Abstract
Histone chaperones are an integral part of the transcription regulatory machinery. We investigated the involvement of histone chaperones and their functional interactions with ATP-dependent chromatin remodeling complexes in the regulation of yeast heat shock genes. Strong functional interaction between the histone chaperone ASF1 and the ATP-dependent chromatin remodeling complex SWI/SNF is exhibited in synergistic diminishment of nucleosome displacement during heat shock in the ΔASF1/ΔSNF2 strain in comparison to individual ASF1 or SNF2 inactivation. A similar but less pronounced effect was observed for ISW1/ASF1 inactivation but not for ASF1/STH1 (RSC complex) combinatorial inactivation. The depletion of Spt16, which is a major subunit of the FACT histone chaperone complex, leads to a severe growth defect phenotype associated with unusual thermotolerance. The acquired thermotolerance in the Spt16-depleted strain is associated with a defect in the reassembly of nucleosomes at the promoters of heat shock genes during sustained heat stress, leading to increased recruitment of the transcriptional activator HSF and RNA polymerase II. The defect in nucleosome assembly associated with Spt16 depletion also leads to an increased tolerance to stress due to an increased concentration of NaCl.
Keywords: Chromatin remodeling, ASF1, SPT16, RSC, ISW1, SWI/SNF
Introduction
Chromatin remodeling is a universal prerequisite for transcription initiation and often extends from the promoter region to the transcription termination site and ranges from single or multiple posttranslational modifications of histones to the shift of nucleosomes and/or complete eviction of histone octamers from functional regions. There are multiple enzymatic activities involved in these processes at different stages (Li et al. 2007), but the actual mechanisms of chromatin remodeling vary among different gene promoters and often are not well characterized. The yeast heat shock genes are an excellent model for the investigation of chromatin remodeling mechanisms because these genes show the most pronounced changes in their promoter regions upon induction by heat stress. It was previously shown by us and others (Erkina and Erkine 2006; Zhao et al. 2005) that, for instance, up to 50 % of histone octamer eviction at the promoter of the HSP82 gene occurs in the first 30 s after temperature increases and peaks at approximately 95 % histone loss after just 8 min of heat stress (Erkina and Erkine 2006). Other heat shock genes can lag for 2–4 min but generally show the same kinetic pattern of chromatin remodeling and transcription activation. The difference likely accounts for the variance in the amount of heat shock factor (HSF) present at different promoters before heat induction as well as in the involvement of other transcriptional activators (Erkina and Erkine 2006; Erkina et al. 2008). While posttranslational histone modification and involvement of ATP-dependent chromatin remodeling activities was demonstrated previously (Erkina and Erkine 2006; Erkina et al. 2010), none of these factors and enzymatic complexes alone can account for the physical displacement of histone octamers from heat shock gene promoters. As previously shown for the yeast PHO5 promoter, which undergoes less pronounced nucleosome loss upon induction of transcription, histone displacement occurs in trans, and the likely mechanism for this process is nucleosome disassembly and reassembly at other locations (Boeger et al. 2004; Korber et al. 2004; Schermer et al. 2005). This concept alone strongly suggests the involvement of histone chaperones (Das et al. 2010), which can interact with different individual components of the nucleosome, thus promoting the disassembly and reassembly processes. The importance of histone chaperones in the initiation of transcription was underscored, for instance, by the fact of demonstration that the malfunction of Spt6, which is critical for reassembly of promoter nucleosomes at the PHO5 promoter, leads to the continuous transcription initiation without the presence of the transcriptional activator (Adkins and Tyler 2006). The presence of other histone chaperones and their critical involvement in the initiation of transcription was demonstrated for a large amount of gene promoters including stress response genes (Jensen et al. 2008; Mason and Struhl 2003; Schwabish and Struhl 2006). Characterization of mechanisms of histone chaperone function remains an important area in the investigation of transcriptional regulation.
Originally, the involvement of chromatin remodeling activities and the function of histone chaperones in transcriptional activation were considered separately, although functional interactions between SWI/SNF and Nap1 were demonstrated in vitro (Lorch et al. 2006) and were presumed to be true for the in vivo situation. More recent reports indicate that the histone chaperones in some instances work in cooperation with other enzymatic activities in vivo. For instance, it was demonstrated that there are functional interactions between SWI/SNF and ASF1 in the regulation of the HO gene (Gkikopoulos et al. 2009), and the recruitment of RSC complex at the HO gene is also dependent on the ASF1 function (Takahata et al. 2009). Functional interactions between the histone chaperone ASF1 and the histone acetyltransferase Rtt109 were also demonstrated (Han et al. 2007; Lin and Schultz 2011). These examples demonstrate that cooperation between different chromatin remodeling activities and histone chaperones is most likely more wide spread than previously thought and that investigation of these interactions is important for the understanding of transcriptional regulation in general and for understanding the regulation of HSP genes in particular.
The significant number of histone chaperones and histone-binding proteins identified in the last decade (Das et al. 2010) suggests differentiation of histone chaperone functions as well as some functional redundancy. Because histone chaperones have different affinities for different histones, they potentially can cooperate and interact functionally as well as physically, yet these ideas require more investigation. The histone chaperone complex FACT (facilitates activation of transcription) was originally presumed to interact with H2A-H2B to determine the removal/deposition of this histone dimer during nucleosome disassembly/reassembly based on in vitro experimental data (Belotserkovskaya et al. 2003), but this specialization was recently subjected to revision (primarily based on the in vivo data), and a new working model was formulated (Formosa 2008; Xin et al. 2009). According to the new model, FACT can interact with both an H2A-H2B dimer and with an H3-H4 tetramer, resulting in an unfolded structurally modified nucleosome, which is a better substrate for further disassembly steps. The idea that in vitro nucleosome assembly/disassembly can be separated into several intermediate steps suggests that each of these steps can be aided by different histone chaperones (Elsasser and D'Arcy 2012). While FACT remains a primary candidate for the H2A-H2B dimer removal/deposition, the chaperoning of H3-H4 histones can be disputed between ASF1 (English et al. 2005, 2006) and Spt6 (Ivanovska et al. 2011) and is likely gene specific. The difficulty in discerning a specific mechanism for the individual chaperones can be partially explained by the presence of unstructured regions that are highly pliable and can participate in multiple target interactions utilizing an “induced fit” (Elsasser and D'Arcy 2012). Characterization of the function of individual histone chaperones in specific gene contexts remains an important task.
In this report, we show that the cooperation of ATP-dependent nucleosome remodelers and histone chaperones is a likely occurrence in chromatin remodeling at least at yeast heat shock gene promoters. While previous reports indicate that the ASF1 and SWI/SNF complexes are directly recruited to the yeast heat shock gene promoters, we show that, individually, any of these activities has little or no effect on chromatin remodeling at the heat shock gene promoters. In contrast to the individual deletions of ASF1 and SNF2, the combination of ΔASF1 and ΔSNF2 mutations has strong synergistic affects and strongly diminishes nucleosome displacement at least at the HSP82 and SSA4 promoters, suggesting that ASF1 and SWI/SNF complexes functionally interact. We show that such functional interactions do not occur between ASF1 and RSC or between ASF1 and SWI1 complexes. Arguably, one of the most interesting observations we report is that the inactivation of Spt16, which is the major subunit of FACT complex, leads to synthetic thermotolerance, with the Spt16-depleted strain exhibiting an increased growth rate of at 39 °C in comparison to 30 °C. This thermotolerant phenotype is correlated with a higher level of Pol II recruitment and with a defect in the nucleosome assembly at the HSP12, HSP82, and SSA4 heat shock gene promoters, suggesting that the FACT complex determines nucleosome deposition at least at some the heat shock gene promoters.
Materials and methods
Strains and cultivation conditions
The strains utilized in this study are indicated in Table 1. Strains ΔASF1 ΔSNF2 (TYE022), ΔASF1 tet-STH1 (TYE020), ΔASF1 ΔISW1 (TYE021), and ΔASF1 tet-SPT16 (TYE019) were constructed by substituting the entire ORF of the ASF1 gene including the promoter and terminator areas (−262 to +1004, relative to the ASF1 gene translation start codon) with the LEU2 cassette (−316 to +1372, relative to the LEU2 gene translation start codon), which was PCR amplified from the PRS315 plasmid with synthetic fusion primers bearing 44 nt at each flank of ASF1 sequence at the 5′ends and 30 nucleotides of LEU2 sequences at the 3′ends. Strains bearing the single mutation were transformed with the LEU2 cassette flanked by ASF1 target sequences according to the previously described procedure (Guldener et al. 1996). The correct chromosomal integration was confirmed by a diagnostic PCR using the ASF1 (−368 to −333) and LEU2 (+92 to +57) primers.
