Abstract
Chaperone Hsp70 can cross the plasma membrane of living cells using mechanisms that so far have not received much research attention. Searching the part of the molecule that is responsible for transport ability of Hsp70, we found a cationic sequence composed of 20 amino acid residues on its surface, KST peptide, which was used in further experiments. We showed that KST peptide enters living cells of various origins with the same efficiency as the full-length chaperone. KST peptide is capable of carrying cargo with a molecular weight 30 times greater than its own into cells. When we compared the membrane-crossing activity of KST peptide in complex with Avidin (KST–Av complex) with that of similarly linked canonical TAT peptide, we found that TAT peptide penetrated SK-N-SH human neuroblastoma cells at a similar rate and efficiency as the KST peptide. Furthermore, KST peptide can carry protein complexes consisting of a specific antibody coupled to the peptide through the Avidin bridge. An antibody to Hsp70 delivered to SK-N-SH cells with high expression level of Hsp70 reduced the protective power of the chaperone and sensitized the cells to the pro-apoptotic effect of staurosporine. We studied the mechanisms of penetration of KST–Av and full-length Hsp70 inside human neuroblastoma SK-N-SH and human erythroleukemia K-562 cells and found that both used an active intracellular transport mechanism that included vesicular structures and negatively charged lipid membrane domains. Competition analysis of intracellular transport showed that the chaperone reduced intracellular penetration of KST peptide and conversely KST peptide prevented Hsp70 transport in a dose-dependent manner.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-014-0554-z) contains supplementary material, which is available to authorized users.
Keywords: Hsp70, Cell-penetrating peptides, Intracellular transport, Membrane, Vesicles
Introduction
In 1986, Tytell described the transport of Hsp70 from squid glia cells to the giant neuron (Tytell et al. 1986), and a few years later, two groups demonstrated that Hsp70 was released from rat embryo cells (Hightower and Guidon 1989) and human glioblastoma T-98G cells (Guzhova et al. 2001). Importantly, in all three cases, living cells were the source of Hsp70.
A great variety of stimuli can cause Hsp70 release from living cells: oxidative stress, heat stress (Evdonin et al. 2004), exercise (Walsh et al. 2001), and treatment with IFN-γ and IL10 (Barreto et al. 2003; Gastpar et al. 2005); some of these stimuli are well-known inducers of heat shock response (Guzhova et al. 2013). The exact mechanisms by which cells release Hsp70 remain unclear although some reports indicate that extracellular transport of the chaperone can occur through canonical and non-canonical protein transport mechanisms (Mambula et al. 2007). Gastpar and colleagues proved that exosomes are involved in extracellular transport of Hsp70, and they showed that the transport particles possessed all the features of that type of vesicle: floating capacity, acetylcholinesterase activity, and specific protein composition (Gastpar et al. 2005). In another study, Hsp70 was shown to be an obligatory component of exosomes, and Hsp70 release from mouse tumor cells was inhibited by factors suppressing exosomal transport (Chalmin et al. 2010). Other Hsp70 translocation pathways including secretory lysosomes and endosomes were also found to be important in the specific context of cancer cell physiology (see Guzhova et al. 2013 for review).
Regardless of whether it is released from living or dying cells or introduced into a cell culture, Hsp70 exerts a variety of effects, mainly based on its ability to cross the plasma membrane. The most important property of exogenous Hsp70 is its cell-penetrating activity, which was demonstrated in studies using normal and transformed mammalian cells of different origins. This activity may be due to the ability of the chaperone to bind acidic plasma membrane lipids through its ATP- and lipid-binding domains (Harada et al. 2007). Interestingly, only acid lipids formed complexes with Hsp70; neutral glyco- or phospholipids did not. Pure Hsp70 was also found to interact with artificial membranes and to form pores, suggesting a possible mechanism for transport of the protein into or from a given cell (Arispe et al. 2002). Lastly, several receptors for Hsp70 were discovered, Lox-1, CD40, and SREC1, pointing to the possibility that more specific interactions mediate the penetration of Hsp70 into a cell (Calderwood et al. 2007).
Intracellular transport of Hsp70 is of considerable practical interest because of the need for stable vehicles capable of delivering biological cargos to the inside of living cells. A number of molecular transporters developed to date constitute a group of relatively short peptides, cell-penetrating peptides (CPPs), consisting of 5–40 amino acid residues which reach their intracellular locales through various mechanisms; the most valuable property of CPPs is their ability to deliver into a target cell covalently or non-covalently associated molecular cargo (Kersemans et al. 2008). CPPs may be useful in gene therapy, treatment for neurological diseases, or cancer treatment, and so there have been substantial efforts to discover the peptides with suitable carrier properties (Bitler and Schroeder 2010). To date, the most promising results in transportation of biological materials to the interior of living cells were obtained using TAT peptide, the fragment of TAT protein constituting the HIV-1 structure (Viscidi et al. 1989). This peptide has been shown to deliver a large variety of cargo, from small particles to proteins, peptides, and nucleic acids, with great efficiency, probably using endocytotic intracellular transport mechanisms (Ignatovich et al. 2003; Brooks et al. 2005). We hypothesized that Hsp70’s ability to penetrate living cells could be used in the design of a new transport peptide which would provide efficient cargo transfer.
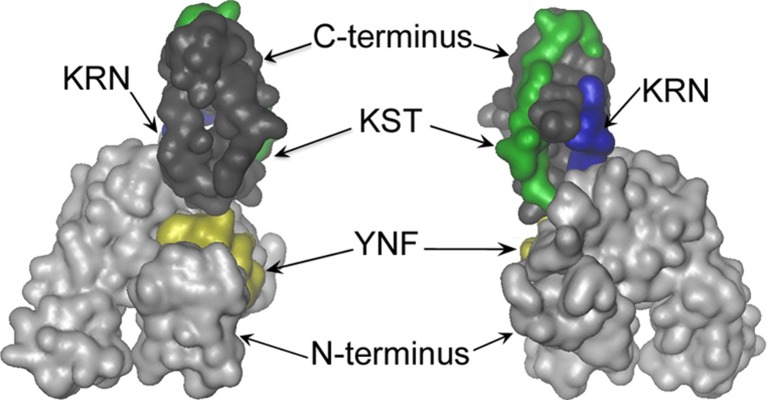
Most CPPs are enriched by positively charged Arg and/or Lys residues and are internalized after initial interactions with negatively charged cell surface glycosaminoglycans which cluster CPPs on outer membrane surfaces (Console et al. 2003; Deshayes et al. 2006). We analyzed three-dimensional images of the human Hsp70 molecule and found several positively charged regions exposed to the solvent. On the base of such analysis, three peptides were chosen: two at the C-terminal domain of the molecule, 493–512 aa, KSTGKANKITITNDKGRLSK (KST peptide), containing six positively charged amino acid residues (five lysines and one arginine) and 415–423 aa, KRNSTIPTK (KRN peptide), with three positively charged residues (two lysines and one arginine) and one at the N-terminal domain, peptide 148–165 aa, YFNDSQRQATKAGVIA (YFN peptide), which had two positively charged amino acid residues (Fig. 1).
Fig. 1.
Localization of KST, KRN, YFN peptides on the Hsp70 molecule (SWISSMODEL based on template: 1yuwA) (Kiefer et al. 2009; Kopp and Schwede 2004). KST peptide, 493–512 aa, KSTGKANKITITNDKGRLSK (green); KRN peptide, 415–423 aa, KRNSTIPTK (blue); and YFN peptide, 148–165 aa, YFNDSQRQATKAGVIA (yellow). C-terminal part is presented in dark gray, N-terminus in light gray. This figure was prepared with VMD 1.9.1 (Humphrey et al. 1996)
The aim of this study was to assay the penetrative activity of the above peptides and their capacity as cargo transporters. Only one of the three peptides, KST, was found to possess penetrative activity similar to that of the whole Hsp70 molecule; KST and Hsp70 were shown to compete for entry to a cell.
