Abstract
Mesenchymal stem cells (MSCs) are under intensive investigation for use in cell-based therapies because their differentiation abilities, immunomodulatory effects, and homing properties offer potential for significantly augmenting regenerative capacity of many tissues. Nevertheless, major impediments to their therapeutic application, such as low proliferation and survival rates remain as obstacles to broad clinical use of MSCs. Another major challenge to evolution of MSC-based therapies is functional degradation of these cells as a result of their exposure to oxidative stressors during isolation. Indeed, oxidative stress-mediated MSC depletion occurs due to inflammatory processes associated with chemotherapy, radiotherapy, and expression of pro-apoptotic factors, and the microenvironment of damaged tissue in patients receiving MSC therapy is typically therapeutic not favorable to their survival. For this reason, any strategies that enhance the viability and proliferative capacity of MSCs associated with their therapeutic use are of great value. Here, recent strategies used by various researchers to improve MSC allograft function are reviewed, with particular focus on in vitro conditioning of MSCs in preparation for clinical application. Preconditioning, genetic manipulation, and optimization of MSC culture conditions are some examples of the methodologies described in the present article, along with novel strategies such as treatment of MSCs with secretome and MSC-derived microvesicles. This topic material is likely to find value as a guide for both research and clinical use of MSC allografts and for improvement of the value that use of these cells brings to health care.
Keywords: Mesenchymal stem cell, Preconditioning, Scaffold, Conditioned medium, Microenvironment, Bioreactor
Introduction
Self-renewal, differentiation, and regeneration capacities are the main characteristics of stem cells making them ideal tools for treatment of some congenital or acquired diseases or for their application in gene therapy, drug delivery, and regenerative medicine (Biffi et al. 2013; Garbern and Lee 2013; Greco and Rameshwar 2012; Law and Chaudhuri 2013; Murphy et al. 2013; Przybyla et al. 2013; Saunders et al. 2013).
Hence, recently, embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSC) have gained intensive research attention in cell therapy experiments (Cai et al. 2013a; Ito et al. 2013; Kuhn et al. 2013; Liu et al. 2013; Shtrichman et al. 2013; Toh et al. 2011).
However, despite the differentiation capacity of the ESCs and iPSCs, potential tumorigenesis, ethical concerns, and graft versus host disease (GVHD) are the major challenges in development and clinical application of these cells (Brind’Amour 2012; Herberts et al. 2011; Knoepfler 2009; Lodi et al. 2011; Malard and Mohty 2014; Mertes and Pennings 2009; Takahashi et al. 2007).
Due to these limitations, mesenchymal stem cells (MSCs) are now much more interested for application in cell-based therapy (Law and Chaudhuri 2013; Murphy et al. 2013; Wei et al. 2013). MSCs are plastic-adherent-multipotent stem cells that are able to differentiate to at least osteo, adipo, and chondrocytes and also several other cell types (Dominici et al. 2006; Li et al. 2013b). They are easily isolated from bone marrow, adipose tissue, peripheral blood, dermis, umbilical cord (UC), umbilical cord blood (UCB), amnion fluid, and placenta, somehow without any invasive procedure (Choudhery et al. 2013; Koliakos et al. 2011; Lee et al. 2010; Lindenmair et al. 2012; Mennan et al. 2013; Ribeiro et al. 2013). Despite some differences between MSCs originated from various sources, they share the main characteristics mentioned above (Al-Nbaheen et al. 2013; Choudhery et al. 2013; Jin et al. 2013). MSCs have paracrine effects with immunomodulatory properties because of their ability to secrete several cytokines and chemokines (Arno et al. 2014; Linero and Chaparro 2014; Song et al. 2013).
However, application of MSCs in cell therapy has been hindered due to various limitations such as their low proliferation rate (Han et al. 2014; Liu et al. 2009; Yoon et al. 2011), restricted life span, and gradual loss of stemness during ex vivo expansion (Fossett and Khan 2012; Liu et al. 2009). Various stress conditions including oxidative stresses imposed through isolation and in vitro expansion of MSCs could induce apoptosis (Wei et al. 2010; Han et al. 2013), resulting in more than 99 % cell death during the first few days after transplantation (Lee et al. 2009b; Toma et al. 2002; Zhang et al. 2001). Moreover, the toxic environment caused by inflammation, chemotherapy, and irradiation of MSCs recipients could result in even higher cell death (Li et al. 2013a; Francois et al. 2013; Xu et al. 2012).
Hence, optimization of MSCs culture condition and their protection against various stresses are very critical to provide sufficient amount of efficient MSCs. Application of some useful guidelines to protect MSCs from premature death would lead to higher engraftment rates. There are several strategies to improve survival rate, potency, and function of MSCs. In fact, methods for improvement of MSCs for cell therapy applications are under intensive studies. Some of these methods would be reviewed and discussed in the following sections.
Preconditioning
Preconditioning-induced protection was first reported by Murry (Murry et al. 1986) when he exposed myocardial stem cells to a sub-lethal ischemic condition that resulted in the heart resistance to severe ischemia.
Different in vitro and in vivo studies reported increased MSCs survival after their preconditioning by cultivation under hypoxic and serum deprivation conditions (He et al. 2009; Hu et al. 2008; Hung et al. 2007; Oh et al. 2010; Wang et al. 2008). Rate of successful engraftment and the differentiation capacity of the preconditioned MSCs are higher than non-preconditioned controls (Jäderstad et al. 2010; Ren et al. 2006). Chemotaxis, proliferation, and migration capacities of MSCs are enhanced following their treatment under optimized sub-lethal stress conditions (Liu et al. 2010; Tang et al. 2009b).
Mechanisms involved in MSCs protection following their preconditioning are not fully determined. However, alteration of intrinsic signaling pathways of some growth factors, cytokines, and chemokines and the expression levels of their receptors (especially CXCR4) due to preconditioning have been reported in several studies (Liu et al. 2010; Lu et al. 2012). The most practical and important preconditioning method applied in various studies is H2O2 preconditioning.
ROS-mediated-oxidative stress is one important reason for apoptosis and death of donor MSCs following engraftment (Li et al. 2013a; Ren et al. 2012; Wei et al. 2010). Hence, various strategies such as preconditioning of MSCs with H2O2 might be useful for their adoptive protection against harmful conditions and could prevent apoptosis induction. In other words, treatment of MSCs with minute concentrations of H2O2 for a short period of time fortifies MSCs against oxidative damages upon their exposure to high concentrations of ROS (Li et al. 2014). Enhanced heart function, induction of neovascularization, and decreased myocardial fibrosis have been reported following transplantation of H2O2 preconditioned MSCs to infracted myocardium of mice (Pendergrass et al. 2013). Higher levels of VEGF, hepatocyte growth factor (HGF), and IL-6 were detected after H2O2 treatment of MSCs (Zhang et al. 2012). H2O2 preconditioning decreases serum deprivation (SD)-induced apoptosis and enhances expression of smooth muscle and endothelial cell markers, resulting in proper myocardial cell differentiation and improvement of ventricular function after ischemia-reperfusion injury (Pendergrass et al. 2013). H2O2 preconditioning activates Notch1 (Notch homolog1) and Wnt11 signaling pathways involved in stem cell differentiation, heart regeneration, and enhancement of early cardiac development (Boopathy et al. 2013). Low H2O2 concentration enhances expression of CXCR4 at transcriptional and translational levels and also promotes migration capability of MSCs by facilitating the interaction between CXCR4 and its ligand, stromal cell-derived factor-1α (SDF-1α). It also prevents induction of apoptosis in MSCs (Li et al. 2009).
It is noteworthy that treatment of MSCs with high concentrations of H2O2 or for a long period of time induces cell damages and death (Li et al. 2014; Pendergrass et al. 2013). Table 1 represents the conditions of hydrogen peroxide preconditioning and potential mechanisms that have been reported in previous studies.
Table 1.