Table 1.
Yeast strains
| Strain | Genotype | Reference |
|---|---|---|
| BY4741 (R1158) | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| ΔASF1 (YSC6273) | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 asf1::kanMX | Open Biosystems |
| Tet-SPT16 (TH_5591) | MATa his3-1 leu2-0 met15-0 pSPT16::kanR-tet07-TATA URA3::CMV-tTA | Open Biosystems |
| ΔSNF2 (YSC6273) | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 snf2::kanMX | Open Biosystems |
| Tet-STH1 (TH_7448) | MATa his3-1 leu2-0 met15-0 pSTH1::kanR-tet07-TATA URA3::CMV-tTA | Open Biosystems |
| ΔISW1 (YSC6273) | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 isw1::kanMX | Open Biosystems |
| ΔASF1 ΔSNF2 (TYE022) | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 snf2::kanMX asf1::LEU2 | This study |
| ΔASF1 tet-STH1 (TYE020) | MATa his3-1 leu2-0 met15-0 pSTH1::kanR-tet07-TATA URA3::CMV-tTA asf1::LEU2 | This study |
| ΔASF1 ΔISW1 (TYE021) | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 isw1::kanMX asf1::LEU2 | This study |
| ΔASF1 tet-SPT16 (TYE019) | MATa his3-1 leu2-0 met15-0 pSPT16::kanR-tet07-TATA URA3::CMV-tTA asf1::LEU2 | This study |
Saccharomyces cerevisiae strains were cultivated at 30 °C to early log phase in rich YPD broth supplemented with 0.04 mg/ml adenine. For heat shock time course experiments, instantaneous up-shift was achieved by rapidly mixing equal volumes of 30 °C culture with a pre-warmed 52 °C medium and then incubating with shaking at 39 °C for the times indicated. If necessary, doxycycline was added to the cultivation media at 10 μg/ml, and cell cultures were grown overnight before heat shock experiments.
Spot dilution assay
Cells were grown at 30 °C until early log phase (OD600 0.3–0.5). Each cell culture was precipitated and resuspended in sterile water to OD600 0.1. Each individual culture was fivefold serially diluted and replicated on individual plates using Sigma Replica Plater (R-2383). Spots were grown for 2 days at the indicated temperatures.
ChIP analysis
Chromatin Immunoprecipitation (ChIP) was performed essentially as previously described (Erkine and Gross 2003) with the exception that protein A magnetic beads were used instead of protein A sepharose beads to precipitate antigen-antibody complexes. Special attention was paid to the consistency of the sonication levels of the cell lysates. Before immunoprecipitation, all samples were tested for the level of DNA fragmentation, and the mean size of DNA fragments was always 500 bp. Antibodies specific for the following epitopes were used: histone H3 total (from AbCam; ab1791); Pol II-YSPT[pS]PS repeat of Pol II C-terminal domain (CTD) (4H8 monoclonal antibody from Upstate Biotechnology; this antibody recognizes both phospho- and non-phospho Pol II according to the manufacturer’s data); HSF (rabbit antibody generated and characterized previously (Erkine et al. 1996)). Immunoprecipitated DNA samples were used for real-time PCR with SYBR Green dye. Because the PHO5 promoter is known to contain positioned nucleosomes (Korber et al. 2004) and its chromatin context does not change during heat shock (Erkine and Gross 2003), signals for individual gene promoters were normalized against the corresponding signal derived from the PHO5 promoter (for histone ChIPs) or the chromosome V intergenic region (in the case of Pol II and HSF ChIPs) and to the input DNA sample. We previously compared (Erkina et al. 2008) normalization of the signal from the HSP12 promoter to these regions and found no differences. The results of ChIPs are presented either as an abundance of the normalized signal relative to the above indicated loci or as a loss (an inverse ration) of the normalized signal (e.g., histone H3 loss, see the beginning of the “Results” section for the rationale). For each DNA sample, at least three consecutive dilutions were analyzed to ensure that the amplification rate was always optimal and the change in amplification signal was proportional to the change in the amount of DNA. In addition, controls without DNA were always included to verify that primer-dimer formation was not detectable or comparable to the amplification from experimental samples. Experiments were typically repeated three times or more; error bars in the figures indicate standard deviations.
Primers for real-time PCR reactions were selected among a significant number of primers based on PCR efficiency. The only primer pairs used were those that gave an amplification rate of at least 1.9 per PCR cycle during linear amplification and did not produce primer-dimers. The sequences of PCR primers used in this study were as follows (coordinates are relative to ATG): PHO5 (−214 to −192; −20 to −48), HSP12 (−304 to −279 or −337-304; −82 to −107), HSP82 (−193 to −167; −37 to −69), SSA4 (−307 to −279; −70 to −98), and chromosome V intergenic region (GCAATCAACATCTGAAGAAAAGAAAGTAGT, CATAATCTGCTGAAAAATGGCGTAAAT).
Results
Deletion of ASF1 leads to temperature sensitivity but chromatin remodeling at the heat shock gene promoters is not affected
The involvement of histone chaperones and chromatin remodeling complexes in heat shock gene regulation were tested by the following two approaches: by analyzing the growth phenotype of the knockout or knockdown strains with inactivated critical subunits of the corresponding complexes and by investigation of nucleosome displacement at selected HSP gene promoters associated with heat stress in the same strains. The growth defect phenotypes were tested not only at 30 °C, which is the normal cultivation temperature for yeast, but also at higher temperatures (33, 36, and 39 °C) to assess temperature sensitivity. We realize that temperature sensitivity, if observed, can be pleiotropic and/or caused by a change in the expression of non-heat shock genes, yet considering critical role of molecular chaperones in coping with stress, we considered temperature sensitivity as a preliminary indication of potential effects of the investigated mutations on the expression of heat shock genes, and thus we followed phenotypic experiments with chromatin immune-precipitations (ChIPs) using variable indicated antibodies, testing chromatin remodeling at the promoters of selected heat shock genes.
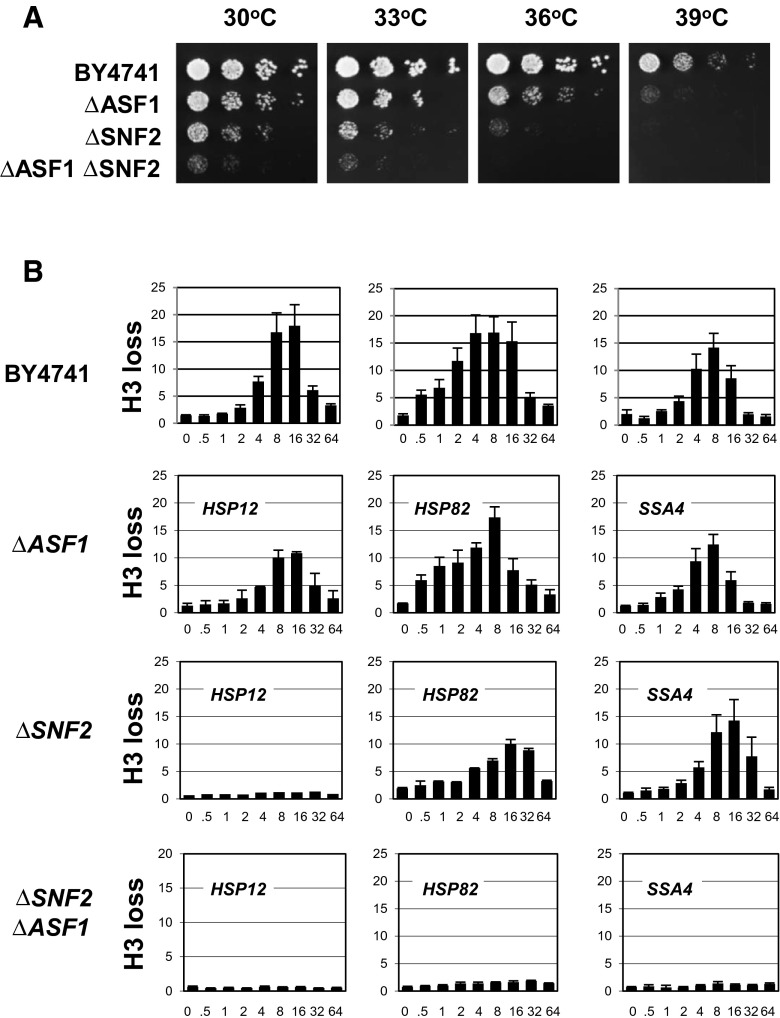
Because ASF1 is a major histone chaperones, is evolutionarily conserved and was previously shown to be recruited to some gene promoters (Gkikopoulos et al. 2009; Takahata et al. 2009), including promoters of heat shock genes (Schwabish and Struhl 2006), we wanted to test whether inactivation of ASF1 by deletion of the ASF1 gene caused any growth defects. We reasoned that if the product of a gene affects the expression of heat shock proteins, which are critical to coping with heat stress, the deletion of the gene should affect the temperature sensitivity of the strain. As Fig. 1a indicates, at 30 °C, the ΔASF1 strain shows only slight differences in comparison to the parental BY4741 strain. Nevertheless, at higher temperatures, especially at 39 °C, the growth defect becomes obvious. These data suggest that ASF1 might be indeed involved in the regulation of heat shock genes.