Materials and methods
Cells
Human erythroblastoma K-562, human epidermoid carcinoma A-431, human glioblastoma T98G, and mouse fibroblasts 3T3 and 3T3-SV-40 were obtained from the Russian Collection of Cell Cultures (St. Petersburg, Russia); human neuroblastoma SK-N-SH and SH-SY-5Y were from Dr. D. Rubinzstein (Cambridge, UK); human immortalized epidermal HaCaT cells were kindly provided by Dr. N. Fusenig (DKFZ, Germany); and human neuronal stem/progenitor cells (SPC) were obtained from Dr. L. Korochkin (Institute of Gene Biology, Russian Academy of Sciences) (Aleksandrova et al. 2004). A-431, T98G, 3T3, 3T3-SV40, HaCaT, and SK-N-SH cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) and K-562 cells in RMPI-1640, and all media were supplemented with 10 % fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin, except for the SPC which were cultured in DMEM with 20 % fetal bovine serum. All media and sera were purchased from PanEco, Russia. Cell viability was estimated using the MTT method (Mosmann 1983).
To explore the effects of the anti-Hsp70 antibody delivered with the aid of KST peptide, we used SK-N-SH cells transfected with pZ-hsp70 + pZem plasmid (plasmids were a kind gift from Prof. M. Jäättelä, Danish Cancer Society), in which expression of Hsp70 was induced by treatment with 25 or 75 μM ZnSO4 overnight. SK-N-SH–Hsp70 cells were cultured in the presence of 100 μg/ml G418.
Proteins, peptides, and antibody
Recombinant human Hsp70 was purified from bacteria transformed with a pMSHsp70 plasmid, as described elsewhere (Guzhova et al. 2011). Hsp70 solution was further detoxified by incubation with Polymyxin B-Agarose gel (Sigma-Aldrich, USA) and sterilized by filtration through a 0.2-μm filter (Millipore, USA). The E-Toxate assay (Sigma-Aldrich, USA) showed that the level of lipopolysaccharide in the final Hsp70 preparation was lower than 2.0 U/ml. For microscopy and for flow cytometry experiments, Hsp70 was conjugated to Alexa488 dye and to Alexa647 f (Invitrogen, USA) according to the manufacturer’s protocol.
Biotinylated KST, KRN, and YFN peptides were synthesized in Diafarm LLC, St. Petersburg, Russia. The biotinylated TAT peptide was a kind gift from Dr. Sergey Burov (Diafarm, Russia).
Anti-Hsp70 antibody was isolated from the culture medium of 3B5 hybridoma cells (Guzhova et al. 1997) using affinity chromatography on Hsp70 attached to N-Succinilimide-Sepharose (Sigma, USA). After precipitation with 35 % ammonium sulfate and dialysis against phosphate-buffered saline (PBS), pure immunoglobulins were biotinylated with EZ-Link Sulfo-NHS-Biotin (Pierce, USA) according to the manufacturer’s instructions.
Confocal microscopy
SK-N-SH and K-562 cells were allowed to settle on poly-L-lysine-coated glass slides. Cells were incubated with 50 μg/ml Alexa488-labeled Hsp70 or with 5 μM biotinylated peptides for 18 h at 37 °C. After incubation with the protein or peptides, the cells were washed with ice-cold PBS, fixed with 4 % paraformaldehyde, and incubated with Avidin–FITC (Sigma, USA). Plasma membranes of cells were visualized by staining with a CellVue® Claret Far Red Fluorescent Cell Linker Mini Kit (Sigma, USA) according to the manufacturer’s instructions. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, USA).
In the other experimental series, we incubated biotinylated KST peptide (5 μM) with Avidin–FITC (Sigma, USA) in a molecular ratio of 1:1 for 30 min and then added the KST–Av–FITC complex to the cells settled on poly-l-lysine-coated glass slides for the time indicated. Cells were washed with cold PBS, fixed with 4 % paraformaldehyde, and stained with CellVue® Claret and DAPI as described above.
To establish the transporter function of KST peptide, it was first incubated with Avidin–FITC (Av–FITC) and biotinylated 3B5 monoclonal antibody in molar ratio 1:2:1.5. After dialysis against serum-free DMEM, the complex was added to SK-N-SH–Hsp70 cells treated with 75 μM ZnSO4 overnight, and cells were incubated with the complex for 18 h. Cells were washed with cold PBS, fixed with 4 % paraformaldehyde, and permeabilized with 0.1 % Triton X-100 followed by staining with secondary anti-mouse antibody conjugated with Cy3 (Jackson ImmunoResearch, USA).
To estimate the contribution of the endocytosis pathway to the intracellular transport of Hsp70 or KST peptide, we transfected SK-N-SH neuroblastoma cells with plasmids encoding rab5-RPF, rab7-RPF, or mito-PAGFP fusion genes (Addgene, USA) and then incubated them with 1 μM Hsp70 labeled with Alexa488 or 1 μM KST–Av–FITC complex. In other experiments, SK-N-SH cells were incubated with 1 μM of Hsp70 labeled with Alexa488 or 1 μM KST–Av–FITC complex and then stained with CytoPainter Lysosomal Staining Kit (Abcam, UK) according to the manufacturer’s protocol.
To reveal the possible contribution of cell surface Hsp70 to endocytosis of full-sized Hsp70 and KST peptide, we transfected K-562 and SK-N-SH cells with pFusionRed-f-mem plasmid (Evrogen, Russia). Cells were heat shocked at 43 °C for 30 min and then were cultured overnight. Next morning, cells were washed with ice-cold PBS incubated with monoclonal cmHsp70.1-FITC antibody on ice (kindly provided by Prof. Gabriele Multhoff, Technical University, Munich, Germany), fixed with 4 % ice-cold paraformaldehyde, and nuclei were stained with DAPI.
Fluorescence images were captured with the use of a Leica TCS SP2 confocal microscope (Leica, Germany). To avoid possible cross interference among the various fluorochromes, images for DAPI, FITC, Alexa488, or deep red were acquired using the sequential image recording method.
Western blotting
K-562 and SK-N-SH cells were heat shocked at 43 °C for 30 min and after overnight incubation were collected and lysed, and the lysates were analyzed with the aid of Western blotting using 3B5 anti-Hsp70 monoclonal antibody; 6C5 anti-GAPDH antibody (Abcam, UK) was employed to represent loading control.
Flow cytometry
Flow cytometry was used to analyze the time- and dose-dependent penetration of the KST–Av–FITC complex. SK-N-SH or K-562 cells were incubated with 5 μM KST–Av–FITC for 30 min, 1 h, 3 h, or 6 h, washed in PBS, and analyzed with the aid of a Coulter Epics XL (Beckman Coulter, USA) flow cytometer using laser with λ = 488 nm. YFN peptide–Av–FITC and Av–FITC were incubated with cells for the same periods of time. In similar experiments, SK-N-SH cells were incubated with 0.2, 1, 5, 10, or 20 μM KST–Av–FITC for 6 h, washed, and analyzed using flow cytometry as described above.
To carry out inhibitor analysis of Hsp70 and KST peptide penetration, K-562 cells and SK-N-SH cells were incubated with 50 μg/ml Alexa-488-labeled Hsp70 or 5 μM KST–Av–FITC in the presence of 100 μM dynasore, an inhibitor of dynamin-dependent endocytosis; 10 μM chlorpromazine, known to suppress clathrin-dependent endocytosis; 50 μM amiloride, which inhibits pinocytosis; 10 μM filipin, known to suppress caveolin-dependent endocytosis; 5 μM cytochalasine D, which disrupts actin-dependent transport; 2 μM noсodazole, which inhibits tubulin-dependent endocytosis; and 5 μM methyl-beta cyclodextrin, known to destroy lipid rafts. After incubation and two washes with PBS, viable cells were gated and analyzed with the aid of the Coulter Epics XL cytometer using a laser with λ = 488 nm.
