cell viability was assessed using the MTT assay, and the results are expressed as MTT OD values. Each value indicates the means ± SD. *P < 0.05, as compared with control group; # P < 0.05, as compared with LPS group
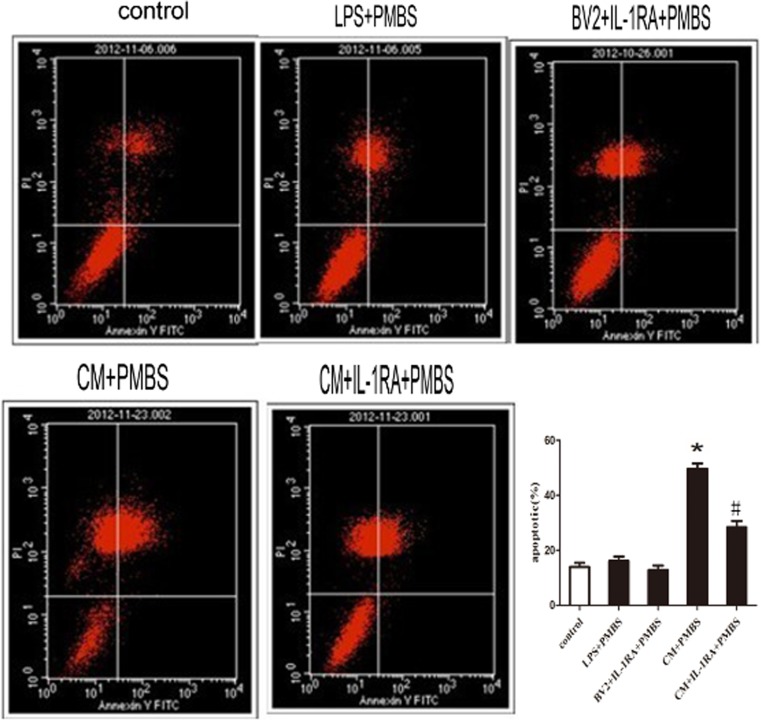
Effect of CM on apoptosis in PC12 cells
Compared with control group, the percentage of apoptotic cells increased after being exposed to CM + PMBS, which was down-regulated by IL-1RA (P < 0.05, Fig. 8).
Fig. 8.
Effect of CM on apoptosis in PC12 cells. After exposure to the CM for 24 h, PC12 cells were stained with annexin V and PI and analyzed by FACS. The apoptotic cells (the annexin V-positive and PI-negative cells) were indicated as the percentage of gated cells. Relative percentage of the annexin V-positive and PI-negative cells. Data are the mean ± SD. *P < 0.05, as compared with control group. # P < 0.05, as compared with (CM + PMBS) group
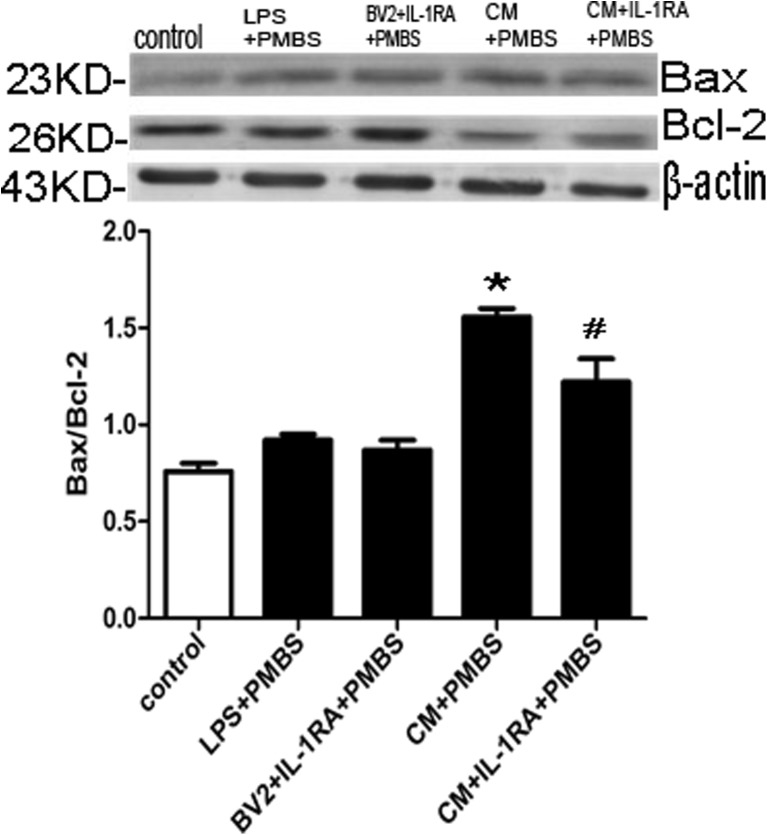
Furthermore, we detected the changes of pro-apoptotic protein (Bax) and anti-apoptotic protein (Bcl-2) which were primary regulators initiating mitochondrial depolarization. The results showed that CM increased Bax to Bcl-2 ratio (P < 0.05; Fig. 9). Pretreatment with 100 ng/ml IL-1RA down-regulated the Bax level and up-regulated the Bcl-2 protein level, resulting in the decrease in the Bax to Bcl-2 ratio (P < 0.05; Fig. 9).