Fig. 1.
Genetic and functional interactions between the histone chaperone ASF1 and the ATP-dependent chromatin remodeling complex SWI/SNF. a Cell patches for the fivefold spot dilution assay were grown for the indicated strains on YPDA plates for 2 days at the indicated temperatures. b ChIPs were performed using an antibody that recognizes total histone H3. The promoter analyzed is indicated in the upper left corner of each panel. Y-axis: loss of histone H3. X-axis: time (0 to 64 min) after switch of temperature from 30 to 39 °C. All real-time PCR values were normalized relative to the input and to the signal from the PHO5 promoter, which is known to contain positioned nucleosomes that do not change during heat shock (Korber et al. 2004). Values represent mean ± S.D. (n ≥ 3). Note: displacement value is an inverse value of abundance
Knowing that promoters of heat shock genes undergo nucleosome clearance upon heat stress (Erkina and Erkine 2006; Erkina et al. 2008, 2010; Zhao et al. 2005), we wanted to determine whether the ASF1 deletion has any effect on nucleosome eviction at promoters of heat shock genes during the induction of transcription. To test that, we performed histone H3 ChIP (Fig. 1b). The results of such experiments are in some cases reported as a drop (1 to 0 or 100 % to 0 %) in histone abundance during gene activation. This form of presentation restricts analysis of the data because the closer the values are to 0 the more difficult it is to see fine differences. Therefore, we have chosen to present the results as an inverse value, which represents the degree of histone loss and ranges from 1 to ∞. This presentation format better conveys fine differential chromatin remodeling, and we have used it before (Erkina and Erkine 2006; Erkina et al. 2008). This form of presentation also allows better monitoring of kinetic shifts of the maximums in nucleosome displacement, which is important for our analysis. At the same time, we realize that while having the above-mentioned advantages, this form of presentation has a tendency to deemphasize histone losses in first minutes or even seconds of heat shock.
As the histone H3 ChIP data presented in Fig. 1b show, during the time course of heat shock, all three heat shock gene promoters (parental strain BY4741) show the typical response of gradual loss of nucleosomes, which peaks at 8–16 min after the temperature increases, followed by gradual attenuation. This pattern reflects an increase of heat shock gene expression upon stress, which is attenuated by feedback loop regulation (Voellmy and Boellmann 2007). The profiles of the histone H3 loss that we observed in the ΔASF1 strain are not significantly different from those of the parental BY4741 strain. Given the difference in the phenotypes (Fig. 1a) and knowing that upon heat shock the ASF1 is recruited to heat shock gene promoters (Schwabish and Struhl 2006), this was an unexpected result. One possible cause for this discrepancy is that the ASF1 deletion is redundantly compensated by the function of the other histone chaperone and/or is functionally coupled with another chromatin remodeling factor(s).
ASF1 and SNF2 interact genetically and functionally at heat shock gene promoters
It was shown previously that chromatin remodeling at heat shock gene promoters depends partially on the function of the SWI/SNF complex, with SNF2 being the major enzymatic subunit (Erkina et al. 2008). To test whether there are any functional interactions between the ASF1 and the SWI/SNF complex, we created the ΔASF1/ΔSNF2 strain and first tested its phenotype and then followed those experiments by histone H3 ChIPs testing the dynamics of nucleosome loss during heat stress. While the ΔSNF2 strain is known to have a mild slow growth phenotype at normal temperature, we wanted to test whether it had any temperature sensitivity. Indeed, at 39 °C, the ΔSNF2 strain ceases to grow (Fig. 1a), which is consistent with the SWI/SNF complex being involved in the regulation of heat shock genes as we showed previously (Erkina et al. 2008). Importantly, the ΔASF1/ΔSNF2 strain exhibits an even stronger growth defect, barely surviving at 30 °C and halting growth with even a mild increase in temperature (Fig. 1a). Comparing the histone loss profiles for the ΔASF1, ΔSNF2, and ΔASF1/ΔSNF2 strains at three reporter genes, we see a strong effect on the HSP12 promoter and a mild decrease and delay in H3 loss in the ΔSNF2 strain for the HSP82 and SSA4 promoters, which we reported previously (Erkina et al. 2008) and use here just as a reference point. The delay and decrease in H3 loss at the HSP82 and SSA4 promoters are reflected not only in the shift of the maximum but also in harmonic change of samples for the whole profile of the time course with decreased values before the maximum and increased after. We also see a very strong distortion of profiles in the ΔASF1/ΔSNF2 strain. Here, the HSP12 promoter shows an absence of histone loss, similar to the ΔSNF2 strain, while the HSP82 and SSA4 promoters show minor histone loss that peaks at 32 min of heat stress for the HSP82 promoter and is barely visible for the SSA4 promoter. The reason for the abnormal behavior of the HSP12 promoter shown here and previously (Erkina et al. 2008) can be explained by differential regulation of this promoter rooted in domination of this promoter by Msn2/Msn4 activators instead of HSF (Boy-Marcotte et al. 1999). Comparison of all three strains indicates that the decline in the histone H3 loss is significantly higher in the ΔASF1/ΔSNF2 strain in comparison to the single-deletion strains, indicating a synergistic effect of double deletion and suggesting functional cooperation between the ASF1 and the SWI/SNF complex. Because the ΔASF1 strain does not show a change in histone displacement during heat shock, the functional interactions between ASF1 and SWI/SNF complex may be indirect. This possibility will be discussed later (see “Discussion”).