Competitive entry of KST–Av–FITC and Hsp70-Alexa647 was achieved as follows: SK-N-SH cells were incubated with 0.2 or 1 μM Hsp70-Alexa647 or 0.2, 1, or 5 μM KST–Av–FITC separately or in combination for 12 h. After three washings with PBS, cells were analyzed by flow cytometry using a CyFlow Space apparatus (Partec GmbH, Germany) equipped with two lasers (λs = 488 and 635 nm).
Statistics
Observations are generally reported as mean ± SE. Student’s t tests were used to evaluate differences between the control and treatment groups; differences were considered to be statistically significant when p < 0.05 (*) or p < 0.01 (**).
Results
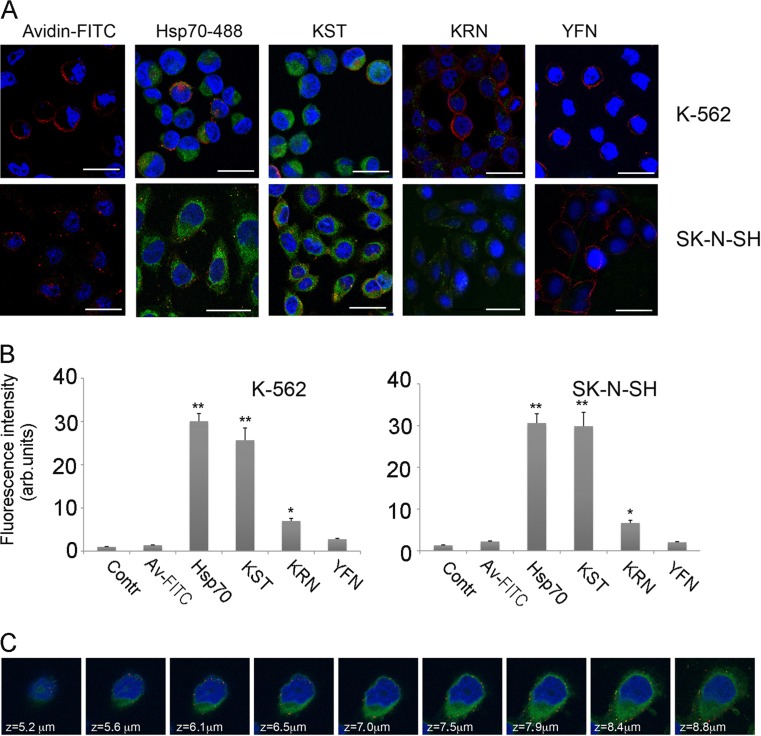
On the basis of the results of sequence alignment of known CPPs and a comparison of their structure with the structure of human Hsp70, we identified three domains in Hsp70 that we chose to test for intracellular transport of a whole protein (Fig. 1). The appropriate peptides were synthesized with a biotin tag on their C-termini. First, the cell penetrative activity of the peptides was analyzed using growing in a suspension K-562 human erythroleukemia cells and adhesive human neuroblastoma SK-N-SH cells. The cell surface was marked with CellVue® Claret Far Red reagent, and we found that this reagent stains big particles on cell membranes as well as the whole membranes (Fig. 2a). Of the three candidate CPPs, only KST peptide demonstrated profound penetration into the cells of both lines with the same efficiency as the full-length chaperone tagged with Alexa488 (Fig. 2a, b). The penetrating capacity of KRN peptide was much lower, and YFN peptide did not penetrate into the cells of either line at all. In further experiments, we focused on the properties of KST peptide using YFN peptide as the inactive control. To prove that KST peptide can penetrate and reside within the cytosol, we used the sequential image recording method with a 0.5-μm step from top to bottom and found the peptide evenly distributed throughout the cytoplasm (Fig. 2c).
Fig. 2.
KST peptide is able to penetrate K562 and SK-N-SH cells with the same efficacy as full-size Hsp70. a K562 cells (upper line) and SK-N-SH cells (lower line) were placed on cover slips and incubated with biotinylated KST, KRN, or YFN peptides, or with Hsp70 labeled with Alexa488 for 18 h at 37 °C, then fixed, permeabilized, and stained with Av–FITC. Nuclei were stained with DAPI, cell surfaces with CellVue® Claret Far Red reagent. Scale bar 5 μm. b K562 and SK-N-SH cells were incubated with studied peptides as described above and than were applied for flow cytometry. Data of five independent experiments are summarized. Differences between control and treatment groups were considered to be statistically significant when p < 0.05 (*) or p < 0.01 (**) according to Student’s t test. c We used sequential image recording with a step of 0.5 μm from top to bottom to demonstrate that KST peptide can penetrate SK-N-SH cells and reside within the cytosol
Notably, KST peptide demonstrated no or minimal toxicity. After 72 h of incubation with KST peptide, we observed 6–7 % dead cells in populations of K-562 and SK-N-SH cells (data not shown).
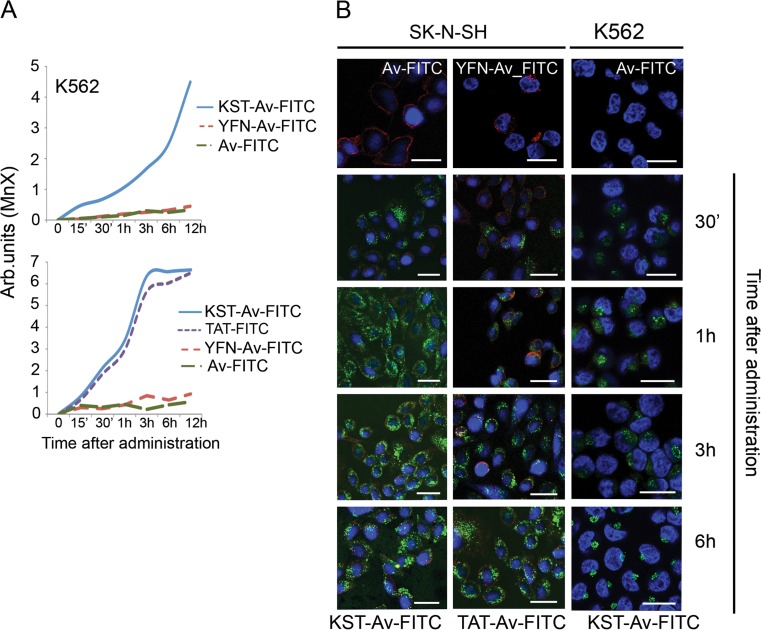
One of the most important properties of CPPs is their ability to deliver certain cargo molecules, including drugs, DNA, RNA, and proteins. To check whether Hsp70-based peptides were able to carry high molecular weight cargoes into living cells, we used a biotin tag to construct a complex with Avidin, a protein with a molecular mass more than 30-fold that of the Hsp70 peptides. The complex containing Av–FITC and the peptides to be investigated, KST–biotin, YFN–biotin, or TAT–biotin, was formed at room temperature, and then, the solution was dialyzed to remove excessive peptide. We used two types of cells, adhesive SK-N-SH human neuroblastoma and K562 human erythroleukemia cells. Various concentrations of KST–biotin–Av–FITC complex were used in experiments with SK-N-SH cells; we found that even at the level of 0.2 μM a reasonable fluorescent signal was detectable with the aid of confocal microscopy and flow cytometry (Fig. S1). Fluorescence increased with increasing concentrations of KST–Av–FITC complex, and in subsequent experiments, we used 5 μM; this value is employed in the studies on the well-established CPP, TAT peptide.