H2O2 preconditioning for enhancing MSCs potentiality
| MSCs sources | H2O2 preconditioning | Mechanisms/altered markers |
|---|---|---|
| Human | 200 μM for 2 h | ↑ Cell differentiation and survival/VEGF, HGF, IL-6 (Zhang et al. 2012) |
| Rat | 100 μM for 48 h | ↓ Apoptosis ↑ endothelial differentiation/α-SMA, early cardiac markers (Pendergrass et al. 2013) |
| Rat | 100 μM Up to 1 week | ↑ Endothelial differentiation/Notch1 and Wint11 (Boopathy et al. 2013) |
| Rat | 20 μM for 24 h | ↑ Cell survival and migration/SDF-1-CXCR4 (Li et al. 2009) |
VEGF vascular endothelial growth factor; HGF hepatocyte growth factor; α-SMA alpha-smooth muscle actin; Wnt11 wingless-related MMTV integration site 11; Notch1 Notch homolog1; CXCR4 chemokine receptor; SDF-1 stromal cell-derived factor-1
Hypoxia
In vitro cultivation of mammalian cells including MSCs is performed under normoxic condition containing 20 % O2. However, the physiological O2 concentration is much less than the in vitro concentration. Oxygen pressure in various tissues from which MSCs are isolated is variable, being 10–15 % in adipose tissue, 1–7 % in bone marrow, and 1.5–5 % in female reproductive tract and birth-associated tissues (Bizzarri et al. 2006; Fischer and Bavister 1993). O2 concentration in MSCs niche is about 2–8 % (Ma et al. 2009). Therefore, cultivation of MSCs under normoxic condition induces oxidative stress, produce reactive oxygen species (ROS) that affect DNA, proteins, and other biomolecule structures, and changes metabolism of the cells (Fehrer et al. 2007; Jackson and Bartek 2009). In contrast, cultivation of MSCs under lower O2 pressure exhibits less chromosomal abnormalities and senescence (Fehrer et al. 2007). For example, O2 concentration of 0.5–1 % reduces apoptosis, increases paracrine effects, and enhances regenerative capacity of bone marrow-derived-MSCs (BM-MSCs) for repairing infarcted myocardium (Hu et al. 2008). Hematopoietic stem cells (HSCs) proliferate much faster when co-cultured with hypoxia-preconditioned-MSCs that secrete higher levels of IL-6 and express hypoxia inducible factor-1 (HIF-1) (Hammoud et al. 2012).
Table 2 represents some reports on hapoxia and its effects on signaling molecules which might be involved in survival, differentiation, and proliferation of MSCs.
Table 2.
Different mechanisms and molecules involved in MSCs behaviors following hypoxic treatment
| MSCs Sources | Hypoxia condition | Mechanisms/altered markers |
|---|---|---|
| Mouse | 1 % for 36 h | ↑ Cell migration/Wnt4 (Leroux et al. 2010) |
| Rat | 2 % for 4–48 h | ↑ Angiogenesis/VEGF, Flk1 VE-cadherin (Li et al. 2002) |
| Mouse | 3 % for 2–24 h | ↑ Cell migration, adhesion, and survival/ HIF-1α, Akt, CXCR7, CXCR4 (Liu et al. 2010) |
| Mouse | 0.5 % for 24 h | ↑ Cell survival/HIF-1α, Bcl-2, Flk1, EPO (Hu et al. 2008) |
| Human | 1 % for 22 h | ↑ Cell migration/HIF-1α, CXCR4, CX3CR1 (Hung et al. 2007) |
| Human | 1 % for 48 h | ↑ Angiogenesis, ↓ osteogenesis/VEGF (Potier et al. 2007a) |
Wnt4 wingless-related MMTV integration site 4; VEGF vascular endothelial growth factor; Flk1 fetal liver kinase 1; VE-cadherin vascular endothelial-cadherin; HIF-1α hypoxia inducible factor 1-α; Akt a type of protein kinase; CXCR4 and 7 and CX3CR1 chemokine receptors; Bcl-2 B-cell lymphoma 2; EPO erythropoietin
Conversely, there are some studies indicating inhibitory effects of hypoxia on differentiation capacity of MSCs isolated from different sources. However, no effects on cell survival and metabolism have been shown (Hass et al. 2011; Potier et al. 2007a). Oxygen concentration, time of exposure to hypoxia, procedure of hypoxia induction, and especially intrinsic differences between various cell types could be the reasons of these discrepancies. It is clear that oxygen tension is an important element in maintenance of MSCs stemness and for determination of their fate (Drela et al. 2014). Overall, preclinical studies on hypoxia preconditioning are under way for improvement of MSCs survival and healing capability.
Serum deprivation (SD)
Serum deprivation and poor nutrition are known stresses, due to which increased MSCs death occurred (Haider and Ashraf 2008; Robey et al. 2008). Therefore, strengthen of MSCs against these stresses might be useful for enhancing their therapeutic efficacy. Various concentrations of fetal bovine serum (FBS) are used in most expansion protocols to supply essential requirements including growth factors, vitamins, and attachment factors that are necessary for cell growth and proliferation (Bieback et al. 2009). However, optimization and standardization of suitable FBS concentration is very difficult because of lot-to-lot variation of FBS. Moreover, the risk of infections and immune reactions must be considered (Sundin et al. 2007). Serum contains complement that upon activation injures MSCs which leads to cell death (Li and Lin 2012). FBS and serum supplements might cause MSCs senescence and aneuploidy as shown in some clinical applications (Tarte et al. 2010). Today, reinforced studies are aimed to minimize FBS application or establish alternative methods or materials for replacing FBS (Halme and Kessler 2006). MSCs cultured in SD condition show normal morphology (Fu et al. 2011). Higher viability rate and clonogenic capacity are observed when SD-treated MSCs are consequently cultivated in presence of FBS (Pochampally et al. 2004). They show suitable tube formation ability when cultured on Matrigel-coated plates that refer to their potentiality for endothelial differentiation (Iwase et al. 2005). They also differentiate to functional endothelial cells after transplantation to ischemic-model-rats (Iwase et al. 2005). SD accelerates somatic cells reprogramming in procedures of iPSCs production because it improves viral infection rate and increases transduction ratio (Chen et al. 2012a).
Conversely, some reports indicate induction of cell death by SD. Intensive and long-time hypoxia/SD condition result in massive cell death because of severe and prolonged restricted nutrients supply (Potier et al. 2007b). SD provokes the onset of apoptosis by increasing caspase-3 activity. It also induces cytochrome C releasing resulting in mitochondrial dysfunction and more apoptotic death (Zhu et al. 2006). SD (or combination of SD and hypoxia) may generate more intracellular ROS, which decreases the integrity and potential of mitochondrial membrane followed by increased Bax/Bcl-2 ratio (Wang et al. 2014a).
However, the reported discrepancies may be due to differences between various methods used for induction of SD condition, especially regarding the exposure time length and also because of the differences between various techniques used to determine cell death or survival. Some related studies and their findings about effects of SD on MSCs are represented by Table 3.
Table 3.
Serum deprivation effects on MSCs fate
| MSCs sources | Serum deprivation (SD) | Mechanisms/altered markers |
|---|---|---|
| Rat | SD for 3 h | ↑ Cell survival/not detected (He et al. 2009) |
| Human | SD for 48 h | ↑ Cell survival and angiogenesis/Akt, IL-6, eNOS (Hung et al. 2007) |
| Rat | – | ↑ Cell survival and differentiation (Iwase et al. 2005) |
Akt a type of protein kinase; eNOS endogenous Nitric oxide synthesis
Pretreatment
MSCs were subjected to pretreatment with other chemicals and biologicals such as SDF-1 (Pasha et al. 2008), IL-1β, transforming growth factor-β (TGF-β) (Luo et al. 2012), and transforming growth factor-α (TGF-α) (Herrmann et al. 2010). Simultaneous pretreatment with IL-1β and TGF-β stimulates secretion of higher levels of VEGF comparing to pretreatment with each of these factors alone. This resulted in better post-ischemic recovery of left ventricular developed pressure (LVDP) (Luo et al. 2012). Pretreatment of MSCs with SDF-1 increases their survival following exposure to H2O2 and improves proliferation and homing ability of transplanted MSCs (Pasha et al. 2008).
Genetic manipulation
Overexpression of cytoprotective genes is a suitable strategy to increase survival of MSCs. There are various reports on upregulation or suppression of important genes involved in vital cell cycles, apoptosis, and cell survival pathways. Hence, various signaling pathways are activated, and the modified networks of biomolecules, chemokines, and cytokines might affect metabolism, survival, differentiation, and migration potencies of MSCs (Hodgkinson et al. 2010).
MSCs overexpressing Akt (MSCs-Akt), a serine-threonine protein kinase with vast cytoprotective effects, showed higher survival rate and significant improvements in cardiac function following their transplantation into ischemic heart tissue. Furthermore, the MSCs-Akt showed higher angiogenesis capability, leading to decreased infarcted size and number of apoptotic cells (Noiseux et al. 2004). Upregulation of Akt enhances secretion of fibroblast growth factor (FGF), VEGF, HGF, and some other cytokines by MSCs (Mirotsou et al. 2007).