The ASF1 and RSC complex interact genetically, but chromatin remodeling at the heat shock gene promoters is affected only by STH1 depletion
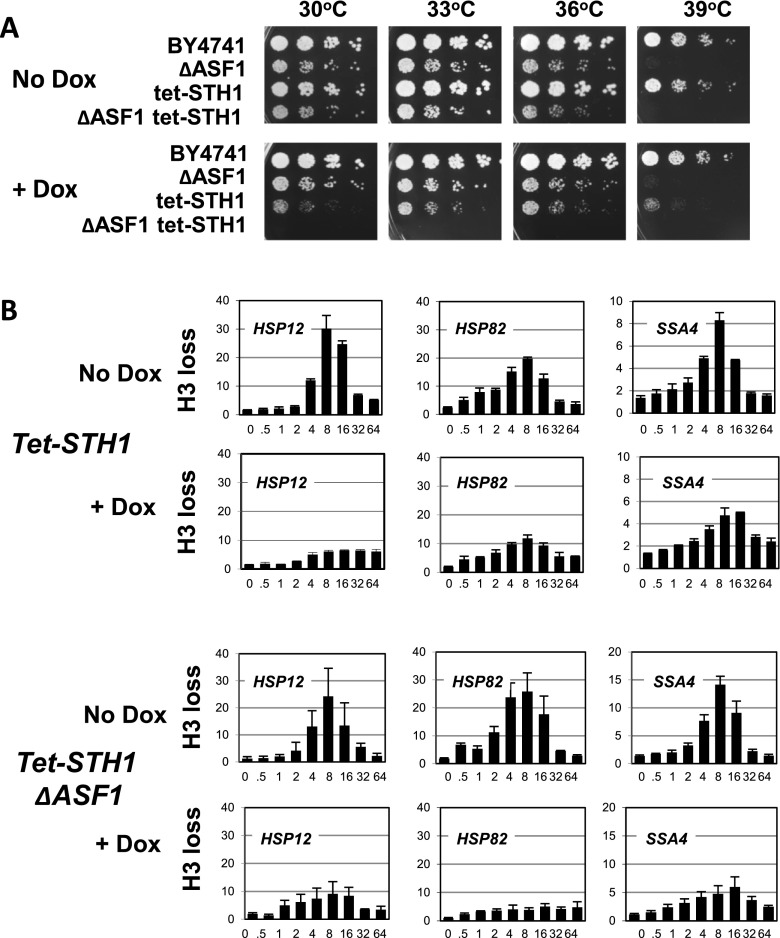
While both ASF1 and the SWI/SNF complex are dispensable for cell growth at 30 °C, creating only a slight growth defect while inactivated, the RSC complex is a major ATP-dependent chromatin remodeling complex that is absolutely essential for growth in any conditions and, as we showed previously (Erkina et al. 2010), is critical for chromatin remodeling at the heat shock gene promoters. To test whether, similarly to the SWI/SNF complex, the RSC complex interacts functionally with ASF1, we created a strain in which the ASF1 deletion is combined with the STH1 gene promoter modification containing the engineered tet-off element. Because the STH1 gene is essential, this latter construct is necessary to grow the strain and later, if desired, to conditionally shut off of STH1 gene expression in the presence of the stable tetracycline analog doxycycline. Analysis of the growth phenotype (Fig. 2a) indicates the expected growth defect of the tet-STH1 strain in the presence of doxycycline, but importantly, unlike the ASF1 or SNF2 deletion strains, the growth rate of the tet-STH1 strain in the presence of doxycycline does not reveal significant temperature dependence, showing a slow growth rate at all tested temperatures in the 30–39 °C interval. That observation indicates that STH1 inactivation might have a more global effect and is not specific for heat shock genes. Analysis of the growth rate phenotypes also revealed that the combination of STH1 depletion and ASF1 deletion is essentially lethal at all growth conditions, which is an indication of possible functional interactions between the RSC complex and ASF1.
Fig. 2.
ASF1 and RSC complex interact genetically but that is not reflected on nucleosome displacement at heat shock gene promoters. a Cell patches for the fivefold spot dilution assay were grown for the indicated strains on the YPDA plates for 2 days at indicated temperatures. b ChIPs using an antibody raised against total histone H3 were performed in the tet-STH1 and tet-STH1/ΔASF1 strains in the absence (No Dox) or presence (+Dox) of doxycycline. For each panel, the time course of heat shock is shown—(0 to 64 min) after switch of temperature from 30 to 39 °C. Data were obtained and represented as described for Fig. 1b
Considering the synthetic lethality of the ΔASF1/tet-STH1 strain in the presence of doxycycline, we wanted to determine whether this combinatorial inactivation of the histone chaperone and the ATP-dependent chromatin remodeling complex had any effect on nucleosome displacement at heat shock gene promoters. Based on the results of histone H3 ChIPs (Fig. 2b), the STH1 inactivation leads to diminishment and delay of histone loss at the heat shock gene promoters, which is reminiscent to the SNF2 deletion effects (Fig. 1b, ΔSNF2). However, comparison of the heat shock-induced histone loss profiles with analogous data for the ΔASF1/tet-STH1 strain showed no significant difference. In the double-inactivation strain, histone loss during the time course of heat stress at all three analyzed promoters was diminished to the same degree and delayed similarly to the single STH1-depleted strain, with the exception of the HSP82 gene promoter, where there is some additive effect. These results indicate that even though there is a strong genetic interaction between ASF1 and the RSC complex as exhibited by their synthetic lethality, the interactions might be gene specific and do not appear to take place at heat shock gene promoters.
ASF1 and ISW1 interact genetically, but not functionally, at heat shock gene promoters
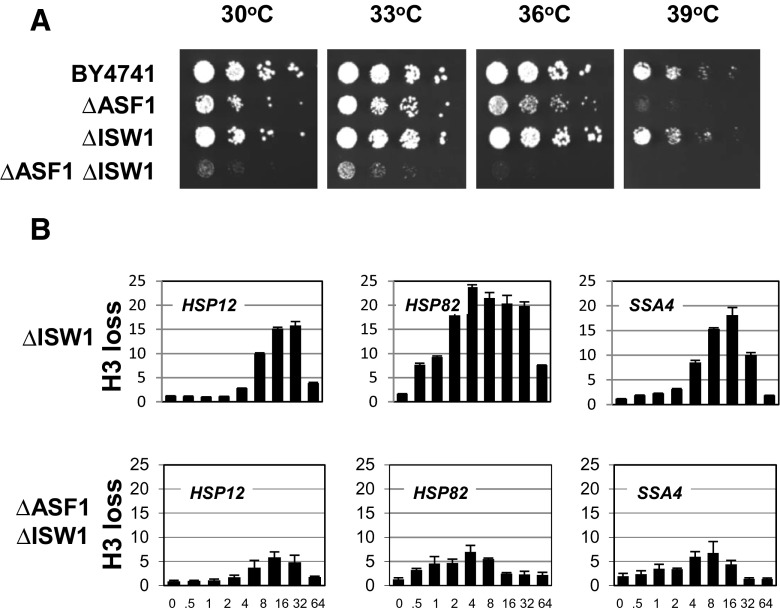
Because we also previously showed the involvement of the ISW1 chromatin remodeling complex in nucleosome displacement events at heat shock gene promoters (Erkina et al. 2010), we wanted to test whether, similarly to the SWI/SNF and RSC complexes, the ISW1 complex, which has ISW1 as a major functional and structural subunit, interacts functionally with ASF1. To investigate this possibility, we created the double-deletion ΔASF1/ΔISW1 strain. Phenotypic analysis (Fig. 3a) of this and the control strains revealed that, while the inactivation of ISW1 alone does not have any effect on the growth rate, the combination of this deletion with ASF1 deletion significantly affects the growth rate, leading not only to slow growth at 30 °C but also to lethality at 36 and 39 °C, suggesting that ISW1 and ASF1 interact genetically. The fact that deletion of ISW1 has no effect on the growth rate is consistent with our previous finding that deletion of ISW1 has an effect only if combined with SNF2 deletion (Erkina et al. 2010). The strong growth defect and the sensitivity to increased temperatures suggest that ASF1/ISW1 interactions may be related to the expression of heat shock genes. To test this relationship, we performed histone H3 ChIPs (Fig. 3b) and found that the degree of histone H3 loss during heat shock was approximately twofold lower in the ASF1/ISW1 double deletion strain than in the ΔISW1 strain (Fig. 3b) or in the ΔASF1 strain (Fig. 1b), which in both single-deletion cases was essentially the same as BY4741 (Fig. 1b). These results indicate some functional interactions but do not signify a synergy in ASF1 and ISW1 functions.
Fig. 3.
Genetic and functional interactions between histone chaperone ASF1 and ATP-dependent chromatin remodeling complex ISW1. a Cell patches for the fivefold spot dilution assay were grown for the indicated strains on the YPDA plates for 2 days at the indicated temperatures. b ChIPs were performed using an antibody that recognizes total histone H3 as described for Fig. 1b. For each panel, the time course of heat shock is shown—(0 to 64 min) after switch of temperature from 30 to 39 °C
Genetic interactions between ASF1 and the FACT complex
The process of nucleosome assembly/disassembly, at least in vitro, can be separated into several intermediate steps with the major ones being the formation of the H3-H4 tetramer and then consecutive addition of two H2A-H2B dimers. Considering that distinct histone chaperones have affinities to different histones, it is reasonable to imagine the cooperation of chaperones with affinities to different histones. Because ASF1 preferentially interacts with histones H3-H4 (English et al. 2005) and considering that the clearest preference for interactions with H2A-H2B histones has been demonstrated for the FACT complex (Belotserkovskaya et al. 2003), we decided to test whether the FACT complex is involved in chromatin remodeling at the heat shock gene promoters and whether it cooperates with ASF1. Because the FACT complex is essential, we utilized a strain containing the tet-off element in the promoter of the SPT16 gene, which encodes the major and essential subunit of the complex. We also created a new strain in which we combined the tet-STH1 construct with the deletion of ASF1.