First, we estimated the parameters of peptide–Av–FITC complex intracellular transport using flow cytometry. We found that the complex containing KST peptide penetrated almost 100 % of the K-562 cell population while that with YFN peptide did not (Fig. 3a). The fluorescence of KST–Av–FITC complex usually became noticeable 15 min after introduction of the complex into the culture. Loading of K-562 cells with the complex lasted approximately 6–8 h; penetration into SK-N-SH cells was faster and the intracellular concentration peaked at 3 h, followed by a plateau. We observed similar complex accumulation kinetics in a variety of mammalian cells including HaCaT, A-431, SPC, T98G, 3T3, and 3T3-SV40, proving that the cargo-delivering efficiency of KST peptide is independent of cell type (Fig. S2). Importantly, the capacity for delivery of KST–Av–FITC to SK-N-SH cells was similar to or even higher than that of the well-established vector TAT peptide, suggesting that this newly developed CPP is highly potent.
Fig. 3.
KST peptide is able to carry cargo exceeding its own molecular weight inside living cells. a K-562 cells (upper panel) and SK-N-SH cells (lower panel) were incubated with KST–Av–FITC, Av–FITC, or YFN–Av–FITC (the latter two were negative controls) for the time indicated. SK-N-SH cells were also incubated with TAT–Av–FITC. Cells were subsequently investigated using flow cytometry. b The same cells placed on cover glasses were incubated with KST–Av–FITC and TAT–Av–FITC during the time indicated, and Av–FITC and YFN–Av–FITC during 6 h, then were fixed and stained with DAPI for nuclei visualization. Scale bar 5 μm
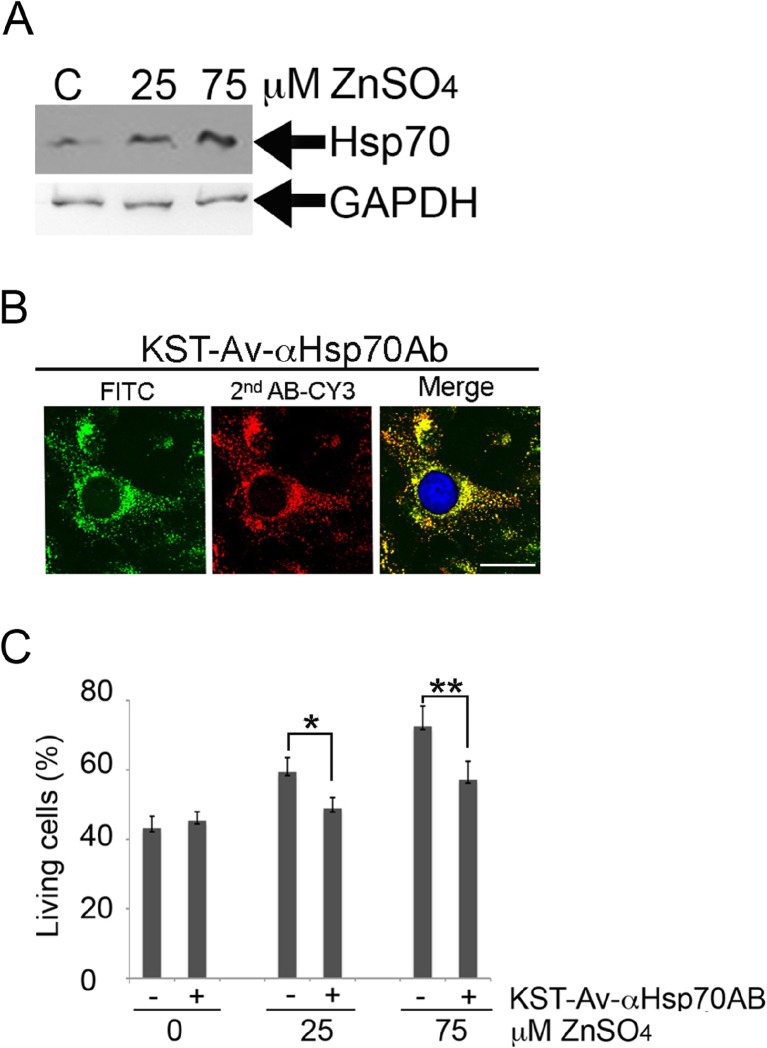
Next, we investigated whether KST peptide could deliver biomolecules without damaging their function. In this part of the study, we targeted the Hsp70 chaperone known to constitute one of the most powerful cell protection systems; this protection is directed against a variety of cytotoxic factors, including those that induce apoptosis (Gabai et al. 1995; Lasunskaia et al. 1997; Jäättelä 1999). The experimental design was as follows. We used human neuroblastoma SK-N-SH cells transfected with a plasmid bearing the hsp70 gene under the control of the metallothionein promoter (SK-N-SH–Hsp70). Western blotting using 3B5 anti-Hsp70 antibody revealed that elevation of Zn2+ concentration in the culture medium caused dose-dependent increases in Hsp70 level (Fig. 4a). As expected, cells pretreated with higher concentrations of Zn2+ were more resistant to the apoptogenic effect of staurosporine (Fig. 4c). We found that the level of apoptosis in a population of SK-N-SH–Hsp70 cells treated with 75 μM ZnSO4 was 20 % lower than in cells with the background level of Hsp70 (0 μM ZnSO4).
Fig. 4.
KST peptide can carry into cells a multimolar complex containing antiHsp70 antibody and thus block the protective effect of Hsp70 in SK-N-SH cells containing different amounts of the chaperone. a SK-N-SH cells were transfected with plasmid containing the hsp70 gene under control of metallothionein promoter (SK-N-SH–Hsp70 cells) and incubated with 25 or 75 μM ZnSO4 to induce Hsp70 synthesis. Cells were investigated with Western blotting. An antibody to GAPDH was used as a loading control. b SK-N-SH–Hsp70 cells were inducted with 75 μM ZnSO4 and incubated with the macromolecular complex KST–Av–αHsp70Ab overnight, then fixed, permeabilized, and stained with secondary anti-mouse antibody conjugated with Cy3 (red). Avidin was labeled with FITC (green). Nuclei were stained with DAPI (blue). Scale bar 1 μm. c SK-N-SH–Hsp70 cells were inducted with 25 or 75 μM of ZnSO4 for 18 h and incubated with KST–Av–αHsp70 complex overnight and then treated with 1 μM staurosporine to induce apoptosis. After 24 h, the amount of living cells was determined with an MTT assay
To determine the capacity of KST peptide to haul functionally active molecules, in this case anti-Hsp70 antibody, we purified antibody from a culture medium of 3B5 hybridoma cells, biotinylated it, and linked the antibody to KST peptide via the Avidin bridge. It has been previously shown that 3B5 antibody possesses titrating activity using an immunoprecipitation assay (Guzhova et al. 1997), making it possible to bind Hsp70 in a living cell. We prepared a complex containing KST peptide–Av–anti-Hsp70Ab-biotinylated (KST–Av-αHsp70Ab) in the molar ratio 1:2:1.5, which was established in preliminary experiments. SK-N-SH–Hsp70 cells were treated with 25 or 75 μM ZnSO4 to accumulate a high Hsp70 content and then incubated with KST–Av–αHsp70Ab complex overnight. After careful washing with PBS, cells were fixed, permeabilized, and stained with secondary anti-mouse antibody conjugated with Cy3 (red). Avidin used in this experiment was labeled with FITC (green) (Fig. 4b). Confocal microscopy data showed that Cy3-labeled antibody co-localized with Av–FITC inside cells and that the distribution of Av–FITC was different from that of the simple KST–Av–FITC complex (Fig. 4b).