Fig. 9.
Effects of CM on Bax and Bcl-2 in PC12 cells. a The cell lysates were immunoblotted with anti-Bax (the first panel), anti-Bcl-2 (the second panel), or β-actin antibodies. b The differences of the protein expressions between the groups were analyzed with Image J. *P < 0.05 versus control group; # P < 0.05, as compared with (CM + PMBS) group
Discussion
In this study, we provide several evidences to support the hypothesis that activation of BV2 microglia by TLR4 signaling pathway is involved in the apoptosis in PC12 cells. Specifically, we treated BV2 microglia with LPS and show that (1) the levels of TLR4, MyD88, and NF-κB significantly increased in BV2 microglia and the release of proinflammatory factor IL-1β significantly increased; (2) PC12 cell viability decreased after CM pretreatment. CM up-regulated the Bax level and down-regulated the Bcl-2 protein levels in PC12 cells which led to PC12 cell apoptosis; (3) Pretreatment of PC12 cells with interleukin-1 receptor antagonist (IL-1RA) for 30 min significantly alleviated CM-induced PC12 cell injury and apoptosis, and inhibited the up-regulation of Bax/Bcl-2. These results indicated that LPS activated BV2 microglia through TLR4/MyD88/NF-κB signaling pathway, then promoted the release of proinflammatory cytokine IL-1β, which led to the apoptosis in PC12 cells.
Microglia, macrophage-like resident immune cells in CNS, are considered to be a first line of defense in CNS and provide tissue defense and protection (Bechade et al. 2013; Bessis et al. 2007). However, excessive acute or chronic microglia activation can trigger severe neuronal damage by the release of cytotoxic factors, including nitric oxide, reactive oxygen species, tumor necrosis factor α, and IL-1 (Surace and Block 2012; Trang et al. 2012). Thus, microglia may be a double-edged sword since the microglia have both neurotoxic and neuroprotective effects. The activated microglia can produce some proinflammatory cytokines such as TNF-α, IL-1β, NO, and ROS. The release and accumulation of these microglia-derived proinflammatory factors are thought to enhance the neuronal damage, especially in neurodegenerative diseases (Gao et al. 2003). However, the damaged neurons secrete the neurotoxic soluble factors and, in turn, induce microglia reactivation (Block and Hong 2005). Taken together, a vicious cycle causing the prolonged neuroinflammation and the progressive neurodegeneration is created (Gao and Hong 2008). Properly activated microglia can be neuroprotective. Being the native immune cells of the CNS, microglia act as the first line of defense, protecting the CNS from invading agents as well as internal enemies. The properly activated microglia can release some useful factors, such brain-derived neurotrophic factor (BDNF) or glial cell-line derived neurotrophic factor (GDNF). There is compelling evidence that activated microglia in the spinal cord release BDNF in response to peripheral nerve lesion, which is crucial for the development of neuropathic pain (Tsuda et al. 2003; Coull et al. 2005; Ulmann et al. 2008). Microglia express many surface receptors, including lipopolysaccharide (LPS) receptor CD14 (Liu and Walter 2005), the purine receptor P2Y6 (Hoarau et al. 2011), the CX3C chemokine fractalkine receptor CX3CR1 (Lee et al. 2010), and the toll-like receptors (TLRs) (Liu et al. 2012), which are all involved in the progression of neurological disease by altering microglia activities (Suzumura 2013). Therefore, it is important to clarify this cellular crosstalk signaling pathways between microglia and neurons for seeking future therapeutic targets of CNS diseases, including pain, infection, neurodegeneration, and stroke.