Equipping MSCs with anti-apoptotic factors including Bcl-2 inhibits mitochondrial apoptotic pathways; hence, it improves cell viability, angiogenesis rate, and the regeneration potential of damaged heart tissue via regulation of VEGF secretion (Li et al. 2007).
Overexpression of heat shock protein 20 (Hsp20) in MSCs (MSCs-Hsp20) using viral vectors enhances their engraftment potential in ischemic condition because of increased number of viable cells. Normally, Hsp20 is expressed in stress conditions to correct configuration of damaged biomolecules such as VEGF, insulin-like growth factor-1 (IGF-1), Akt, and FGF to retain their biological functions. It also improves angiogenesis potential and survival of cardiomyocytes under oxidative stress (Wang et al. 2009).
Transient overexpression of nuclear factor related (erythroid-derived 2)-like 2 (Nrf2) in MSCs results in increased level of super oxide dismutase (SOD) and heme oxygenase-1 (HO-1), which protect the cells against apoptosis induced by oxidative stress and hypoxic conditions (Mohammadzadeh et al. 2012). Nrf2 is an oxidation/reduction-sensitive transcription factor that plays important role in antioxidant defense (Kensler et al. 2007; Lee and Johnson 2004).
Overexpression of HO-1 in MSCs using adenovirus expression system fortifies these cells against cytotoxic effects of H2O2 and inhibits the apoptosis induced by oxidative stress (Hamedi-Asl et al. 2012). In addition, overexpression of Lipocalin-2 (Lcn2) in MSCs protects them against stressful microenvironments through upregulation of various antioxidant and growth factors (Halabian et al. 2013).
Genetic manipulation of MSCs with recombinant vectors encoding HIF-1α (MSCs-HIF-1α) reinforces their supportive functions on hematopoietic stem cells (HSCs). The MSCs-HIF-1α are resistant against hypoxic, oxidative, and serum-deprived stress conditions (Kiani et al. 2014).
There are further studies regarding cytoprotective effects of overexpressing other genes in MSCs. Table 4 summarizes the results of the mentioned studies.
Table 4.
Summary of genes used for increment of MSCs survival
| MSCs sources | Overexpressed genes | Mechanisms/altered markers |
|---|---|---|
| Mice | AKt | ↑ Cell survival and angiogenesis (Noiseux et al. 2004) |
| Mice | Akt | ↑ Endothelial differentiation/FGF, VEGF, HGF (Mirotsou et al. 2007) |
| Rat | Bcl2 | ↓ Apoptosis, ↑ cell survival and angiogenesis (Li et al. 2007) |
| Rat | Hsp20 | ↑ Endothelial differentiation and cell survival/VEGF, IGF-1, Akt, and FGF (Wang et al. 2009) |
| Human | Nrf2 | ↑ Cell survival/SOD and HO-1 (Mohammadzadeh et al. 2012). |
| Human | HO1 | ↓ Apoptosis and ↑ cell survival (Hamedi-Asl et al. 2012) |
| Rat | Lcn2 | ↑ antioxidant elements and growth factors (Halabian et al. 2013) |
| Human | HIF-1α | ↑ Cell survival, paracrine effects (Kiani et al. 2014) |
| Rat | TNFR | ↓ Apoptosis, ↑ endothelial differentiation/TNF-, IL-1beta and IL-6 (Bao et al. 2008) |
| Rat | IGI-1 | ↑ Paracrine effects and homing/SDF-1α, Akt, and Bcl.xl (Haider et al. 2008) |
| Rat | VEGF | ↑ Endothelial differentiation/VEGF and desmin (Gao et al. 2007) |
| Human | Hsp70 | ↑ Cell survival and differentiation, ↓ apoptosis (McGinley et al. 2011) |
| Rat | Survivin (SVV) | ↑ Cell differentiation/VEGF, FGF (Liu et al. 2011) |
| Human | FGF-2 | ↑ Cell proliferation and differentiation/MAPK (Cai et al. 2013b) |
| Rat | SDF-1 | ↑ Cell differentiation/VEGF, Akt (Tang et al. 2009a) |
| Rat | SIRT1 | Cell survival and potential/FGF, Ang1 (Chen and Wang 2014) |
| Human | TERT | ↑ Cell proliferation and differentiation (Liu et al. 2012) |
Akt a type of protein kinase; FGF fibroblast growth factor; VEGF vascular endothelial growth factor; HGF hepatocyte growth factor; Bcl-2 B-cell lymphoma 2; Nrf2 nuclear factor related (erythroid-derived 2)-like 2, Hsp20 and 70 heat shock protein 20 and 70, IGF-1 insulin-like growth factor-1; SOD super oxide dismutase; HO-1 heme oxygenase-1; Lcn2 lipocalin 2; HIF-1α hypoxia inducible factor-1α; TNFR tumor necrosis factor receptor; Bcl.xl B-cell lymphoma extra large; MAPK mitogen-activated protein kinase; SDF-1 stromal cell-derived factor 1; SIRT1 NAD-dependent deacetylase sirtuin-1; Ang 1 angiopoietin 1; TERT telomerase reverse transcriptase; SVV Survivin
Despite progressions and encouraging results of the genetically manipulated MSCs, there is a major safety concern in their clinical application and therefore, they are still not approved to be used. Hence, conducting further studies to address this concern is essential.
Improvement of MSCs culture conditions and microenvironments
In order to provide sufficient numbers of MSCs for therapeutic applications, they are cultured and expanded in vitro. However, there is not a single method for cultivation of MSCs and various culture conditions and supplements are used in different laboratories. Various cultivation parameters including nutrients, culture medium, gas pressure (O2, CO2, and N2 %), temperature, and humidity influence the fate of MSCs (Van Der Sanden et al. 2010). There is always a balance between self-renewal and differentiation potential of MSCs (Krinner et al. 2010; Singh and Schwarzbauer 2012). This is influenced by several intrinsic and extrinsic parameters including growth factors and surrounding extracellular matrix (Krinner et al. 2010; Singh and Schwarzbauer 2012). Therefore, their cultivation conditions must be refined according to the purpose of the expansion. In vivo, MSCs reside and grow in their niche, a highly specialized three dimensional microenvironment (Ehninger and Trumpp 2011). Many in vitro studies have been conducted to reproduce the stem cell niche (Ehninger and Trumpp 2011; Jones and Wagers 2008; Ponader and Burger 2010; Scadden 2006; Scaglione et al. 2011). Although the exact resemblance of the niche dynamic structure in cell culture is impossible, proper changes in cultivation conditions may enhance their appropriateness for cell therapy purposes. Various experiments which have been conducted to achieve these purposes are reviewed here.
Non-adherent or suspension cultivation
MSCs are usually cultivated as adherent cells. However, they are also capable of proliferating and differentiating under suspension culture conditions, in which they form colonies or spheroids with enhanced cell-cell interactions. MSCs show more supportive effects on each other when cultivated as non-adherent cells spheroids because of higher communicative and paracrine interactions (Bartosh et al. 2010; Cheng et al. 2012). Interestingly, suspended cultivation of MSCs induces their proliferation without the need to any excessive growth factors or other supplements (Reynolds and Weiss 1992).
Aging and high glucose levels negatively interfere with MSCs self-renewal capacity and their proliferation rate (Choudhery et al. 2012a; Cramer et al. 2010; Han et al. 2012; Stolzing et al. 2010). However, MSCs isolated from old rats followed by suspended cultivation retain their clonogenic and self-renewal capacity even at presence of high glucose concentrations (Stolzing et al. 2012). These cells express pluripotency markers and are much primitive than those cultured under adherent conditions.
MSCs show faster proliferation rate and shorter doubling time when cultured in suspension. Increased nitric oxide (NO) contents, an efficient factor for suppression of immune reactions, gives the suspended MSCs more immunomodulatory potencies which cause better engraftment and higher regeneration capability after allogeneic transplantation (Chen et al. 2012b). Cultivation under suspension conditions retains MSCs stemness and induces expression of pluripotency markers (Amiri et al. 2014a; Higuchi et al. 2012).
Hanging drops technique is a conventional method for culturing cells under suspension conditions or their differentiation (Banerjee and Bhonde 2006). Several biomolecules and synthetic polymers for inhibition of cell attachment and suspension cultivation of various cell types have been developed and reported by different studies (Amiri et al. 2014b; Asakura et al. 1992; Ishihara et al. 1999).
Scaffolds
MSCs are increasingly used in regeneration medicine for repairing various damaged tissues. A wide range of biomaterials have been developed to fit prerequisites of MSCs for this purpose. Being either synthetic or natural, these scaffolds are mostly originated from collagen, fibrin, agarose, alginate, and silk (Willerth and Sakiyama-Elbert 2008).