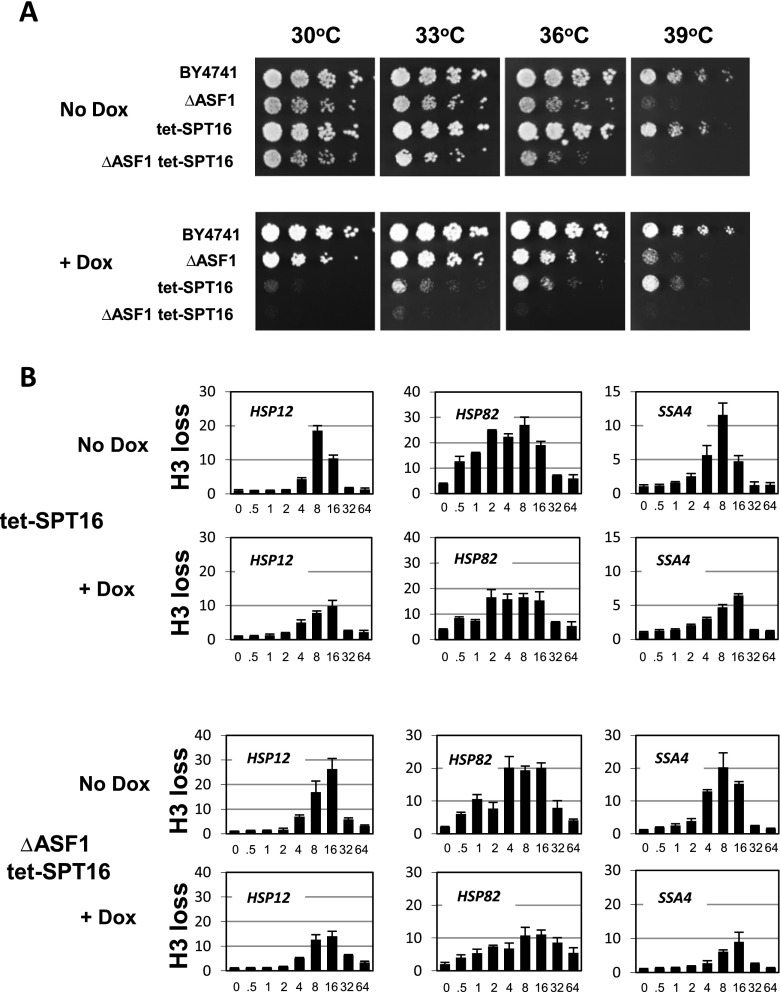
First, we tested the growth phenotypes and found that at 30 °C, the inhibition of expression of the SPT16 gene by doxycycline creates a slow growth phenotype, which is converted into lethality when the SPT16 inactivation is combined with the ASF1 deletion (Fig. 4a). These results suggest that FACT and ASF1 may interact functionally. Examination of growth at higher temperatures revealed a highly unusual situation. While the presence of doxycycline created synthetic lethality for the combinatorial tet-SPT16/ΔASF1 strain at any tested temperature, the tet-SPT16 strain gained viability with the increase of temperature and, in fact, at 39 °C, it did not grow much slower than the parental WT strain, in contrast to its growth at 30 °C (Fig. 4a, +Dox tet-SPT16). These are unexpected results considering that mutations usually create growth defect and/or temperature sensitivity. That was certainly true for almost all mutants we have analyzed so far. We hypothesized that the unusual acquired thermotolerance of the Spt16-deficient strain is likely associated with abnormality of the expression of heat shock genes, the majority of which give encode molecular chaperones and other proteins that help cells to cope with high temperatures, which are conducive to the formation of misfolded proteins. Based on phenotypic analysis of both the tet-SPT16 and tet-SPT16/ΔASF1 strains, we conclude that both ASF1 and Spt16 are likely involved in the regulation of heat shock genes. The synthetic lethality acquired by the inactivation of ASF1 and Spt16 together suggests that they might interact functionally.
Fig. 4.
Genetic interactions between histone chaperones ASF1 and FACT. a Cell patches for the fivefold spot dilution assay were grown for the indicated strains on the YPDA plates for 2 days at the indicated temperatures. b ChIPs using an antibody raised against total histone H3 were performed in the tet-SPT16 and tet-SPT16/ΔASF1 strains in the absence (No Dox) or presence (+Dox) of doxycycline. For each panel, the time course of heat shock is shown—(0 to 64 min) after switch of temperature from 30 to 39 °C. Data were obtained and represented as described for Fig. 1b
To test whether the inactivation of SPT16 alone or in combination with the ASF1 deletion affects chromatin remodeling events at heat shock gene promoters, we again used histone H3 ChIPs in the heat shock time course format. These experiments (Fig. 4b) indicate that the inactivation of SPT16 alone diminishes nucleosome loss during heat stress at all three tested promoters and delays reaching histone loss maximum in this process, which is indicated by the shift of the maximum from 8 min of heat stress to 16 min for the HSP12 and SSA4 promoters. This shift in the maximum is not as obvious for the HSP82 promoter, most likely because the peak of histone loss for this gene is very broad and spans from 4 to 16 min of the stress. These experiments indicate that the FACT complex is likely involved in the processes of nucleosome assembly/disassembly and relocation. These results are consistent with the function of the FACT complex in transcription regulation in cooperation with the elongation complex (Mason and Struhl 2003) and at the promoter of the HO gene (Takahata et al. 2009). The combinatorial inactivation of both ASF1 and SPT16 exhibits virtually identical gene-specific patterns of histone loss as in the case of the single inactivation of SPT16. These results indicate that, although Spt16 is involved in nucleosome displacement at heat shock genes, the genetic interactions between ASF1 and Spt16 that we observed phenotypically are not consistent with their functional interaction at the promoters of heat shock genes. However, the distortion of the kinetic profiles of histone loss during the first hour of heat shock clearly suggest that FACT by itself is important at least for the acute phase of stress response at heat shock gene promoters.
Synthetic thermotolerance created by Spt16 depletion is rooted in the defect of nucleosome reassembly at heat shock gene promoters during sustained heat stress
The highly unusual phenotype of acquired thermotolerance observed for the single SPT16 inactivation deserved further investigation. We hypothesized that the inactivation of SPT16, especially at higher temperatures, leads to the increased level of molecular chaperones necessary to counteract misfolded proteins. To test whether this might be the case, we first wanted to test whether Spt16 is directly involved in the chromatin remodeling events at the heat shock gene promoters. Our Spt16 ChIP data (Fig. 5a) indicate that after switching cells from 30 to 37 °C for 16 min, which is the time of maximal transcriptional response to heat shock (Erkina and Erkine 2006; Erkine et al. 1999), there is an increase in the abundance of Spt16 at promoters of the HSP82 and SSA4 genes. For the HSP12 promoter, the increase in abundance was not as pronounced although it was noticeable. The reason for a diminished reaction of the HSP12 promoter might lie in the involvement of different activators in the regulation of this gene in comparison to the other two. While HSP82 and SSA4 are primarily regulated by HSF, the HSP12 gene is under the influence of not only HSF but also Msn2/4, with the latter activators dominating over HSF (Amoros and Estruch 2001; Boy-Marcotte et al. 1999). Considering the increased abundance of Spt16 at the promoters of heat shock genes upon heat stress, we conclude that Spt16 is physically involved in chromatin remodeling events at least at the HSP82 and SSA4 promoters.
Fig. 5.