Microglia, like other monocyte-derived cells, possess a full complement of TLRs. TLR4 recognizes pathogen-associated molecular patterns; trigger the activation of innate immunity and the release of cytotoxic factors. Endotoxin LPS, as an exemplary ligand for TLR4, could bind with TLR4 and initiate the activation of microglia. Therefore, our first aim was to confirm the activation of the TLR4/MyD88 signal pathway, which could mediate the microglia activation following LPS stimulation in vitro. MyD88 is a central adaptor protein for the majority of TLRs, acting as a link between the receptors and downstream signaling molecules. One such molecule is the transcription factor NF-κB, which requires phosphorylation of IκB as a prerequisite for its activation. NF-κB plays a well-characterized role in regulating cytokine production. Our previous studies found that TLR4/MyD88 signaling pathway was involved in traumatic brain injury in vivo (Mao et al. 2012), BV2 microglia injury following the oxygen and glucose deprivation (OGD)/reoxygenation in vitro (Bechade et al. 2013; Qin et al. 2013), and digestive diseases (De Paola et al. 2012). The TLR4-activated downstream signaling pathway, including MyD88 activation and NF-κB translocation, could promote microglia activation and the production of proinflammatory cytokines. In the present study, we found that levels of TLR4 and MyD88 proteins in the BV2 microglia both increased after LPS stimulation in vitro. Meantime, increased protein levels of NF-κB was accompanied by a reduction of IκB-α protein and an increase of p-IκB-α protein, supporting the idea that TLR4-mediated signaling events occur in BV2 microglia activation following LPS stimulation in vitro.
Another important aim of this research was to determine whether LPS activation of TLR4 in microglia cells had an effect on the neuron viability and apoptosis and its mechanism. Lehnardt et al. firstly confirmed the neurotoxic effects of the TLR4 signaling pathway in microglia (Lehnardt et al. 2003). LPS caused neuronal injury in co-cultures prepared from wild-type mice but did not induce neuronal injury in co-cultures derived from TLR4 mutant mice (Lehnardt et al. 2003). Increased TLR4 expression could be involved in excessive neuroinflammation during brain hypoxia/ischemia (Hughes 2012; De Paola et al. 2012; Kilic et al. 2008; Yao et al. 2013). Therefore, TLR4 abnormal expression or excessive immune reaction would cause damage to the CNS. Activation of microglia is interpreted as an inflammatory response to infection, and microglia cytokines are known to induce neuronal death in models of neurodegeneration (Tanga et al. 2005). Our group reported that TLR4 /MyD88 signaling pathway is involved in traumatic brain injury (Mao et al. 2012). In vitro studies, with microglia co-cultured with neurons, Schisandrin B may exert neuroprotective activity by attenuating the microglia-mediated neuroinflammatory response by inhibiting the TLR4/MyD88/NF-κB signaling pathway (Zeng et al. 2012). Since TLR4 is an essential upstream sensor for LPS and may mediate the NF-κB activation, and NF-κB activation increases gene transcription and protein synthesis of proinflammatory cytokines such as IL-1β, we hypothesis that IL-1β released from activated BV2 microglia is a key agent inducing the neuron injury. In most cases, IL-1β, as a cytokine, is typically elevated during neurodegenerative disease states and further promotes CNS inflammation and apoptosis. The increased level of IL-1β is always observed before neuron death, which could alter the function of neurons (Esser et al. 1996; Guo et al. 1998; Chao et al. 1995), IL-1β could lead neuronal injury, and N-methly-d-a spartate (NMDA) receptor antagonists could block this neurotoxicity. Prolong exposure to IL-1β generally lead to chronic inflammation and neuronal degeneration, which culminate into devastating CNS disease. In primary neuronal cultures, IL-1β increased glutamate production and excitotoxicity (Ye et al. 2013). IL-1β might be an important mediator between microglia and neurons. We would like to express that the IL-1β could lead to neuronal death.