At the third stage, we assessed the survival of SK-N-SH–Hsp70 cells following exposure to 25 or 75 μM ZnSO4, then to KST–Av–αHsp70Ab complex, and lastly incubation with 1 μM staurosporine. Incubation with the KST–Av–αHsp70AB reduced the cell survival rate by 11.3 and 15.5 % in cells treated with 25 and 75 μM ZnSO4, respectively (Fig. 4c). We conclude that anti-Hsp70 antibody was able to partially neutralize the protective power of the chaperone and thus reduce the cell survival rate.
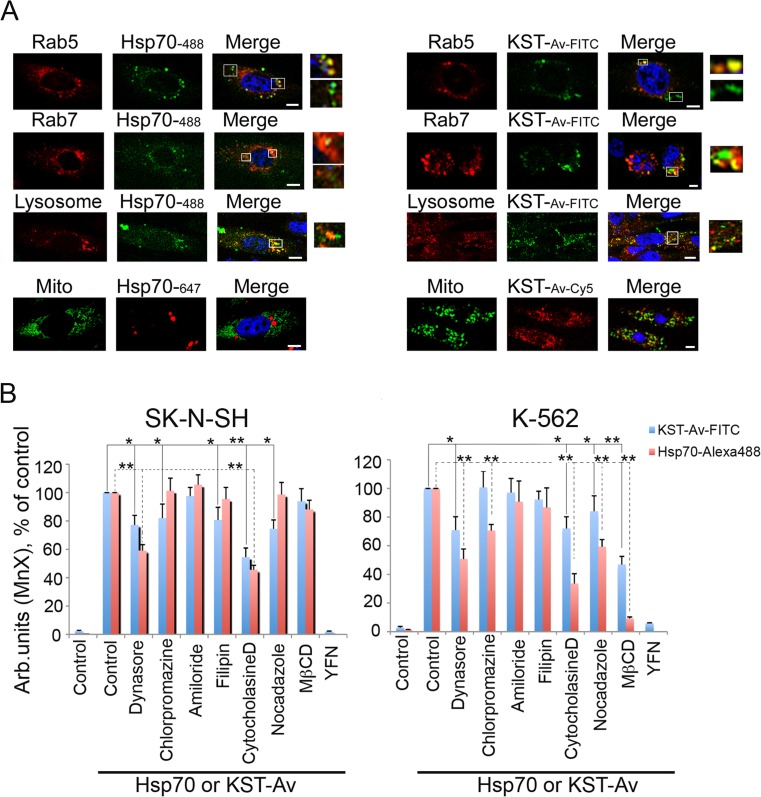
We then addressed the issue of whether KST peptide includes the domain necessary for the intracellular transport of Hsp70 itself by investigating the mechanisms employed by the KST–Av–FITC complex and full-length Hsp70 to enter a cell body. Because vesicular transport seems to be the most common intracellular transport mechanism, we used specific endosome markers. SK-N-SH cells were transfected with plasmids bearing either rab5-RFP (early endosome marker) or rab7-RFP (late endosome marker); alternatively, cells were stained with the Lysosomal Staining Kit, revealing the next step of the endocytosis pathway. When endosomes or lysosomes became visible, we introduced KST–Av–FITC or Hsp70-Alexa488 into the culture medium. Additionally, we used SK-N-SH cells transfected with mito-PAGFP plasmid (GFP-based mitochondrial marker) which were also incubated with both polypeptides. Confocal microscopy data showed that one part of the pools of full-length Hsp70 and the KST–Av–FITC complex was associated with vesicular structures and the other was not (Fig. 5a, see inserts), suggesting that endocytosis may be only one of the mechanisms used in the transport of these molecules into SK-N-SH cells. Mitochondria labeling used as a negative control has shown no colocalization of polypeptides with mitochondria (Fig. 5a).
Fig. 5.
KST–Av complex penetrates inside living cells using different mechanisms. a SK-N-SH cells were transfected with plasmids bearing either rab5-RFP (early endosome marker) or rab7-RFP (late endosome marker) or stained with Lysosomal Staining Kit, revealing next step of endocytosis; when endosomes or lysosomes became visible, KST–Av–FITC or Hsp70-Alexa488 were introduced into the culture medium. Additionally, SK-N-SH cells transfected with mito-PAGFP plasmid (GFP-based mitochondrial marker) incubated with both polypeptides were studied with the aid of confocal microscopy. Scale bar 1 mm. b SK-N-SH cells (left) and K562 cell (right) were incubated with KST–Av–FITC or Hsp70-Alexa488 in the presence of intracellular transport inhibitors and investigated with the help of flow cytometry. Data from three independent experiments are summarized. Differences between control and treatment groups were considered to be statistically significant when p < 0.05 (*) or p < 0.01 (**) according to Student’s t test
To explore the mechanisms of penetration of both structures in more detail, we performed inhibition analysis. Dynasore was used to inhibit dynamin-dependent endocytosis, and chlorpromazine was used to block clathrin-dependent endocytosis. Filipin suppressed the appearance of Hsp70 or KST–Av–FITC using caveolin-dependent endocytosis, and amiloride suppressed pinocytosis. Cytocholasine D was used as a blocker of actin-dependent endocytosis, and nocodazole was used to inhibit the assembly of microtubules that then serve as cage “rails” for endosomes. Finally, methyl β-cyclodextrin (MBCD) was used to destroy lipid rafts, another mechanism by which proteins may penetrate living cells. Hsp70 and KST–Av use active transport mechanisms because incubation at 4 °C completely blocked their entry into the cell (data not shown). Amiloride, an inhibitor of passive transport, had no effect on intracellular transport of either polypeptide (Fig. 5b). It is important to note that Hsp70 and the KST–Av complex use different mechanisms for entering adhesive cells and those growing in suspension. In SK-N-SH cells, both polypeptides use mainly receptor-mediated endocytosis. The quantity of cells incorporating KST–Av–FITC or Hsp70 was reduced by 23.2 and 41.1 %, respectively, as the result of treatment with dynasore. To enter K-562 cells, KST peptide and Hsp70 employed lipid rafts; their destruction with MBCD reduced transport of both polypeptides by 95 and 41 %, respectively. Lastly, caveolin-dependent endocytosis was not involved in the transport of protein/peptide structures into cells of either line (Fig. 5b).
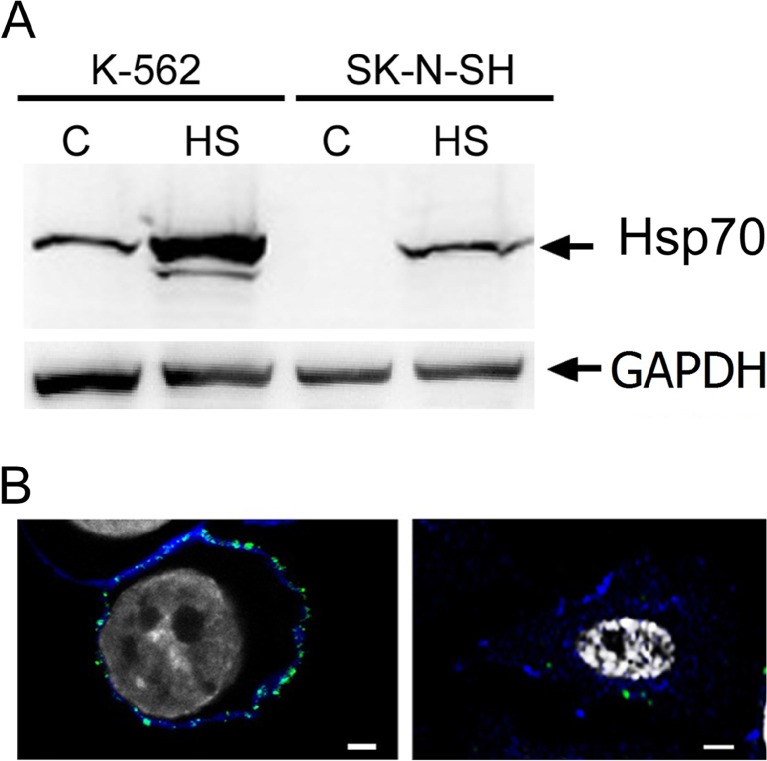
Since a high level of Hsp70 in tumor cells was found to increase the endocytosis of some molecules like transferrin into tumor cells (Vega et al. 2010) and the latter usually expose Hsp70 on their surface (Multhoff et al. 1995), we tested the cytoplasmic and plasma membrane-bound levels of Hsp70 in control and heat-shocked K-562 and SK-N-SH cells. Western blot analysis showed that in K-562 cells in which both Hsp70 and KST–Av–FITC employ mainly the lipid raft-dependent pathway, the Hsp70 level was much higher than in SK-N-SH cells in which both polypeptides use mostly clathrin-dependent endocytosis (Fig. 6a). To reveal the surface Hsp70 in cells of both lines, we first transfected them with pFusionRed-f-mem plasmid and, 2 days later, when the plasma membrane became visible, cells were exposed to heat shock and stained with cmHsp70.1 monoclonal antibody. We observed a high level of Hsp70 on the surface of K-562 cells while SK-N-SH cells demonstrated just single spots on the plasma membrane (Fig. 6b).