Furthermore, to reproduce and mimic natural niche of MSCs and for delivery of engineered cells, three-dimensional (3D) scaffolds have been developed. The most important criteria of these scaffolds are being biocompatible, nontoxic, and biodegradable. These biomaterials are used for manipulation of the complex interactions between cells and their matrix to obtain favorable differentiated status of the cells (El-Amin et al. 2003; Meinel et al. 2004).
These scaffolds support seeded MSCs and direct their commitment toward functional differentiated cells (Donzelli et al. 2007). Sometimes, standardized mixtures of a number of scaffolds show better performance in improving MSCs potentiality (Whu et al. 2009).
Collagen scaffolds impregnated with MSCs prevent expression of growth inhibitory molecules such as Nogo-A that results in enhanced axonal plasticity (Mahmood et al. 2014). It has been shown that regeneration of traumatic brain injury lesions is obtained more quickly by MSCs scaffold comparing to application of MSCs alone (Mahmood et al. 2014). Neuronal differentiation and fast repairing of damaged brain tissues are promoted by chitosan scaffolds (Shi et al. 2012).
Extracellular matrix (ECM) components isolated from cardiac tissue form biocompatible scaffolds which support long-lasting growth of MSCs while maintaining their viability and differentiation capability (Eitan et al. 2009). These cells seem to be more potential for regeneration of infarcted heart.
In general, scaffolds contribute to optimize MSCs microenvironment and modulate the complex interactions between cells and the matrix around, which finally leads to well-controlled growth and behavior of cells.
Three-dimensional and dynamic culturing
Isolation of MSCs from different tissues and their cultivation by conventional monolayer culturing methods alter their naive niche that might affect the fate of MSCs (Ehninger and Trumpp 2011). Since conventional methods cannot control the growth and proliferation rate of MSCs well, their self-renewal capacity and differentiation potencies are gradually lost (Lund et al. 2009).
However, comparing to monolayer cell cultivation, different chemical and physical events are found within 3D environments that cells are originated from them. Encapsulation of cells in some proper biomaterials and formation of 3D spheroids are simple methods that have been developed to generate 3D culturing modules (Cardoso et al. 2012; Ramsey 2011).
Cultivation of MSCs under naive or engineered 3D conditions provides suitable environment for controlling and predicting cell growth (Lund et al. 2009). Cell surface markers, extracellular matrices, and paracrine effects of MSCs might be affected by 3D culturing, which could influence cell-cell interactions and finally change the cells’ fate (Huebsch et al. 2010). 3D cultivation facilitates positive interactions between the three main elements in microenvironment including extracellular matrix, cells, and signaling pathways and molecules (Whu et al. 2009).
Design of dynamic culture condition in appropriate spinner flasks improves MSCs differentiation capability, viability, and therapeutic effects following transplantation (Frith et al. 2009). Application of perfusion flasks that have two phases not only leads to perfect distribution of supplements and nutrients but also results in appropriate elimination of waste and toxic metabolites (Baker and Chen 2012). Intelligent special flasks have been designed to monitor cell metabolism dynamically (Lavrentieva et al. 2010). These flasks have a sensor dye with luminescence lifetime for recording pH and dissolved O2 percentage in culture medium.
Bioreactors
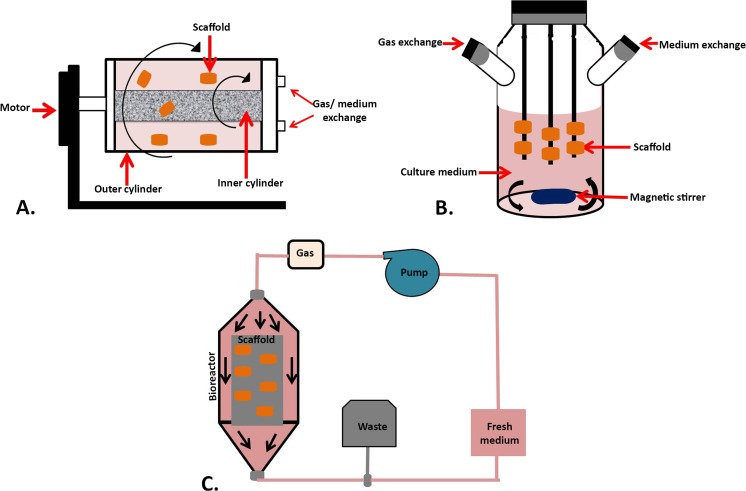
To provide sufficient numbers of MSCs for various applications, cultivation must be scaled up. Some bioreactors have been designed for this purpose. Bioreactors facilitate medium circulation and stimulate cells to form a functional tissue. These instruments reduce contamination potential, handling steps and permit monitoring of environmental parameters such as temperature, pH, O2, and CO2 concentration (Carpentier et al. 2011). Rotating wall vessels, perfusion systems, and spinner flasks are the three most common bioreactor types.
Rotating wall vessels have low turbulence and shear stress and provide well-controlled oxygenation. These bioreactors are composed of two concentric cylinders; first, to supply media and nutrients and second, to circulate and exchange the gasses (Sikavitsas et al. 2002). Spinner flasks have cylindrical container and stirring element to refresh and mix culture medium. These kinds of bioreactors accelerate MSCs differentiation when compared with static cultivation system (Godara et al. 2008). Spinner flasks and rotating wall vessels minimize gradients of metabolite and nutrient concentrations and prepare static conditions. But perfusion, bioreactors provide dynamic-controlled conditions with more uniform media that allow physical stimulation of cells and environmental monitoring. These systems are designed for continuous or non-continuous media perfusion through the scaffolds that performed by especial pumping system (Godara et al. 2008; Yeatts and Fisher 2011).
Enhanced cell seeding, fast and controlled cell expansion, and efficient oxygen, metabolites, and nutrients exchange are main factors which must be considered when designing a bioreactor (Godara et al. 2008). The schematic structures of bioreactors are represented by Fig. 1.
Fig. 1.
The structure of different types of bioreactors. a Rotating wall vessels bioreactor. This kind of bioreactor is designed base on horizontal rotating vessels containing inner and outer cylinders to supply media and circulate and exchange the gasses. The scaffolds may attach on outer vessels wall or not. b Spinner flasks bioreactor. It has cylindrical container contains impregnated scaffolds and magnetic stirring bare or paddle to refresh and mix culture medium. c Perfusion bioreactor. Its dynamic systems are made of pumping system to perfuse fresh medium through the scaffolds continuously or non-continuously
Miscellaneous
Secretome or conditioned medium and microvesicles (MVs) derived from MSCs are also two recent practical strategies to improve MSCs-based therapies.
Secretome or conditioned medium
As mentioned before, paracrine effects of MSCs are mediated due to their ability to secrete several cytokines and chemokines (Devine et al. 2003; Ren et al. 2008). Therefore, culture medium of MSCs, which is called secterome or conditioned medium, contains these biological factors that could successfully be used in regenerative medicine. There are evidences that show regulatory roles of secretome in various critical pathways in cells (J Salgado et al. 2010). Various studies reported that cultivation of stem cells in the presence of MSCs secretome improves their viability, differentiation, and regeneration capacities (Angoulvant et al. 2011; Katsha et al. 2010; Osugi et al. 2012).
However, some biomolecules and growth factors involved in regeneration process are also favorable for metastasis and tumor formation (Hoeben et al. 2004; Lee et al. 2012; Paschos and Bird 2010). For example, hepatocyte growth factor (HGF) plays critical role in motility of hepatocytes and some other cell types. Although its function is very important in liver regeneration, it also involves in neoplasm formation, cellular invasion, and metastasis (Hoeben et al. 2004). VEGF not only accelerates wound healing but also ameliorates formation of tumoral stroma (Hoeben et al. 2004). Tumor growth factor beta and matrix metalloproteinase 7 (MMP-7, matrilysin), a member of MMP family, may be involved in migration and invasion of colorectal cancer cells in early tumor metastatic phase (Paschos and Bird 2010; Lee et al. 2012).
Therefore, the positive effects of MSCs for regenerative therapies especially during remission or in presence of tumor are controversial (Wang et al. 2014b; Wong 2011; Zimmerlin et al. 2013). Hence, injection of optimized volume of conditioned medium instead of individual MSCs might be practical (Cantinieaux et al. 2013) and addresses the concerns related to the probable risks of MSCs engraftment. Secretome and its therapeutic effects are subjected for ongoing intensive studies.