In the present study, the results showed that the secretion of IL-1β increased significantly in the supernatant of LPS-treated BV2 microglia cells. For convenience, we named the supernatant of 10 μg/ml LPS-treated BV2 cells as the conditioned medium (CM). We observed that PC12 cell viability decreased after PC12 cells were co-cultured with CM for 24 h. Meanwhile CM up-regulated the pro-apoptosis Bax protein level and down-regulated anti-apoptosis Bcl-2 protein level in PC12 cells. We speculated that proinflammatory cytokine IL-1β in CM induced PC12 cells injury and apoptosis. In order to exclude the interference of LPS in CM, 10 μg/ml antibiotic PMBS (LPS-specific antagonist) was added to CM. PMBS completely eliminate the neurotoxic effects of LPS (Lehnardt et al. 2008). We verified that PMBS alone did not affect the PC12 cell viability and the release of IL-1β from BV2 microglia following LPS stimulation.
IL-1R is expressed on the surface of PC12 cell by immunocytochemistry and Western blot methods. PC12 cells have the potential to sense extracellular cytokine stimuli such as IL-1β (Shu et al. 2007). IL-1β released from the activation of spinal microglia enhanced the AMPA response in neurons through IL-1R activation which led to acute and chronic pain, and IL-1R antagonist (IL-1RA) can abolish these effects of IL-1β (Gustafson-Vickers et al. 2008; Kawasaki et al. 2008; Liu et al. 2013). IL-1β-induced apoptosis was mediated by mitochondria, through up-regulation of Bax and down-regulation of Bcl-2 and Bcl-xL to initiate caspase-9 activity, leading to the activation of caspase-3 in this apoptotic process (Wang et al. 2005). IL-1R is expressed in the PC12 cells and IL-1β has toxic effect on PC12 cells by binding with IL-1R. We found in this study that pretreatment of PC12 cells with IL-1RA for 30 min could alleviate CM-induced PC12 cell injury and apoptosis and depress the up-regulation of Bax/Bcl-2. Therefore, we suppose that the downstream proinflammatory cytokine IL-1β of TLR4 signaling pathway in BV2 microglia bind with IL-1R on the surface of PC12 cell, which could lead to PC12 cell injury and apoptosis.
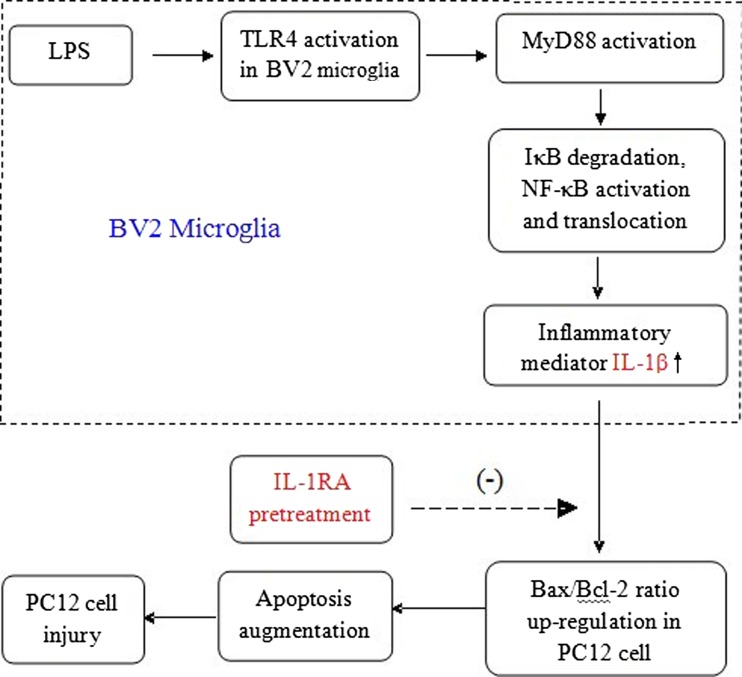
In conclusion, the levels of TLR4, MyD88, and NF-κB proteins in BV2 microglia all increased after stimulation of BV2 microglia with LPS and promote the release of inflammatory mediator IL-1β. The CM containing IL-1β lead to PC12 cell apoptosis and injury by binding with IL-1R. The study establishes a link between BV2 microglia activation and PC12 cell injury and apoptosis in vitro (Fig. 10). These results indicate that the activation of TLR4/MyD88/NF-κB signaling pathway in microglia may be involved in neuronal damage, and microglia are the source of neuroinflammation. The research about bidirectional interaction between neurons and microglia is important for understanding of acute and chronic neuroinflammation, and gives us clues for future therapeutic strategy against CNS diseases such as neurodegenerative disorders, pain, CNS infection, stroke, and trauma.