Fig. 6.
Levels of total and membrane-bound Hsp70 in K-562 and SK-N-SH cells. a K-562 and SK-N-SH cells were heat shocked at 43 °C for 30 min and recovered overnight, and Hsp70 levels were compared using Western blotting. Control and heat-stressed cells were collected and lysed, and expression of Hsp70 and GAPDH (loading control) was measured with the aid of Western blotting. b K-562 and SK-N-SH cells were transfected with pFusionRed-f-mem plasmid to visualize plasma membrane (blue). Two days later, cells were heat shocked at 43 °C for 30 min and after overnight incubation were washed with ice-cold PBS and incubated with monoclonal cmHsp70.1-FITC (green). Nuclei were stained with DAPI (white). Scale bar 1 μm
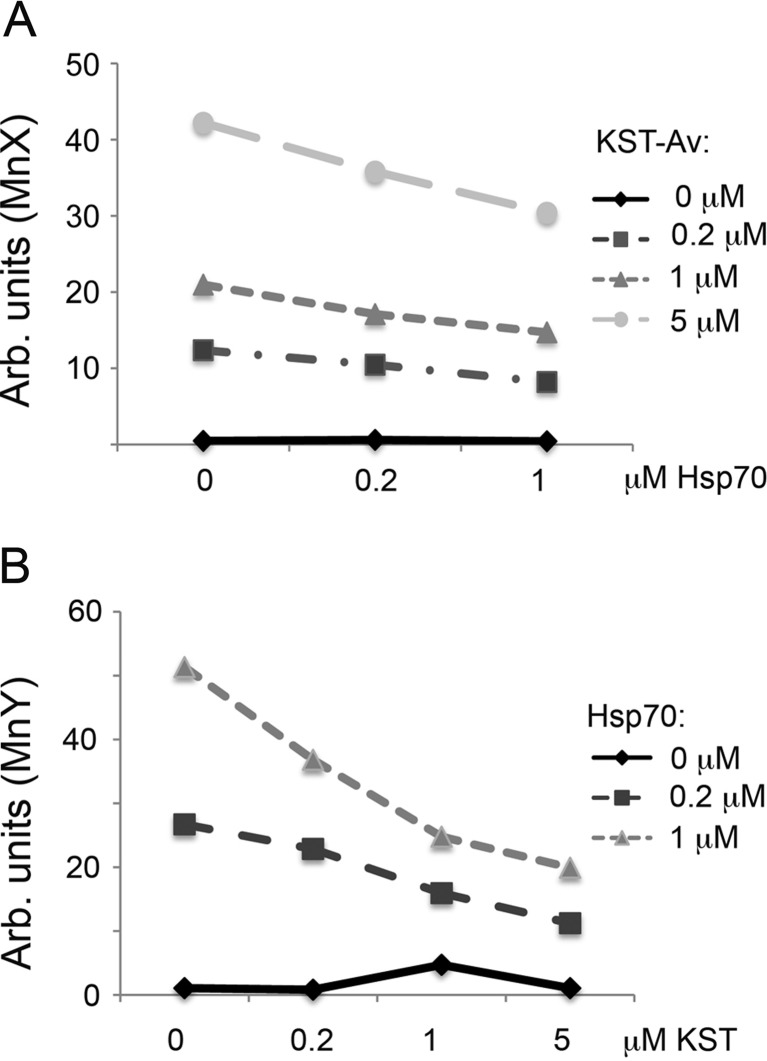
Competitive analysis of Hsp70 and KST–Av–FITC penetration was conducted using SK-N-SH cells. Cells were incubated either alone with Hsp70 or KST–Av at the concentrations indicated in Fig. 7, or in combination with molar ratios Hsp70:KST–Av of 0.2:0.2, 0.2:1, 0.2:5, 1:0.2, 1:1, and 1:5. The Hsp70 used in these experiments was labeled with Alexa647 (deep red). Cells were incubated with the separate polypeptides or their mixtures for 12 h, after which they were washed and subjected to flow cytometry analysis using two lasers, λ = 488 nm and λ = 633 nm. Increasing the molar ratio of KST–Av–FITC to Hsp70-Alexa647 resulted in a dose-dependent reduction of the share of cells accepting the full-length chaperone (Fig. 7). Conversely, increasing the ratio of Hsp70 in the mixture lowered the number of cells which received the complex by almost half, suggesting that Hsp70 and KST peptide compete for possible binding sites on the cell surface and that KST peptide may constitute a part of the Hsp70 molecule responsible for the latter ability to penetrate inside living cells.
Fig. 7.
KST–Av–FITC complex and full-size Hsp70 compete for penetration into SK-N-SH cells. a, b KST–Av–FITC complex and Hsp70 labeled with Alexa647 were mixed in a KST–Av–FITC:Hsp70 molar ratio of 0.2:0.2, 0.2:1, 1:0.2, 1:1, 5:0.2, and 5:1 μM and analyzed with flow cytometry
Discussion
The data reported during the last two decades indicate that the Hsp70 chaperone can enter living cells and/or be released from them using canonical and non-canonical transport pathways. Although it was argued that Hsp70 does not have a secretion signal (Calderwood et al. 2007), there is at least one direct way of crossing the plasma membrane which does not require the involvement of vesicular transport. Alder and colleagues showed that Hsp70 can cross artificial bilateral membranes (Alder et al. 1990); another group reported that the chaperone itself may behave as a membrane channel (Vega et al. 2008). We suggested that Hsp70 possesses motifs resembling those found in well-established membrane-crossing proteins like HIV-1 TAT protein. Taking these results as a starting point, the objectives of this study were to design CPPs with some of the features of the Hsp70 molecule and then explore their transport properties and ability to deliver cargo into living cells. We found three sequences on the Hsp70 molecule surface containing increased quantities of positively charged amino acids, lysine and arginine (Fig. 1). Peptides with these sequences were synthesized, and their uptake by erythroleukemia K-562 growing in suspension and adhesive neuroblastoma SK-N-SH cells was assessed.
The longest of the three sequences, KST peptide, was found to penetrate the cells most effectively and was therefore used in further experiments. Notably, the number of positively charged amino acids in KST peptide was higher than in the other two, KRN and YFN peptides. Although the efficient uptake of several cationic CPPs requires at least eight positive charges (El-Sayed et al. 2009), KST peptide has only six. All known CPPs contain 5–40 amino acid residues and can be divided into subgroups according to their origin or sequence characteristics; they are not cell type or tissue specific, and penetrative capacity is mostly dependent on the positively charged sequences of amino acids at physiological pH (arginine and lysine) and electrostatic interactions with a negatively charged cell surface (before internalization) (Lindgren and Langel 2011). This class of peptides includes penetratin (Derossi et al. 1994), TAT peptide (Jeang et al. 1999), and polyarginine (Futaki et al. 2001). In addition to cationic CPPs, amphipathic and hydrophobic peptides have also been discovered (Koren and Torchilin 2012; Wang et al. 2014). Irrespective of their origin, the applications of CPPs include delivery of genes, proteins, drugs, and even 200-nm-sized imaging reagents (Ziegler et al. 2005; Bolhassani 2011).