The synthetic thermotolerance of the Spt16-depleted strain is determined by the defect of nucleosome assembly at heat shock gene promoters during sustained stress, leading to increased expression of molecular chaperones. a ChIPs using anti-SPT16 antibody were performed for the tet-SPT16 cell culture grown at 30 °C or heat shocked at 37 °C for 16 min, and data for the HSP12, HSP82, and SSA4 promoters are shown. For each panel, Y-axis: abundance of SPT16 relative to the chromosome V intergenic region. X-axis: data for 30 °C and for 37 °C shown. b ChIPs using an anti-histone H3 antibody were performed for the tet-SPT16 cell culture consistently grown at 37 °C in the absence (−Dox) or presence (+Dox) of doxycycline. For each panel, Y-axis: abundance of histone H3 (inverse ration of histone loss) relative to chromosome V intergenic region. X-axis: data shown for cultures consistently grown at 37 °C in the absence (−Dox) or presence (+Dox) of doxycycline. c ChIPs using an anti-HSF antibody were performed for the tet-SPT16 cell culture consistently grown at 37 °C in the absence (−Dox) or presence (+Dox) of doxycycline. For each panel: Y-axis: abundance of HSF relative to the chromosome V intergenic region. X-axis: as in b. d ChIPs using an anti-Pol II antibody were performed for the tet-SPT16 cell culture consistently grown at 37 °C in the absence (−Dox) or presence (+Dox) of doxycycline. For each panel: Y-axis: abundance of Pol II relative to the chromosome V intergenic region. X-axis: as in b. For all sections of the figure (a, b, c, and d), values represent mean ± S.D. (n ≥ 3). The p values calculated using Student’s t test assuming equal variance are shown in the corner of each subpanel
We then wanted to test whether the depletion of Spt16 has any effect on the nucleosome occupancy of the promoters during sustained stress associated with growth at higher temperatures. The experimental setup in this case is different than the previous (Figs. 1, 2, 3, and 4) when we used the acute stress conditions (heat shock within the 0–64 min time scale). For the new setup, we prepared samples from cells consistently grown at 37 °C either with or without doxycycline. The results of the histone H3 ChIPs (Fig. 5b) indicate that cells grown at 37 °C show a reduced degree of histone H3 abundance at the HSP12, HSP82, and SSA4 promoters when SPT16 is depleted (+Dox). Note that the histone abundance format is different than the histone loss format for Figs. 1, 2, 3, and 4. Although these effects are not as pronounced as the loss of histone H3 during acute heat stress (Figs. 1, 2, 3, and 4), considering sustained heat stress (cultivation at 37 °C), the data shown in Fig. 5b certainly indicate that the depletion of Spt16 leads to a lesser efficiency of the nucleosome reassembly at heat shock gene promoters.
Considering the decreased abundance of histone H3 at the HSP12, HSP82, and SSA4 promoters in the Spt16-depleted cells during sustained stress, we hypothesized that the relative promoter vacancy caused by Spt16 depletion and the defect in nucleosome reassembly over the HSP promoters may lead to increased recruitment of the transcriptional activator HSF1 and, as a consequence, to the initiation of transcription by Pol II. To test this, we again employed ChIPs using anti-HSF and anti-Pol II antibodies. As our data show (Fig. 5c, d), the abundance of both HSF1 and Pol II is significantly increased at all three reporter promoters upon Spt16 depletion (+Dox). The relatively mild effect at the SSA4 promoter might be a result of the special situation with the SSA4 promoter, which contains the URS1 sequence and is regulated by the Ume6-Sin3-Rpd3 repressor complex (Kadosh and Struhl 1997). Because yeast genes do not generally exhibit a paused RNA polymerase phenomenon, our data and specifically the increased recruitment of Pol II suggest that there is an overexpression of molecular chaperones in the Spt16-depleted cells.
The heat response in yeast is regulated at the transcriptional level primarily by HSF expressed from a single HSF1 gene. The observed thermotolerance effect associated with Spt16 depletion could be a result of specific HSF-Spt16 interactions. However, it is known that other transcriptional activators such as Msn2 and Msn4 as well as Yap1 and others respond to other types of stresses (Estruch 2000). To test whether Spt16 depletion affects other regulatory circuitries, we wanted to determine whether the Spt16-depleted cells acquire tolerance to other stresses as well. We found that similar to its response to heat stress, the Spt16-depletion strain becomes somewhat tolerant to osmotic stress caused by increased NaCl concentrations. This tolerance is apparent if the growth phenotype of the parental BY4741 strain is compared to that of the tet-SPT16 strain in the presence of doxycycline (Fig. 6). While there is an obvious retardation of the tet-SPT16 strain growth in the presence of doxycycline in YPDA medium without NaCl, with increased NaCl concentration, the difference from the parental strain decreases. This phenotypic effect is not exhibited for stress induced by increased formamide concentration. The similarities of the effects caused by heat stress (Fig. 4a) and the increase of NaCl concentration (Fig. 6) suggests that the reason for the synthetic stress tolerance is likely not a specific Spt16-HSF interaction but rather a more general response related to the defect in nucleosome reassembly during transcription attenuation. Together, our data allow us to make some connections between our phenotypic and biochemical data and formulate a molecular model that explains the stress tolerance induced by Spt16 depletion, which is discussed below.
Fig. 6.
The Spt16 depletion creates tolerance to stresses associated with increased NaCl concentrations. Cell patches for the fivefold spot dilution assay were grown for 2 days for the indicated strains on either YPDA plates or YPDA plates supplemented with NaCl or formamide at the indicated concentrations
Discussion
The main goal of this study was to test whether histone chaperones are involved in chromatin remodeling events at heat shock gene promoters during heat induction and whether these histone chaperones functionally interact with each other and with the ATP-dependent chromatin remodeling complexes. Considering that there are strong indications that, during transcriptional induction, the gene-promoter nucleosomes are removed in trans by complete detachment from DNA (Boeger et al. 2004; Korber et al. 2004; Schermer et al. 2005) and considering that histone chaperones are critical for nucleosome assembly/disassembly (Das et al. 2010), it is reasonable to think that they can interact functionally with the ATP-dependent chromatin remodeling complexes (Duina 2011; Elsasser and D'Arcy 2012). These interactions could be stepwise, starting with the ATP-dependent complexes modifying the nucleosome structure, making it more available for histone chaperone interactions and ending with histone chaperones opening it up, transferring to a new location and detaching from histones, thus completing the assembly of a nucleosome at a new location. This type of mechanism is potentially consistent with previously demonstrated functional interactions between ASF1 and SWI/SNF complexes for the HO promoter (Gkikopoulos et al. 2009; Takahata et al. 2009).
Because chromatin remodeling mechanisms are often gene specific, we restricted our investigations to focus on the promoters of heat shock genes, which are a useful model for extremely fast and robust nucleosome displacement. The choice of the heat shock genes also allowed us to perform the initial steps of our search, analyzing the growth phenotypes caused by the inactivation of single factors belonging to a specific complex or by a combinatorial inactivation and testing whether the new phenotypes are affected by an increase in the cultivation temperature. We reasoned that a temperature-sensitive phenotype may be indicative of changes in the expression level of molecular chaperones, which are responsible for dealing with temperature-induced protein misfolding.
Functional interactions between ATP-dependent chromatin remodeling complexes and histone chaperone ASF1
Because we had previously identified the SWI/SNF, RSC and ISW1 complexes as being involved in chromatin remodeling at heat shock gene promoters (Erkina et al. 2008, 2010), we wanted to test whether combining the inactivation of these complexes with the deletion of ASF1, which was also shown to be directly recruited to these promoters (Schwabish and Struhl 2006), creates a synthetic phenotype. For all of the combinatorial inactivations—SNF2/ASF1, STH1/ASF1, and ISW1/ASF1—we found that the genetic interactions manifested in either an extremely slow growth phenotype (SNF2/ASF1, ISW1/ASF1) or lethality (STH1/ASF1). These interactions manifested as growth defects that may indicate functional interplay but not necessarily at heat shock gene promoters. The fact that all three tested combinations yielded a strong phenotypic result allows us to think that interactions between histone chaperones and ATP-dependent chromatin remodelers might be more common than previously thought.
To determine whether the genetic interactions we identified between ASF1 and the ATP-dependent chromatin remodelers at heat shock gene promoters have any relation to the chromatin remodeling at stress genes, we followed the phenotypic investigations by histone H3 ChIP to test the extent and kinetic profiles of nucleosome loss in response to heat stress. A combination of these approaches allowed us to conclude that only SWI/SNF and ASF1 functionally interact at the heat shock gene promoters. In the case of the SNF2/ASF1 combinatorial inactivation, the outcome is especially strong and synergistic, resulting in a severe reduction of histone H3 loss (Fig. 1b). This synergism suggests that SNF2 and ASF1 together are critical for nucleosome displacement at heat shock gene promoters. Mechanistically, such a requirement makes sense because both of these factors have very distinct functions and may complement each other. For instance, while the function of the SWI/SNF complex is ATP-driven and is known to be targeted by gene activators to specific promoters, ASF1 does not have phosphatase activity, and there are no known mechanisms of ASF1 targeting. The fact that the double-inactivation strain survives, although barely, and shows minimal but detectable nucleosome displacement for genes encoding major molecular chaperones—HSP82 and HSP70 (encoded by the SSA4 gene)—suggests some redundancy in the function of histone chaperones as well as ATP-dependent chromatin remodelers, which is understandable considering the absolute importance of promoter chromatin clearing for gene expression, at least in yeast.