Fig. 10.
Activation of BV2 microglia could trigger a strong inflammatory reaction in PC12 cell apoptosis by toll-like receptor signaling pathway. LPS-treated BV2 microglia had increased levels of TLR4, MyD88, NF-κB, and release of inflammatory mediator IL-1 β significantly increased. The inflammatory mediator up-regulated Bax to Bcl-2 ratio to promote PC12 cell apoptosis and injury. IL-1RA pretreatment protected PC12 cell from IL-1β-induced injury through down-regulating Bax to Bcl-2 ratio to suppress apoptosis
Acknowledgments
This project was supported by grants from the National Natural Science of China (81171041), the Natural Science foundation of Jiangsu Province (BK2011197), Key Subject of Colleges and Universities Natural Science Foundation of Jiangsu Province (13KJA320001), the Natural Science fund for colleges and universities of Jiangsu Province (12KJD320005), the Innovation Project of Nanjing Military Region (ZD13), the University Graduate Student Science and Technology Innovation Project of Jiangsu Province (XYYC-1214), and sponsored by Qing Lan Project.
Competing interests
The authors have declared that no competing interests exist.
Footnotes
Xiao-jing Dai and Na Li contributed equally to this work.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Peterson PK, Lokensgard JR. Toll-like receptors in defense and damage of the central nervous system. J Neuroimmune Pharm. 2007;2:297–312. doi: 10.1007/s11481-007-9071-5. [DOI] [PubMed] [Google Scholar]
- Arslan F, Keogh B, McGuirk P, et al. TLR2 and TLR4 in ischemia reperfusion injury. Mediat Inflamm. 2010;2010:704202. doi: 10.1155/2010/704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Okun E, Tang SC, et al. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- Baron R. Mechanisms of disease: Neuropathic pain-A clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- Bechade C, Cantaut-Belarif Y, Bessis A. Microglial control of neuronal activity. Front Cell Neurosci. 2013;7:32. doi: 10.3389/fncel.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, et al. Microglial control of neuronal death and synaptic properties. Glia. 2007;55(3):233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong J. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Brea D, Blanco M, Sobrino T, et al. The levels of expression of toll-like receptors 2 and 4 in neutrophils are associated with the prognosis of ischaemic stroke patients. Rev Neurol. 2010;52:12–19. [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, et al. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: Involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- De Paola M, Mariani A, Biqini P, et al. Neuroprotective effects of toll-like receptor 4 antagonism in spinal cord cultures and in a mouse model of motor neuron degeneration. Mol Med. 2012;18:971–981. doi: 10.2119/molmed.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BJ, Ma WW, He LL, et al. Soybean isoflavone alleviates beta-amyloid 1–42 induced inflammatory response to improve learning and memory ability by down regulation of Toll-like receptor 4 expression and nuclear factor-kappaB activity in rats. Int J Dev Neurosci. 2011;29:537–542. doi: 10.1016/j.ijdevneu.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Esser R, Glienke W, von Briesen H, et al. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with human immunodeficiency virus. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, et al. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003;24(8):395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Guo H, Jin YX, Ishikawa M, et al. Regulation of betachemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines. Scand J Immunol. 1998;48:502–508. doi: 10.1046/j.1365-3083.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- Gustafson-Vickers SL, Lu VB, Lai AY, et al. Long-term actions of interleukin-1beta on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol Pain. 2008;4:63. doi: 10.1186/1744-8069-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, et al. Activation and control of CNS innate immune responses in health and diseases: a balancing act finely tuned by neuroimmune regulators (NIReg) CNS Neurol Disord Drug Targets. 2011;10(1):25–43. doi: 10.2174/187152711794488601. [DOI] [PubMed] [Google Scholar]