To determine whether KST peptide could be classified as a CPP, we used its biotin tag and formed a complex, KST–Av–FITC. Using flow cytometry and confocal microscopy, we showed that the complex, which has a molecular weight of more than 60 kDa, was able to penetrate the cells of both lines, K-562 and SK-N-SH, within the first 15 min of observation (see Fig. 3). Importantly, the rate of penetration of the KST peptide into SK-N-SH cells was comparable to that of a canonical CPP, TAT peptide, the first discovered and most studied of all CPPs (Viscidi et al. 1989).
Next, we checked whether KST peptide could deliver a given physiologically active therapeutic molecule into a living cell. Intracellular Hsp70 was chosen as the target since its anti-apoptotic activity had been earlier established (Komarova et al. 2004); furthermore, knockdown of the protein sensitized cells to the inducers of apoptosis (Afanasyeva et al. 2007) or even caused spontaneous apoptosis in tumor cells (Nylandsted et al. 2000). We chose the 3B5 monoclonal antibody which has previously been shown to immunoprecipitate the Hsp70 efficiently (Guzhova et al. 1997) to neutralize the protein. The purified and biotinylated IgG was linked to KST peptide via an Avidin bridge, and finally, the KST–Av–αHsp70Ab complex was depleted of biotinylated KST. SK-N-SH cells with a high Hsp70 (SK-N-SH–Hsp70) content were found to be more resistant to the apoptosis-inducing effect of staurosporine than control cells or untreated SK-N-SH–Hsp70 cells (Fig. 4a); incubation of cells with the complex bearing anti-Hsp70 antibody reduced the protective effect of the chaperone (Fig. 4c). Confocal microscopy showed that the complex was found inside SK-N-SH–Hsp70 cells (Fig. 4b), so we suggest that the antibody carried by KST peptide retained its activity. Unlike KST–Av–FITC complex, which was able to penetrate into 100 % of cells (Fig. 3), the KST–Av–αHsp70Ab complex penetrated only inside 30 % of SK-N-SH–Hsp70 cells. We suggest that to improve the penetration properties of KST peptide, other complex constructs should be tested to overcome limitations associated with Avidin specificity and biotin linking. Fusion constructs in which the coding sequence for KST peptide is attached to a gene of interest may be a powerful alternative to Avidin–biotin bridges; similar complexes have been developed for coupling of TAT peptide to Hsp70 (Wheeler et al. 2003).
Another question we addressed was whether the KST domain is responsible for the penetrative capacity of full-size Hsp70. There is at least some evidence which supports this possibility: full-length Hsp70 and KST peptide use the same mechanism for entering cells. In a recent study, we showed that Hsp70 has several mechanisms for entering a single cell, and the balance between these pathways is different in adhesion and suspension cells (Shevtsov et al. 2014). Clathrin-dependent endocytosis was prevalent in adhesive C6 glioblastoma cells, suggesting a receptor-like mechanism, whereas in K-562 cells growing in suspension, this mechanism was less important, but the destruction of lipid rafts resulted in significant suppression of intracellular transport of the chaperone (Shevtsov et al. 2014). In this study, we compared the penetration capacities of full-length Hsp70 labeled with Alexa488 and the KST–Av–FITC complex in SK-N-SH cells. We found that the molecules behaved similarly; in some cases, they were associated with vesicular structures and in some they were not (Fig. 5a). Inhibitory analysis with substances interfering with protein transport proved the similarity of the endocytotic mechanism they used for crossing the plasma membrane. Using inhibitory analysis in K-562 cells, we observed that endocytosis-dependent and endocytosis-independent entry mechanisms contributed to cell penetration; however, lipid rafts seemed to be a more potent entry mechanism for both molecules.
It is currently difficult to establish the most prevalent CPP uptake mechanism in living cells. CPPs appear to penetrate mammalian cells using multiple pathways, including direct translocation through the membrane bilayer and endocytosis-mediated uptake (Wang et al. 2014). Formation of pores in plasma membrane and inverted micelles were also observed; however, the latter is typical of peptides enriched with hydrophobic amino acids rather than cationic peptides (Madani et al. 2011). It is noteworthy that CPPs use different cell penetration mechanisms depending on whether they are bound to a cargo or in free form. For example, when attached to a large cargo, TAT peptide is mostly entrapped by endosomal vesicles, whereas it redistributes throughout the cell cytosol when coupled to a smaller cargo molecule (Tunnemann et al. 2006) and it uses macropinocytosis when entering a cell alone (Koren and Torchilin 2012). Like the majority of CPPs recognized to date, KST peptide also uses different mechanisms to penetrate living cells in different states; when associated with cargo, it employs clathrin-dependent endocytosis (Fig. 5b) which is thought to be mediated by a specific receptor.
The existence of specific receptors for Hsp70 is still controversial, though a number of candidate molecules have been proposed, including CD91 (Basu et al. 2001), CD40 (Becker et al. 2002), CD14 (Asea et al. 2000), and members of the TLR family, particularly TLR2 and TLR4 (Asea et al. 2002). Hsp70 can interact with three members of the scavenger receptor family, namely LOX-1, SPEC-1, and FEEL-1/CLEVER-1 (Delneste et al. 2002; Thériault et al. 2005, 2006) as well as with receptors from the c-type lectin family such as CD94 and NKG2D (Gross et al. 2003; Calderwood et al. 2007). In this study, we did not search for specific receptors on the surface of SK-N-SH and K-562 cells; however, if we assume the presence of one or more of these receptors on the surface of these cells, the full-length chaperone and the KST–Av complex should compete for entry into SK-N-SH cells. We conducted a competition analysis and found that the KST–Av complex dose dependently suppressed penetration of Hsp70 into cells, while Hsp70 inhibited the entry of KST–Av into cells (Fig. 7).
Interestingly, the total amount of Hsp70 as well as the level of the chaperone exposed on a cell surface did not correlate with the capacity to endocytose KST–Av–FITC and Hsp70. In SK-N-SH cells, endocytosis was the preferable pathway and Hsp70 was expressed at an undetectable level in control conditions and at a low level even upon heat shock. In heat-shocked cells, Hsp70 appeared only as single spots on the cell surface. K-562 cells where endocytosis was not the main pathway for both polypeptides had a very high level of Hsp70 both total and located at the plasma membrane (Fig. 6). This is in contradiction with the data of De Maio et al. who showed that positive correlation between a high content of Hsp70 and the efficiency of transferrin endocytosis in HepG2 cells (Vega et al. 2010). This discordance can be explained by the fact that transferrin and Hsp70 have different receptors: transferrin receptor demonstrates high affinity to the protein while affinity of SREC-1, LOX-1, and/or TLR2/TLR4 suggested to be Hsp70 receptors (Delneste et al. 2002; Asea et al. 2002) is much lower.
In conclusion, the sequence of 493–512 aa on the surface of the Hsp70 molecule may be a signal for penetration of the whole chaperone into living cells, and the corresponding peptide, KST, is a novel CPP with a cargo-carrying capacity at least equal to that of commercial peptide-based vectors.