The redundancy of function of the ATP-dependent chromatin remodeling complexes is obvious for the SWI/SNF complex based on the viability of the ΔSNF2 strain, which allows additional considerations. Because the individual inactivation of SNF2 has a relatively minor effect on chromatin remodeling at the heat shock gene promoters, and the deletion of ASF1 does not noticeably affect histone loss at the same promoters (Fig. 1b), it is possible to imagine that the interactions between the ASF1 and SWI/SNF complex are indirect and that the actual direct functional pair with ASF1 is another ATP-dependent chromatin remodeling complex that targets the same promoter nucleosomes. In this case, the double inactivation of ASF1 and SNF2 will affect both redundant pathways, leading to a synergistic effect on nucleosome displacement. Because the RSC complex and ISW1 do not exhibit any synergism with ASF1, other chromatin remodelers could be interacting with ASF1.
The ISW1 and ASF1 genetic interactions are not very pronounced and may reflect a more general and not localized and targeted mode of ISW1 function. Because the ISW1 complex is known to be important for proper nucleosome spacing (Gangaraju and Bartholomew 2007; Whitehouse and Tsukiyama 2006), this chromatin remodeling complex can constantly work to reorganize nucleosome positions before, during, and after gene activation. ASF1 in this situation may aid ISW1 in providing a nucleosome assembly/disassembly platform or work independently as occurred at the analyzed heat shock gene promoters. This interpretation of the ISW1 mechanism of action is consistent with our observation that although histone loss at all three analyzed promoters is diminished in the ASF1/ISW1 double-inactivation mutant, there is no obvious kinetic shift in reaching the maximum of histone loss.
FACT complex function is required for nucleosome displacement during acute stress and is related to nucleosome reassembly at heat shock gene promoters during sustained stress
The involvement of FACT and ASF1 was demonstrated during inactivation of the HO promoter (Takahata et al. 2009), and we wanted to determine whether heat shock gene promoters behave similarly. An additional rationale for testing the functional interactions of ASF1 and FACT was the idea that each histone chaperone has an affinity for different histones (Belotserkovskaya et al. 2003; English et al. 2005) and thus can potentially cooperate in nucleosome assembly/disassembly. While FACT, with its documented affinity to the H2A-H2B dimer, can initiate nucleosome disassembly, the ASF1 can complete it by destabilizing the H3-H4 interactions. We found a clear indication of the involvement of the FACT complex in the regulation of heat shock gene promoters reflected both in the phenotype and in the changes in the chromatin remodeling dynamics of stress gene promoters in the case of Spt16 depletion (Fig. 4b) as well as in the increased abundance of Spt16 at the analyzed promoters during sustained heat stress conditions (Fig. 5a). Although the double inactivation of Spt16 and ASF1 is lethal, the effect on nucleosome displacement at the heat shock gene promoters does not indicate any cooperation (Fig. 4b). The absence of cooperation is consistent with the previous demonstrations that the FACT complex in vivo, unlike in vitro, is not as specialized in terms of interactions with different histones, and the actual mechanism of FACT action lies in general nucleosome structure modification (Formosa 2008; Xin et al. 2009). The reason for the absence of cooperation between ASF1 and FACT, based on our results, might be the redundant involvement of FACT, ASF1, and SWI/SNF and other ATP-dependent chromatin remodelers and histone chaperones in nucleosome displacement at heat shock gene promoters during acute stress. Additionally, these factors appear to be involved in chromatin remodeling associated with different stages of the reaction to stress. Based on our results, while ASF1 cooperates with the SWI/SNF complex and, similarly to FACT, is important for nucleosome disassembly during the initial acute stress, FACT complex functionality at the analyzed promoters differs from that of ASF1, and the difference lies in the process of nucleosome reassembly during a prolonged attenuation phase of reaction to the heat stress. One reason for the difference could be that the FACT complex has a significantly higher Kon then Koff in comparison to the ASF1 while interacting with histones, thus releasing histones faster during the dissociation process. That would be consistent with the defect of the STP16-depleted strain in nucleosome reassembly at heat shock gene promoters during sustained stress conditions (Fig. 5b). The participation of Spt16 in nucleosome reassembly is consistent with the previous reports documenting defects in nucleosome assembly leading to the aberrant activation of the SRG1 (Takahata et al. 2009) and HSP104 genes (Jensen et al. 2008) in strains bearing SPT16 mutations.
Thermotolerance of cells with Spt16 depletion occurs due to compensation for nucleosome loss by a defect in nucleosome reassembly during the attenuation phase of sustained stress
The phenotypic effect of Spt16 depletion is highly unusual, and its explanation has important implications for understanding the regulatory mechanisms of stress genes. It was previously established that the expression of hundreds of yeast genes including the majority of heat shock genes is under the control of the transcriptional activator HSF (Hahn et al. 2004), which, in turn, is upregulated during stressful conditions at multiple levels including nuclear transport, monomer-to-trimer transition, multiple site phosphorylation, and feedback loop regulation by molecular chaperone interactions with the activation domains of HSF (Morimoto 1998; Voellmy 2004). Our data emphasize the critical importance of nucleosome occupancy of heat shock gene promoters for proper stress gene expression and the maintenance of protein homeostasis.
Using the known facts about the heat shock gene regulation in combination with our results allows us to make some connections between our phenotypic and biochemical data (Figs. 4 and 5) and formulate a molecular model explaining the thermotolerance induced by Spt16 depletion (Fig. 4a). We showed that depletion of Spt16 leads to the initial defect of nucleosome loss during the acute phase of heat stress (Fig. 4b). Given that, at least in yeast, the nucleosome occupancy of heat shock gene promoters and the expression level of these genes exhibit inverse ratio relationships (Erkine and Gross 2003), the slow growth phenotype at 30 °C is likely the result of the lower expression of stress genes, the products of which are necessary for the maintenance of protein homeostasis even at normal temperatures (Morimoto 1998; Voellmy 2004). With the increase in temperature and activation of HSF, the expression level of stress genes increases. The next phase in the regulation of expression is attenuation with reassembly of nucleosomes at heat shock gene promoters (Fig. 7), which in the case of Spt16 depletion cannot be completed due to the defect in nucleosome reassembly at heat shock gene promoters. That leaves promoters open and leads to increased binding of the activator (Fig. 5c) and Pol II (Fig. 5d). Because yeast cells, unlike cells of higher eukaryotes, do not have paused polymerase phenomenon at least for heat shock genes, the amount of Pol II is directly proportional to the expression level, which we demonstrated previously (Erkina and Erkine 2006; Erkine and Gross 2003). That allows us to suggest that higher Pol II level leads to a higher expression level of molecular chaperones, which in turn compensates for the original decreased expression level caused by a defect in nucleosome loss. The end result is that the growth rate of the mutant at 39 °C is comparable to that of the WT despite the severe growth retardation at 30 °C. A similar acquired tolerance for increased NaCl concentrations (Fig. 6) suggests that the Spt16 depletion effect is not limited to the function of HSF but rather has a more general effect related to the assembly of nucleosomes across a spectrum of gene promoters expressed under different stress conditions. This conclusion is in good agreement with the recently reported data on transcription-coupled defects in nucleosome assembly at the SER3 gene promoter in strains bearing mutant versions of the SPT16 allele (Hainer et al. 2012) and with the report of a defect in the reassembly of nucleosomes during transcriptional downregulation of the HSP104 gene associated with the temperature-sensitive spt16-11 allele (Jensen et al. 2008).
Fig. 7.
Molecular model showing the ASF1-SWI/SNF cooperation in nucleosome disassembly at yeast heat shock gene promoters during the acute stress and FACT complex participation in nucleosome reassembly during the attenuation phase. a Processes in the WT strain: ASF1 and SWI/SNF complex cooperate in the process of nucleosome removal leading to the increased molecular chaperone expression during the acute stress, while FACT participates in nucleosome reassembly leading to the shutdown of transcription. b Inactivation of FACT leads to incomplete nucleosome reassembly and partial expression of molecular chaperones during the attenuation phase, thus causing a synthetic thermotolerance
The defect in nucleosome assembly at heat shock gene promoters causing increased expression level of heat shock genes and leading to synthetic thermotolerance and resistance to other stresses including tolerance to high salt concentrations (Fig. 6) is an important phenomenon that can be connected to a mechanism of carcinogenesis (Dai et al. 2007). In this mechanism, the increased level of heat shock gene expression protects mutation-bearing malignant cells from being recognized by the organism’s defense system. Supporting this concept of carcinogenesis, specific inhibitors of HSF are being recognized and developed as potential anticancer drugs (Whitesell and Lindquist 2009). The mutations that inactivate Spt16, which is conserved among species, might lead to a consistently higher level of expression of heat shock genes, similar to what we observed in our study, leading to the promotion of carcinogenesis via the proposed mechanism (Dai et al. 2007).