MSCs-derived microvesicles
Microvesicles (MVs) are the plasma membrane-derived circular fragments shed from the surface of MSCs and other cell types or released from endosomal compartment (Camussi et al. 2010; Muturi et al. 2013; Ratajczak 2011). These structures harbor various cell-derived components including surface receptors and ligands, messenger RNAs (mRNAs), microRNAs, proteins, and even some organelles. It seems that MVs play role in transferring messages from parental cells to other cells regarding proliferation, cell immunity, transcription, and other vital cell events (Bruno et al. 2009; Collino et al. 2010). Bi-directional information exchange between stem cells and injured cells by MVs may be one of the mechanisms involved in stem cells’ positive effects on cell reprogramming and reproduction (Camussi et al. 2013).
Presence of antigenic markers including CD44 and CD29 and some adhesion molecules on the surface of MVs originated from MSCs has been reported. These MVs are uptaken by renal tubular cells and participate in their repairing following glycerol-induced acute kidney injury (AKI) (Bruno et al. 2009).
Application of MVs instead of intact MSCs is more promising because of the lower risk of malignant transformation (Baglio et al. 2012; Tetta et al. 2011). It is also possible that MVs originated from engineered MSCs express more surface molecules, growth factors, microRNA (miRNA), and mRNA which may promote neovascularization of damaged tissues and inhibit apoptosis of target cells (Ratajczak 2011).
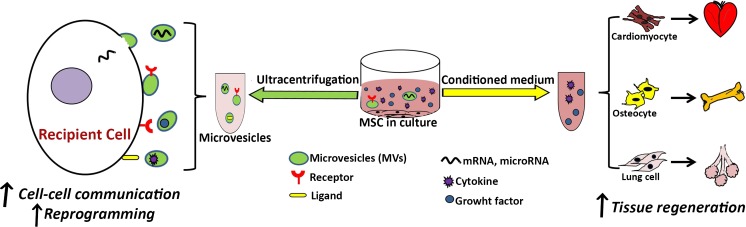
In brief, MVs and their functions in cell-cell communication should be more defined and could become a vehicle for cell-derived therapeutics. Figure 2 describes the MVs roles in cell-cell communication and cell reprogramming.
Fig. 2.
Effects of secretome or conditioned medium and MSCs-derived microvesicles (MVs) on tissue regeneration and cell fate. Harvested conditioned medium that contains growth factors and cytokines improves cell differentiation and tissue regeneration capacities. MVs containing various mRNAs, microRNAs, receptors, and ligands facilitate cell-cell communication and consequently improve their potentiality
Other challenges against MSCs-based cell therapies and promising strategies to overcome them
Due to impaired regeneration potential of MSCs caused by cellular senescence following their multiple passages, application of lower passages of MSCs is recommended for cell therapy purposes (Choo et al. 2014; Burova et al. 2013; Moll, 2012). In addition, replicative and stress-induced premature senescence would be caused in MSCs by unfavorable microenvironments, mainly oxidative stress (Cui et al. 2011). Decreased proliferation rate, genetic instability, and aneuploidy are the main consequences of replicative senescence (Estrada et al. 2013). Hence, many in vitro attempts are underway to decrease senescence of MSCs and subsequently improve their viability.
One of the strategies for prevention of premature cellular senescence induction is pretreatment of MSCs with resveratrol, which may act through inhibiting reduction of senescence-associated proteins and NAD-dependent deacetylase sirtuin-1 (SIRT1) (Choi et al. 2014). Another strategy is to overexpress wild-type p53 inducible phosphatase-1 (Wip1) (Lee et al. 2009a). This protein is a stress modulator, which prevents morphologic changes in MSCs via downregulation of signaling pathways involved in the process of premature senescence (Lee et al. 2009a).
High-calorie resources like glucose- or nutrient-enriched culture media would affect proliferation rate and clonogenicity of MSCs by induction of replicative senescence (Stolzing et al. 2006). Hence, application of low-glucose level culture medium resulted in increased fibroblastoid colony-forming unit (CFU-F) capacity of MSCs in terms of their size and number (Stolzing et al. 2006).
More recently, we also found that overexpression of Lcn2 in bone marrow-derived MSCs decreased their senescence under sub-lethal doses of oxidative stress (Bahmani et al. 2014). This finding suggested that overexpression of Lcn2 would be beneficial to retain the fitness of MSCs for cell therapy purposes in elderly people, who are susceptible to many diseases in which autologous MSC-based therapy may be applicable.
Tissue-specific progenitor cells proliferate and replace lost or injured cells in response to traumatic influences. However, the pool of these progenitor cells is depleted by various situations such as microbial challenge, toxicant exposure, mechanical or thermal injury, and a wide range of other stressors—along with inflammation triggered by the primary insult, which also impairs the normal function of the affected tissues (Seaberg et al. 2003). Therefore, these stressors and their effects on stem cells, particularly MSCs, must be identified to maintain MSCs pools or optimize their expansion protocols (Mansouri et al. 2012; Tower 2012). In addition, for selective tissue regenerative medicine or preserving MSCs pool, application of some new target tissue-based strategies and modulation of the target tissues to secrete more specific chemokines to facilitate MSCs homing might be possible (Naderi‐Meshkin et al. 2014).
Moreover, aging, senescence, and accumulated damages may exhaust stem cell pools in old age (Boyette and Tuan 2014). It has been shown that MSC can be amenable to overcome the challenges (Gharibi et al. 2014). Definition of aging and longevistatic process could progress interventions to extent life span along with stem cell, especially MSCs, in this field (Haines et al. 2013).
Mahmood Choudhery et al. reported less wound healing and proliferation rate after transplantation of MSCs isolated from older donors (Choudhery et al. 2012a). When compared to young donor-derived MSCs, the aged donor-derived MSCs were capable of producing lower amount of some growth factors such as VEGF and SDF-1, but express higher levels of p53, p21, p16, and Bax, which resulted in apoptosis and senesces (Choudhery et al. 2012a). However, in vitro caloric restriction of MSCs isolated from aged (24 months) mice improved expression of genes-regulating viability, along with improvements in indices of population doubling (PD) and superoxide dismutase (SOD) activity (Choudhery et al. 2012b). Moreover, in contrast to MSCs grown in nutrient-rich medium, engraftment of the caloric-restricted aged MSCs significantly improved left ventricular tissue damage of aged mice, which had sustained surgically induced myocardial infarction (Choudhery et al. 2012b).
In another study, enhanced expression of pro-angiogenesis growth factors has been reported following cultivation of diabetic donor-derived MSCs in the presence of cardiomyocytes-conditioned medium (Khan et al. 2013). This improved heart-repairing properties of the MSCs after their transplantation into diabetic animal (Khan et al. 2013).
The immunosuppressive characteristics of MSCs made them also suitable for use in allogeneic and autologous organ transplantation (Tan et al. 2012). Therefore, one of the most promising strategies in the general scope of regenerative medicine would be the engraftment of stem cells capable of differentiating into selected tissue within the same range and functional outcomes as native progenitor phenotypes. There are some other challenges against MSCs-based cell therapy including allergic reaction, embolization upon infusion, lung entrapment, and alteration of proinflammatory gene expression after MSC transplantation (Meier et al. 2013; Wang et al. 2013; Nystedt et al. 2013). Enhancing the immunomodulatory properties of MSCS via various strategies might address the concern dealing with long-term graft survival and alloantibody production.
Two practical strategies which resulted in improvement of MSCs’ immunosuppressive effects included activation or increasing the expression of toll-like receptor 3 (TLR3) by supplementing MSCs culture media with poly (I:C) (Van den Akker et al. 2013) and induction of nitric oxide (NO) produced by nitric oxide synthase (NOS) (Ma et al. 2013).
Recently, improvement of recovery procedure and decreased inflammation and vacuole formation have been reported following low-level laser therapy (LLLT) as an adjuvant care for MSC transplantation in cell therapy of peripheral nerve injury animal model (Yang et al. 2013).
Microarray analysis of cDNA expression of LLLT-affected MSCs indicated changing in expression of several genes involved in cellular viability pathways such as apoptosis and proliferation (Wu et al. 2012; Barboza et al. 2014).