Electronic supplementary material
(PDF 3851 kb)
Acknowledgments
We thank Dr. N. Fusenig (DKFZ, Germany) for HaCaT cells; Dr. Marja Jäättelä for providing us with hsp70 plasmid; Dr. Oleg Demidov (INSERM, France) for rab-5, rab-7, and mito-PAGFP plasmids; Mr. Michael Vorobyev and Dr. Gregory Shtein (Institute of Cytology of RAS, Russia) for the help with confocal microscopy experiments; and Ms. Ekaterina Fedorova for technical assistance. This work was supported by grants from the Russian Foundation of Basic Research 14-08-00164 and 13-04-01299 and of the Molecular and Cell Biology Program of the Russian Academy of Sciences.
References
- Afanasyeva EA, Komarova EY, Larsson LG, Bahram F, Margulis BA, Guzhova IV. Drug-induced Myc-mediated apoptosis of cancer cells is inhibited by stress protein Hsp70. Int J Cancer. 2007;121(12):2615–2621. doi: 10.1002/ijc.22974. [DOI] [PubMed] [Google Scholar]
- Alder GM, Austen BM, Bashford CL, Mehlert A, Pasternak CA. Heat shock proteins induce pores in membranes. Biosci Rep. 1990;10(6):509–518. doi: 10.1007/BF01116611. [DOI] [PubMed] [Google Scholar]
- Aleksandrova MA, Revishchin AV, Podgornyi OV, Poltavtseva RA, Marei MV, Korochkin LI, Sukhikh GT. Transplantation of cultured neural cells from human fetuses into the brain of rats exposed to acute hypoxia. Bull Exp Biol Med. 2004;137(3):262–265. doi: 10.1023/B:BEBM.0000031565.35782.31. [DOI] [PubMed] [Google Scholar]
- Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7(4):330–338. doi: 10.1379/1466-1268(2002)007<0330:LIDTCA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70:role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277(17):15028–15034 [DOI] [PubMed]
- Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222(2):97–104. doi: 10.1016/S0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14(3):303–313. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158(7):1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitler BG, Schroeder JA. Anti-cancer therapies that utilize cell penetrating peptides. Recent Patent Anticancer Drug Discov. 2010;5(2):99–108. doi: 10.2174/157489210790936252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta. 2011;1816(2):232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Brooks H, Lebleu B, Vivès E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57(4):559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581(19):3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–571. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console S, Marty C, García-Echeverría C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transductiondomains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278(37):35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17(3):353–362. doi: 10.1016/S1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269(14):10444–10450. [PubMed] [Google Scholar]
- Deshayes S, Plénat T, Charnet P, Divita G, Molle G, Heitz F. Formation of transmembrane ionic channels of primary amphipathic cell-penetrating peptides. Consequences on the mechanism of cell penetration. Biochim Biophys Acta. 2006;1758(11):1846–1851. doi: 10.1016/j.bbamem.2006.08.010. [DOI] [PubMed] [Google Scholar]
- El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11(1):13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evdonin AL, Guzhova IV, Margulis BA, Medvedeva ND. Phospholipase c inhibitor, u73122, stimulates release of hsp-70 stress protein from A431 human carcinoma cells. Cancer Cell Int. 2004;4(1):2. doi: 10.1186/1475-2867-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276(8):5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Zamulaeva IV, Mosin AF, Makarova YM, Mosina VA, Budagova KR, Malutina YV, Kabakov AE. Resistance of Ehrlich tumor cells to apoptosis can be due to accumulation of heat shock proteins. FEBS Lett. 1995;375(1–2):21–26. doi: 10.1016/0014-5793(95)01152-5. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65(12):5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LA, Multhoff G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003;8(4):348–360. doi: 10.1379/1466-1268(2003)008<0348:HSPRIA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2(2):132–139. doi: 10.1379/1466-1268(1997)002<0132:MSPHIW>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914(1–2):66–73. doi: 10.1016/S0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Lazarev VF, Kaznacheeva AV, Ippolitova MV, Muronetz VI, Kinev AV, Margulis BA. Novel mechanism of Hsp70 chaperone-mediated prevention of polyglutamine aggregates in a cellular model of Huntington disease. Hum Mol Genet. 2011;20(20):3953–3963. doi: 10.1093/hmg/ddr314. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia. 2013;29(5):399–408. doi: 10.3109/02656736.2013.807439. [DOI] [PubMed] [Google Scholar]
- Harada Y, Sato C, Kitajima K. Complex formation of 70-kDa heat shock protein with acidic glycolipids and phospholipids. Biochem Biophys Res Commun. 2007;353(3):655–660. doi: 10.1016/j.bbrc.2006.12.068. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138(2):257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD—visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Ignatovich IA, Dizhe EB, Pavlotskaya AV, Akifiev BN, Burov SV, Orlov SV, Perevozchikov AP. Complexes of plasmid DNA with basic domain 47-57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278(43):42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- Jäättelä M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248(1):30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Jeang KT, Xiao H, Rich EA. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem. 1999;274(41):28837–28840. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- Kersemans V, Kersemans K, Cornelissen B. Cell penetrating peptides for in vivo molecular imaging applications. Curr Pharm Des. 2008;14(24):2415–2447. doi: 10.2174/138161208785777432. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EY, Afanasyeva EA, Bulatova MM, Cheetham ME, Margulis BA, Guzhova IV. Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones. 2004;9(3):265–275. doi: 10.1379/CSC-27R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Schwede T. The SWISS-MODEL repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004;32:D230–D234. doi: 10.1093/nar/gkh008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18(7):385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Lasunskaia EB, Fridlianskaia II, Guzhova IV, Bozhkov VM, Margulis BA. Accumulation of major stress protein 70 kDa protects myeloid and lymphoid cells from death by apoptosis. Apoptosis. 1997;2(2):156–163. doi: 10.1023/A:1026460330596. [DOI] [PubMed] [Google Scholar]
- Lindgren M, Langel U. Classes and prediction of cell penetrating peptides. Methods Mol Biol. 2011;683:3–19. doi: 10.1007/978-1-60761-919-2_1. [DOI] [PubMed] [Google Scholar]
- Madani F, Lindberg S, Langel U, Futaki S, Gräslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011;2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43(3):168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61(2):272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jäättelä M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A. 2000;97(14):7871–7876. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov MA, Komarova EY, Meshalkina DA, Bychkova NV, Aksenov ND, Abkin SV, Margulis BA, Guzhova IV. Exogenously delivered heat shock protein 70 displaces its endogenous analogue and sensitizes cancer cells to lymphocytes-mediated cytotoxicity. Oncotarget. 2014;5(10):3101–3114. doi: 10.18632/oncotarget.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thériault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579(9):1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Thériault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol. 2006;177(12):8604–8611. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- Tunnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. FASEB J. 2006;20(11):1775–1784. doi: 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- Tytell M, Greenberg SG, Lasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986;363(1):161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and its release into extracellular environment in membrane-associated form that activates macrophages. J Immunol. 2008;180(6):4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Vega VL, Charles W, De Maio A. A new feature of the stress response: increase in endocytosis mediated by Hsp70. Cell Stress Chaperones. 2010;15(5):517–527. doi: 10.1007/s12192-009-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi RP, Mayur K, Lederman HM, Frankel AD. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989;246(4937):1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6(4):386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang Y, Zhang X, Zhang W, Guo S, Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J Control Release. 2014;174:126–136. doi: 10.1016/j.jconrel.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Dunsmore KE, Wong HR. Intracellular delivery of HSP70 using HIV-1 Tat protein transduction domain. Biochem Biophys Res Commun. 2003;301(1):54–59. doi: 10.1016/S0006-291X(02)02986-8. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Nervi P, Durrenberger M, Seelig J. The cationic cell-penetrating peptide CPP TAT derived from the HIV-1 protein Tat is rapidly transported into living fibroblasts: optical, biophysical and metabolic evidence. Biochemistry. 2005;44(1):138–148. doi: 10.1021/bi0491604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 3851 kb)