- Hughes V. Microglia: the constant gardeners. Nature. 2012;485(7400):570–572. doi: 10.1038/485570a. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol. 2003;23:15–44. doi: 10.1615/CritRevImmunol.v23.i12.20. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, et al. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, et al. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31(1):33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Lee S, Vravel NH, Konerth ME, et al. CX3CR1 deficiency alters microglial activation and reduces β-amyloid deposition in two Alzheimer's disease mouse models. Am J Pathol. 2010;177(5):2552–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22(7):2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, et al. A vicious cycle involving release of heat shock protein60 from injured cells and activation of toll-Like receptor 4 mediates neurodegeneration in the CNS. Neuroscience. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Walter S. Staqi M, at al: LPS receptor (CD14): a receptor for phagocytosis of Alzheimer's amyloid peptide. Brain. 2005;128(8):1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28(2):131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Jiang CY, Fujita T, et al. Enhancement by interleukin-1beta of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal horn neurons. Mol Pain. 2013;9(1):16. doi: 10.1186/1744-8069-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Mao SS, Hua R, Zhao XP, et al. Exogenous administration of PACAP alleviates traumatic brain injury in rats through a mechanism involving the TLR4/MyD88/NF-κB pathway. J Neurotrauma. 2012;29(10):1941–1959. doi: 10.1089/neu.2011.2244. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. TLR-mediated innate immune recognition. Semin Immunol. 2007;19:1–2. doi: 10.1016/j.smim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptor. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Lofrumento DD, Saponaro C, et al. Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson's-like disease. Immunopharmacol Immunotoxicol. 2008;30:729–740. doi: 10.1080/08923970802278557. [DOI] [PubMed] [Google Scholar]
- Qin X, Sun ZQ, Dai XJ, et al. Toll-like receptor 4 signaling is involved in PACAP-induced neuroprotection in BV2 microglial cells under OGD/reoxygenation. Neurol Res. 2012;34:610–615. doi: 10.1179/1743132812Y.0000000028. [DOI] [PubMed] [Google Scholar]
- Qin X, Sun ZQ, Zhang XW, et al. TLR4 signaling is involved in the protective effect of propofol in BV2 microglia against OGD/reoxygenation. J Physiol Biochem. 2013;64(4):707–718. doi: 10.1007/s13105-013-0247-6. [DOI] [PubMed] [Google Scholar]
- Rajagopal N. Inhibition of toll-like receptor signaling in primary murine microglia. J Neuroimmune Pharm. 2008;3:5–11. doi: 10.1007/s11481-007-9097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rev P. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Schulz J. Woolf CJ: The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Shu HF, Wang BR, Bi H, et al. PC12 cells express IL-1 receptor type I and response to IL-1 stimulation. Respir Physiol Neurobiol. 2007;157:187–195. doi: 10.1016/j.resp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Smith HS. Activated microglia in nociception. Pain Physicians. 2010;13:295–304. [PubMed] [Google Scholar]
- Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MR, Wen YR, Decosterd I, et al. Do glial cells control pain? Neuron Glia Biol. 2007;3:255–268. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A. Neuron-Microglia Interaction in Neuroinflammation. Curr Protein Pept Sci. 2013;14(1):16–20. doi: 10.2174/1389203711314010004. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102(16):5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. ATP receptor gate microglia signaling in neuropathic pain. Exp Neurol. 2012;234:354–361. doi: 10.1016/j.expneurol.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tozaki-Saitoh H, Inoue K. Purinergic system, microglia and neuropathic pain. Curr Opin Pharmacol. 2012;12:74–79. doi: 10.1016/j.coph.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. Neuroscience. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar P, Kullmann JS, Thilo B, Claussen MC, Rothhammer V, Jacobi H, Sellner J, Waak J, Weber SS, Waldenmaier A, et al. Regulation of astrocyte inflammatory responses by the Parkinson's disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang MW, Tashiro S, et al. IL-1β acts in synergy with endogenous IL-1β in A375-S2 human melanoma cell apoptosis through mitochondrial pathway. J Korean Med Sci. 2005;20:555–561. doi: 10.3346/jkms.2005.20.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Kan EM, Lu J, et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation. 2013;10:23. doi: 10.1186/1742-2094-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Huang Y, Zhao L, et al. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. Neurochemistry. 2013;125(6):897–908. doi: 10.1111/jnc.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng KW, Zhang T, Fu H, et al. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur J Pharmacol. 2012;692(1–3):29–37. doi: 10.1016/j.ejphar.2012.05.030. [DOI] [PubMed] [Google Scholar]