Conclusions
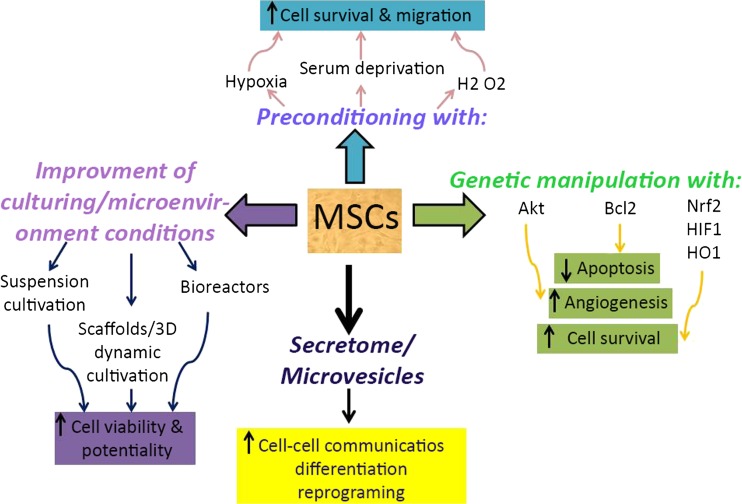
MSCs are attractive cells in several fields of cell therapy. However, there are some critical challenges against MSCs-based cell therapies. Low survival and limitations in vitro culture conditions are two important challenges. Here, we discussed some strategies used to increase potentiality and survival of MSCs (Fig. 3). However, more ongoing studies should be conducted in this regard. Knowledge and understanding of these conditions will contribute to the improvement of MSCs-based cell therapy.
Fig. 3.
Schematic representation of strategies used to improve MSCs potentiality and their therapeutic efficiency. Preconditioning under sub-lethal stresses including hypoxia, serum deprivation, and oxidative stress enhances MSCs properties such as survival and migration capability. Genetic manipulation and equipping with cytoprotective genes (Akt, Bcl2, Nrf2, HIF-1α, and HO1) lead to enhanced angiogenesis and survival rate and decreased apoptosis of MSCs. Improvement of culturing and microenvironment conditions increases MSCs survival rate and potentiality. Some of the most important methods (suspension cultivation, scaffolds, 3-dimesional dynamic culturing, and bioreactors) are highlighted here. Secretome and MSCs-derived microvesicles (MSCs-MVs) are recent strategies which may improve MSCs communication, differentiation, and reprogramming ability
References
- Al-Nbaheen M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev Rep. 2013;9:32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri F, Halabian R, Dehgan Harati M, Bahadori M, Mehdipour A, Mohammadi Roushandeh A, Habibi Roudkenar M (2014a) Positive selection of Wharton’s jelly-derived CD105+ cells by MACS technique and their subsequent cultivation under suspension culture condition: a simple, versatile culturing method to enhance the multipotentiality of mesenchymal stem cells Hematology [DOI] [PubMed]
- Amiri F, Halabian R, Salimian M, Shokrgozar MA, Soleimani M, Jahanian-Najafabadi A, Roudkenar MH (2014b) Induction of multipotency in umbilical cord-derived mesenchymal stem cells cultivated under suspension conditions Cell Stress and Chaperones:1–10 [DOI] [PMC free article] [PubMed]
- Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant. 2011;30:95–102. doi: 10.1016/j.healun.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, Jeschke MG (2014) Effect of human Wharton’s jelly mesenchymal stem cell paracrine signaling on keloid fibroblasts stem cells translational medicine:sctm. 2013–0120 [DOI] [PMC free article] [PubMed]
- Asakura S, Hurley RW, Skorstengaard K, Ohkubo I, Mosher DF. Inhibition of cell adhesion by high molecular weight kininogen. J Cell Biol. 1992;116:465–476. doi: 10.1083/jcb.116.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy Frontiers in physiology 3 [DOI] [PMC free article] [PubMed]
- Bahmani B, Roudkenar MH, Halabian R, Jahanian-Najafabadi A, Amiri F, Jalili MA (2014) Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress Cell Stress and Chaperones:1–9 [DOI] [PMC free article] [PubMed]
- Baker BM, Chen CS. Deconstructing the third dimension—how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Bhonde RR. Application of hanging drop technique for stem cell differentiation and cytotoxicity studies. Cytotechnology. 2006;51:1–5. doi: 10.1007/s10616-006-9001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C, Guo J, Lin G, Hu M, Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc J. 2008;42:56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- Barboza CAG, Ginani F, Soares DM, Henriques ÁCG, Freitas RdA (2014) Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells Einstein (São Paulo) 12:75–81 [DOI] [PMC free article] [PubMed]
- Bartosh TJ, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- Biffi A, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Bizzarri A, Koehler H, Cajlakovic M, Pasic A, Schaupp L, Klimant I, Ribitsch V. Continuous oxygen monitoring in subcutaneous adipose tissue using microdialysis. Anal Chim Acta. 2006;573:48–56. doi: 10.1016/j.aca.2006.03.101. [DOI] [PubMed] [Google Scholar]
- Boopathy AV, Pendergrass KD, Che PL, Yoon Y-S, Davis ME. Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res & therapy. 2013;4:1–15. doi: 10.1186/scrt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette LB, Tuan RS. Adult stem cells and diseases of aging. J Clin Med. 2014;3:88–134. doi: 10.3390/jcm3010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brind’Amour K (2012) Ethics and induced pluripotent stem cells embryo project encyclopedia
- Bruno S, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burova E, Borodkina A, Shatrova A, Nikolsky N (2013) Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium oxidative medicine and cellular longevity 2013 [DOI] [PMC free article] [PubMed]
- Cai J et al. (2013a) Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells Cell Regeneration 2 [DOI] [PMC free article] [PubMed]
- Cai T-Y, Zhu W, Chen X-S, Zhou S-Y, Jia L-S, Sun Y-Q. Fibroblast growth factor 2 induces mesenchymal stem cells to differentiate into tenocytes through the MAPK pathway. Molecular medicine reports. 2013;8:1323–1328. doi: 10.3892/mmr.2013.1668. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283–287. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- Cantinieaux D, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8:e69515. doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso TC, et al. Isolation and characterization of Wharton’s jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012;12:18. doi: 10.1186/1472-6750-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier B, Layrolle P, Legallais C. Bioreactors for bone tissue engineering. Int J Artif Organs. 2011;34:259–270. doi: 10.5301/ijao.2011.6333. [DOI] [PubMed] [Google Scholar]
- Chen H, J. A. W (2014) GW25-e1653 SIRT1 enhances therapeutic efficacy of aged mesenchymal stem cells in rat myocardial infarction via lightening MSCs aging and heightening stress resistance. J Am Coll Cardiol 64
- Chen M, et al. Serum starvation induced cell cycle synchronization facilitates human somatic cells reprogramming. PLoS One. 2012;7:e28203. doi: 10.1371/journal.pone.0028203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-D, et al. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther. 2012;3:40. doi: 10.1186/scrt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N-C, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biogeosciences. 2012;33:1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Choi MR, Kim SH, Ohn T, Jung KH, Chai YG. Resveratrol relieves hydrogen peroxide-induced premature senescence associated with SIRT1 in human mesenchymal. Stem cells Mol & Cell Toxicol. 2014;10:29–39. [Google Scholar]
- Choo KB, et al. Oxidative stress-induced premature senescence in Wharton’s jelly-derived mesenchymal stem cells. J Med Sci. 2014;11:1201. doi: 10.7150/ijms.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti‐apoptosis capabilities. Cell Biol Int. 2012;36:747–753. doi: 10.1042/CBI20110183. [DOI] [PubMed] [Google Scholar]
- Choudhery MS, et al. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair‐infarcted myocardium. J Cell Mol Med. 2012;16:2518–2529. doi: 10.1111/j.1582-4934.2012.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy. 2013;15:330–343. doi: 10.1016/j.jcyt.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Collino F, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C, et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19:1875–1884. doi: 10.1089/scd.2010.0009. [DOI] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H (2011) Oxidative stress, mitochondrial dysfunction, and aging Journal of signal transduction 2012 [DOI] [PMC free article] [PubMed]
- Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Society for Cellular Therapy position statement Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Donzelli E, et al. Mesenchymal stem cells cultured on a collagen scaffold: in vitro osteogenic differentiation. Arch Oral Biol. 2007;52:64–73. doi: 10.1016/j.archoralbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Drela K et al. (2014) Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner Cytotherapy [DOI] [PubMed]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan Y, Sarig U, Dahan N, Machluf M. A cellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility tissue engineering part C. Methods. 2009;16:671–683. doi: 10.1089/ten.TEC.2009.0111. [DOI] [PubMed] [Google Scholar]
- El-Amin S, Lu H, Khan Y, Burems J, Mitchell J, Tuan R, Laurencin C. Extracellular matrix production by human osteoblasts cultured on biodegradable polymers applicable for tissue engineering. Biomaterials. 2003;24:1213–1221. doi: 10.1016/s0142-9612(02)00451-9. [DOI] [PubMed] [Google Scholar]
- Estrada J et al. (2013) Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy Cell death & disease 4:e691 [DOI] [PMC free article] [PubMed]
- Fehrer C, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Fischer B, Bavister B. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Fossett E, Khan W (2012) Optimising human mesenchymal stem cell numbers for clinical application: a literature review Stem cells international 2012 [DOI] [PMC free article] [PubMed]
- Francois S, Mouiseddine M, Allenet-Lepage B, Voswinkel J, Douay L, Benderitter M, Chapel A (2013) Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects BioMed research international 2013 [DOI] [PMC free article] [PubMed]
- Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential tissue engineering part C. Methods. 2009;16:735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- Fu W-L, et al. Proliferation and apoptosis property of mesenchymal stem cells derived from peripheral blood under the culture conditions of hypoxia and serum deprivation. Chinese Medical Journal-Beijing. 2011;124:3959. [PubMed] [Google Scholar]
- Gao F, He T, Wang H, Yu S, Yi D, Liu W, Cai Z. A promising strategy for the treatment of ischemic heart disease: mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can J Cardiol. 2007;23:891–898. doi: 10.1016/s0828-282x(07)70845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration cell. Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibi B, Farzadi S, Ghuman M, Hughes FJ (2014) Inhibition of Akt/mTOR attenuates age‐related changes in mesenchymal stem cells STEM CELLS [DOI] [PubMed]
- Godara P, McFarland CD, Nordon RE. Design of bioreactors for mesenchymal stem cell tissue engineering. J Chem Technol Biotechnol. 2008;83:408–420. [Google Scholar]
- Greco SJ, Rameshwar P. Mesenchymal stem cells in drug/gene delivery: implications for cell therapy. Ther Deliv. 2012;3:997–1004. doi: 10.4155/tde.12.69. [DOI] [PubMed] [Google Scholar]
- Haider HK, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider KH, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- Haines DD, Juhasz B, Tosaki A. Management of multicellular senescence and oxidative stress. J Cell Mol Med. 2013;17:936–957. doi: 10.1111/jcmm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabian R, Tehrani HA, Jahanian-Najafabadi A, Roudkenar MH. Lipocalin-2-mediated upregulation of various antioxidants and growth factors protects bone marrow-derived mesenchymal stem cells against unfavorable microenvironments. Cell Stress and Chaperones. 2013;18:785–800. doi: 10.1007/s12192-013-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- Hamedi-Asl P, et al. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress and Chaperones. 2012;17:181–190. doi: 10.1007/s12192-011-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud M, et al. Combination of low O2 concentration and mesenchymal stromal cells during culture of cord blood CD34+ cells improves the maintenance and proliferative capacity of hematopoietic stem cells. J Cell Physiol. 2012;227:2750–2758. doi: 10.1002/jcp.23019. [DOI] [PubMed] [Google Scholar]
- Han J, Mistriotis P, Lei P, Wang D, Liu S, Andreadis ST. Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential. Stem Cells. 2012;30:2746–2759. doi: 10.1002/stem.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Fan L, Heng BC, Zigang GE. Apoptosis and metabolism of mesenchymal stem cells during chondrogenic differentiation in vitro. Int J Tissue Regen. 2013;4:61–64. [Google Scholar]
- Han S-M et al. (2014) Enhanced proliferation and differentiation of Oct4-and Sox2-overexpressing human adipose tissue mesenchymal stem cells Experimental & molecular medicine 46:e101 [DOI] [PMC free article] [PubMed]
- Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A, Jiang Y, Gui C, Sun Y, Li J, Wang JA. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can J Cardiol. 2009;25:353–358. doi: 10.1016/s0828-282x(09)70094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberts CA, Kwa M, Hermsen H. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- Higuchi O, Okabe M, Yoshida T, Fathy M, Saito S, Miyawaki T, Nikaido T. Stemness of human Wharton’s jelly mesenchymal cells is maintained by floating cultivation cellular reprogramming. Formerly “Cloning and Stem Cells”. 2012;14:448–455. doi: 10.1089/cell.2012.0020. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21:1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K‐Akt pathway in hypoxic endothelial cells to inhibit apoptosis. Increase Survival, and Stimulate Angiogenesis Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Ishikawa E, Iwasaki Y, Nakabayashi N. Inhibition of fibroblast cell adhesion on substrate by coating with 2-methacryloyloxyethyl phosphorylcholine polymers. J. Biomater Science Polymer Edition. 1999;10:1047–1061. doi: 10.1163/156856299x00676. [DOI] [PubMed] [Google Scholar]
- Ito D, Yagi T, Suzuki N (2013) [Progress in induced pluripotent stem cell research for age-related neurodegenerative diseases] Brain and nerve = Shinkei kenkyu no shinpo 65:283–288 [PubMed]
- Iwase T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- J Salgado A, L Reis R, Sousa N, M Gimble J (2010) Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine Current stem cell research & therapy 5:103–110 [DOI] [PubMed]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäderstad J, Brismar H, Herlenius E. Hypoxic preconditioning increases gap-junctional graft and host communication. Neuroreport. 2010;21:1126–1132. doi: 10.1097/WNR.0b013e328340a77b. [DOI] [PubMed] [Google Scholar]
- Jin HJ, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nature Reviews Molecular Cell Biology. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2010;19:196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Khan M, et al. Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Res Ther. 2013;4:58. doi: 10.1186/scrt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani AA, Abdi J, Halabian R, Roudkenar MH, Amirizadeh N, Soleiman Soltanpour M, Kazemi A. Over expression of HIF-1α in human mesenchymal stem cells increases their supportive functions for hematopoietic stem cells in an experimental co-culture model. Hematology. 2014;19:85–98. doi: 10.1179/1607845413Y.0000000093. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliakos I, Tsagias N, Karagiannis V. Mesenchymal cells isolation from Wharton’s jelly, in perspective to clinical applications. J Biol Res. 2011;16:194–201. [Google Scholar]
- Krinner A, Hoffmann M, Loeffler M, Drasdo D, Galle J. Individual fates of mesenchymal stem cells in vitro. BMC Syst Biol. 2010;4:73. doi: 10.1186/1752-0509-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn LT, et al. Developmental-like bone regeneration by human embryonic stem cell-derived mesenchymal cells. Tissue Eng A. 2013;20:365–377. doi: 10.1089/ten.tea.2013.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrentieva A, Majore I, Kasper C, Hass R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun Signal. 2010;8:18. doi: 10.1186/1478-811X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. American journal of stem cells. 2013;2:22. [PMC free article] [PubMed] [Google Scholar]
- Lee J-M, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. BMB Rep. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee MO, Moon BH, Shim SH, Fornace AJ, Cha HJ. Senescent growth arrest in mesenchymal stem cells is bypassed by Wip1‐mediated downregulation of intrinsic stress signaling pathways. Stem Cells. 2009;27:1963–1975. doi: 10.1002/stem.121. [DOI] [PubMed] [Google Scholar]
- Lee KA, et al. Analysis of changes in the viability and gene expression profiles of human mesenchymal stromal cells over time. Cytotherapy. 2009;11:688–697. doi: 10.3109/14653240902974032. [DOI] [PubMed] [Google Scholar]
- Lee MW, Jang IK, Yoo KH, Sung KW, Koo HH. Stem and progenitor cells in human umbilical cord blood. Int J Hematol. 2010;92:45–51. doi: 10.1007/s12185-010-0619-4. [DOI] [PubMed] [Google Scholar]
- Lee SK, et al. Phosphatase of regenerating liver‐3 promotes migration and invasion by upregulating matrix metalloproteinases‐7 in human colorectal cancer cells. Int J Cancer. 2012;131:E190–E203. doi: 10.1002/ijc.27381. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436–3443. doi: 10.1182/blood-2012-03-420612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. Bcl‐2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- Li S, Deng Y, Feng J, Ye W. Oxidative preconditioning promotes bone marrow mesenchymal stem cells migration and prevents apoptosis. Cell Biol Int. 2009;33:411–418. doi: 10.1016/j.cellbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Y, Deng Z. Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem cell research & therapy. 2013;4:1–3. doi: 10.1186/scrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal. Stem cells Cytotechnology. 2013;65:323–334. doi: 10.1007/s10616-012-9497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Xu Y, Gao C, Zhai Y (2014) Adaptive protection against damage of preconditioning human umbilical cord-derived mesenchymal stem cells with hydrogen peroxide Genetics and molecular research: GMR 13 [DOI] [PubMed]
- Lindenmair A, et al. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells. 2012;1:1061–1088. doi: 10.3390/cells1041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One. 2014;9:e107001. doi: 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TM, Wu YN, Guo XM, Hui JHP, Lee EH, Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009;18:1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- Liu N, et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med. 2011;9:105. doi: 10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TM, et al. Molecular basis of immortalization of human mesenchymal stem cells by combination of p53 knockdown and human telomerase reverse transcriptase overexpression. Stem Cells Dev. 2012;22:268–278. doi: 10.1089/scd.2012.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang R, He Z, Gao W-Q. Generation of functional organs from stem cells. Cell Regeneration. 2013;2:1. doi: 10.1186/2045-9769-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:1–20. doi: 10.1186/1756-9966-30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Li Y, Sheng Z, Wang Y. Preconditioning of stem cells for the treatment of myocardial infarction. Chin Med J (Engl) 2012;125:378–384. [PubMed] [Google Scholar]