Acknowledgments
We thank Pam Crowell for the critical reading of the manuscript and helpful suggestions and Tim Formosa for the anti-Spt16 antibody. This work was supported by the National Science Foundation [MCB-1029254] and the National Institutes of Health [R03 MH097538].
References
- Adkins MW, Tyler JK. Transcriptional activators are dispensable for transcription in the absence of Spt6-mediated chromatin reassembly of promoter regions. Mol Cell. 2006;21:405–416. doi: 10.1016/j.molcel.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Amoros M, Estruch F. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol Microbiol. 2001;39:1523–1532. doi: 10.1046/j.1365-2958.2001.02339.x. [DOI] [PubMed] [Google Scholar]
- Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D. FACT facilitates transcription-dependent nucleosome alteration. Science. 2003;301:1090–1093. doi: 10.1126/science.1085703. [DOI] [PubMed] [Google Scholar]
- Boeger H, Griesenbeck J, Strattan JS, Kornberg RD. Removal of promoter nucleosomes by disassembly rather than sliding in vivo. Mol Cell. 2004;14:667–673. doi: 10.1016/j.molcel.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Boy-Marcotte E, Lagniel G, Perrot M, Bussereau F, Boudsocq A, Jacquet M, Labarre J. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol Microbiol. 1999;33:274–283. doi: 10.1046/j.1365-2958.1999.01467.x. [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Tyler JK, Churchill ME. The histone shuffle: histone chaperones in an energetic dance. Trends Biochem Sci. 2010;35:476–489. doi: 10.1016/j.tibs.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duina AA. Histone chaperones Spt6 and FACT: similarities and differences in modes of action at transcribed. Genes Genet Res Int. 2011;2011 doi: 10.4061/2011/625210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser SJ, D'Arcy S. Towards a mechanism for histone chaperones. Biochim Biophys Acta. 2012;1819:211–221. doi: 10.1016/j.bbagrm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CM, Maluf NK, Tripet B, Churchill ME, Tyler JK. ASF1 binds to a heterodimer of histones H3 and H4: a two-step mechanism for the assembly of the H3-H4 heterotetramer on DNA. Biochemistry. 2005;44:13673–13682. doi: 10.1021/bi051333h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkina TY, Erkine AM. Displacement of histones at promoters of Saccharomyces cerevisiae heat shock genes is differentially associated with histone H3 acetylation. Mol Cell Biol. 2006;26:7587–7600. doi: 10.1128/MCB.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkina TY, Tschetter PA, Erkine AM. Different requirements of the SWI/SNF complex for robust nucleosome displacement at promoters of heat shock factor and Msn2- and Msn4-regulated heat shock genes. Mol Cell Biol. 2008;28:1207–1217. doi: 10.1128/MCB.01069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkina TY, Zou Y, Freeling S, Vorobyev VI, Erkine AM. Functional interplay between chromatin remodeling complexes RSC, SWI/SNF and ISWI in regulation of yeast heat shock genes. Nucleic Acids Res. 2010;38:1441–1449. doi: 10.1093/nar/gkp1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkine AM, Gross DS. Dynamic chromatin alterations triggered by natural and synthetic activation domains. J Biol Chem. 2003;278:7755–7764. doi: 10.1074/jbc.M211703200. [DOI] [PubMed] [Google Scholar]
- Erkine AM, Adams CC, Diken T, Gross DS. Heat shock factor gains access to the yeast HSC82 promoter independently of other sequence-specific factors and antagonizes nucleosomal repression of basal and induced transcription. Mol Cell Biol. 1996;16:7004–7017. doi: 10.1128/mcb.16.12.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkine AM, Magrogan SF, Sekinger EA, Gross DS. Cooperative binding of heat shock factor to the yeast HSP82 promoter in vivo and in vitro. Mol Cell Biol. 1999;19:1627–1639. doi: 10.1128/mcb.19.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol Rev. 2000;24:469–486. doi: 10.1111/j.1574-6976.2000.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Formosa T. FACT and the reorganized nucleosome. Mol Biosyst. 2008;4:1085–1093. doi: 10.1039/b812136b. [DOI] [PubMed] [Google Scholar]
- Gangaraju VK, Bartholomew B. Dependency of ISW1a chromatin remodeling on extranucleosomal DNA. Mol Cell Biol. 2007;27:3217–3225. doi: 10.1128/MCB.01731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkikopoulos T, Havas KM, Dewar H, Owen-Hughes T. SWI/SNF and Asf1p cooperate to displace histones during induction of the Saccharomyces cerevisiae HO promoter. Mol Cell Biol. 2009;29:4057–4066. doi: 10.1128/MCB.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer SJ, Charsar BA, Cohen SB, Martens JA. Identification of mutant versions of the Spt16 histone chaperone that are defective for transcription-coupled nucleosome occupancy in Saccharomyces cerevisiae. G3 (Bethesda) 2012;2:555–567. doi: 10.1534/g3.112.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhou H, Li Z, Xu RM, Zhang Z. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J Biol Chem. 2007;282:28587–28596. doi: 10.1074/jbc.M702496200. [DOI] [PubMed] [Google Scholar]
- Ivanovska I, Jacques PE, Rando OJ, Robert F, Winston F. Control of chromatin structure by spt6: different consequences in coding and regulatory regions. Mol Cell Biol. 2011;31:531–541. doi: 10.1128/MCB.01068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MM, Christensen MS, Bonven B, Jensen TH. Requirements for chromatin reassembly during transcriptional downregulation of a heat shock gene in Saccharomyces cerevisiae. FEBS J. 2008;275:2956–2964. doi: 10.1111/j.1742-4658.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/S0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- Korber P, Luckenbach T, Blaschke D, Horz W. Evidence for histone eviction in trans upon induction of the yeast PHO5 promoter. Mol Cell Biol. 2004;24:10965–10974. doi: 10.1128/MCB.24.24.10965-10974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Lin LJ, Schultz MC. Promoter regulation by distinct mechanisms of functional interplay between lysine acetylase Rtt109 and histone chaperone Asf1. Proc Natl Acad Sci U S A. 2011;108:19599–19604. doi: 10.1073/pnas.1111501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y, Maier-Davis B, Kornberg RD. Chromatin remodeling by nucleosome disassembly in vitro. Proc Natl Acad Sci U S A. 2006;103:3090–3093. doi: 10.1073/pnas.0511050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PB, Struhl K. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol. 2003;23:8323–8333. doi: 10.1128/MCB.23.22.8323-8333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Schermer UJ, Korber P, Horz W. Histones are incorporated in trans during reassembly of the yeast PHO5 promoter. Mol Cell. 2005;19:279–285. doi: 10.1016/j.molcel.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Schwabish MA, Struhl K. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol Cell. 2006;22:415–422. doi: 10.1016/j.molcel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Takahata S, Yu Y, Stillman DJ. FACT and Asf1 regulate nucleosome dynamics and coactivator binding at the HO promoter. Mol Cell. 2009;34:405–415. doi: 10.1016/j.molcel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat Struct Mol Biol. 2006;13:633–640. doi: 10.1038/nsmb1111. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin Ther Targets. 2009;13:469–478. doi: 10.1517/14728220902832697. [DOI] [PubMed] [Google Scholar]
- Xin H, Takahata S, Blanksma M, McCullough L, Stillman DJ, Formosa T. yFACT induces global accessibility of nucleosomal DNA without H2A-H2B displacement. Mol Cell. 2009;35:365–376. doi: 10.1016/j.molcel.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Herrera-Diaz J, Gross DS. Domain-wide displacement of histones by activated heat shock factor occurs independently of Swi/Snf and is not correlated with RNA polymerase II density. Mol Cell Biol. 2005;25:8985–8999. doi: 10.1128/MCB.25.20.8985-8999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]