- Lund AW, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng B Rev. 2009;15:371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y et al. (2012) SURGICAL RESEARCH REVIEW Surgery
- Ma T, Grayson WL, Fröhlich M, Vunjak‐Novakovic G. Hypoxia and stem cell‐based engineering of mesenchymal tissues. Biotechnol Prog. 2009;25:32–42. doi: 10.1002/btpr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y (2013) Immunobiology of mesenchymal stem cells Cell Death & Differentiation [DOI] [PMC free article] [PubMed]
- Mahmood A, Wu H, Qu C, Mahmood S, Xiong Y, Kaplan D, Chopp M. Down-regulation of Nogo-A by collagen scaffolds impregnated with bone marrow stromal cell treatment after traumatic brain injury promotes axonal regeneration in rats. Brain Res. 2014;1542:41–48. doi: 10.1016/j.brainres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Malard F, Mohty M (2014) New insight for the diagnosis of gastrointestinal acute graft-versus-host disease mediators of inflammation 2014 [DOI] [PMC free article] [PubMed]
- Mansouri L, Xie Y, Rappolee DA. Adaptive and pathogenic responses to stress by stem cells during development. Cells. 2012;1:1197–1224. doi: 10.3390/cells1041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, O’Brien T (2011) Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia Stem Cell Res Ther 2:12 [DOI] [PMC free article] [PubMed]
- Meier RP, et al. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013;11(3):1348–64. doi: 10.1016/j.scr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Meinel L, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S (2013) Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord BioMed research international 2013 [DOI] [PMC free article] [PubMed]
- Mertes H, Pennings G. Cross-border research on human embryonic stem cells: legal and ethical considerations. Stem Cell Rev Rep. 2009;5:10–17. doi: 10.1007/s12015-008-9046-9. [DOI] [PubMed] [Google Scholar]
- Mirotsou M, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh M, et al. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress and Chaperones. 2012;17:553–565. doi: 10.1007/s12192-012-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll G et al (2012) Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 30(7):1565–74 [DOI] [PubMed]
- Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine Experimental & molecular medicine 45:e54 [DOI] [PMC free article] [PubMed]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Muturi HT, Dreesen JD, Nilewski E, Jastrow H, Giebel B, Ergun S, Singer BB. Tumor and endothelial cell-derived microvesicles carry distinct CEACAMs and influence T-cell behavior. PLoS One. 2013;8:e74654. doi: 10.1371/journal.pone.0074654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi‐Meshkin H, Bahrami AR, Bidkhori HR, Mirahmadi M, Ahmadiankia N (2014) Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy Cell biology international [DOI] [PubMed]
- Noiseux N, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2004;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Nystedt J, et al. Cell surface structures influence lung clearance rate of systemically infused mesenchymal stromal cells. Stem Cells. 2013;31(2):317–26. doi: 10.1002/stem.1271. [DOI] [PubMed] [Google Scholar]
- Oh JS, et al. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett. 2010;472:215–219. doi: 10.1016/j.neulet.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng A. 2012;18:1479–1489. doi: 10.1089/ten.tea.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos KA, Bird NC. Liver regeneration and its impact on post-hepatectomy metastatic tumour recurrence. Anticancer Res. 2010;30:2161–2170. [PubMed] [Google Scholar]
- Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- Pendergrass KD, Boopathy AV, Seshadri G, Maiellaro-Rafferty K, Che PL, Brown ME, Davis ME. Acute preconditioning of cardiac progenitor cells with hydrogen peroxide enhances angiogenic pathways following ischemia-reperfusion injury. Stem Cells Dev. 2013;22:2414–2424. doi: 10.1089/scd.2012.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochampally RR, Smith JR, Ylostalo J, Prockop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103:1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- Ponader S, Burger JA. Modeling the marrow stem cell niche in vitro: is proximity the key to reproduction? Haematologica. 2010;95:e5. doi: 10.3324/haematol.2010.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Andriamanalijaona R, Pujol J-P, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- Przybyla LM, Theunissen TW, Jaenisch R, Voldman J (2013) Matrix remodeling maintains embryonic stem cell self‐renewal by activating atat3 stem cells 31:1097–1106 [DOI] [PMC free article] [PubMed]
- Ramsey F (2011) A simple hanging drop cell culture protocol for generation of 3D spheroids Journal of Visualized Experiments [DOI] [PMC free article] [PubMed]
- Ratajczak MZ. The emerging role of microvesicles in cellular therapies for organ/tissue regeneration. Nephrol Dial Transplant. 2011;26:1453–1456. doi: 10.1093/ndt/gfr165. [DOI] [PubMed] [Google Scholar]
- Ren H, et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- Ren G, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem cells translational medicine. 2012;1:51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Ribeiro A, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem cell research & therapy. 2013;4:125. doi: 10.1186/scrt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Faiola F, Wang J. Concise review: pursuing self‐renewal and pluripotency with the stem cell factor Nanog. Stem Cells. 2013;31:1227–1236. doi: 10.1002/stem.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Scaglione S, Giannoni P, Quarto R (2011) Cell-biomaterial interactions reproducing a Niche ADVANCES IN REGENERATIVE MEDICINE:363
- Seaberg RM, Van Der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26(3):125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Shi W, et al. BDNF blended chitosan scaffolds for human umbilical cord MSC transplants in traumatic brain injury therapy. Biomaterials. 2012;33:3119–3126. doi: 10.1016/j.biomaterials.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Shtrichman R, Germanguz I, Eldor JI. Induced pluripotent stem cells (iPSCs) derived from different cell sources and their potential for regenerative and personalized medicine. Current molecular medicine. 2013;13:792–805. doi: 10.2174/1566524011313050010. [DOI] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three‐dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation—lessons from chondrogenesis. J Cell Sci. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, et al. The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells Dev. 2013;23:654–663. doi: 10.1089/scd.2013.0277. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal. Stem cells Rejuvenation research. 2006;9:31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Sellers D, Llewelyn O, Scutt A. Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs. 2010;191:453–465. doi: 10.1159/000281826. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Bauer E, Scutt A. Suspension cultures of bone-marrow-derived mesenchymal stem cells: effects of donor age and glucose level. Stem Cells Dev. 2012;21:2718–2723. doi: 10.1089/scd.2011.0406. [DOI] [PubMed] [Google Scholar]
- Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tan J, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- Tang J, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- Tang YL, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarte K, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7:105–115. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh WS, Lee EH, Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev Rep. 2011;7:544–559. doi: 10.1007/s12015-010-9222-6. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult. Murine heart Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tower J (2012) Stress and stem cells. WIREs Dev Biol 1:789–802 [DOI] [PMC free article] [PubMed]
- van den Akker F, de Jager S, Sluijter J (2013) Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of toll-like receptors Mediators of inflammation 2013 [DOI] [PMC free article] [PubMed]
- Van Der Sanden B, Dhobb M, Berger F, Wion D. Optimizing stem cell culture. J Cell Biochem. 2010;111:801–807. doi: 10.1002/jcb.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JA et al (2008) Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 29:74–82 [DOI] [PubMed]
- Wang X, et al. Hsp20‐engineered mesenchymal stem cells Are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. Human mesenchymal stem cell transplantation changes proinflammatory gene expression through a nuclear factor‐κB‐dependent pathway in a rat focal cerebral ischemic model. J Neurosci Res. 2013;91(11):1440–9. doi: 10.1002/jnr.23267. [DOI] [PubMed] [Google Scholar]
- Wang F et al. (2014a) Cytoprotective effect of melatonin against hypoxia/serum deprivation-induced cell death of bone marrow mesenchymal stem cells in vitro European journal of pharmacology [DOI] [PubMed]
- Wang X, Mei J, Liao C, Zhang L, Li M, Ding Q, Li S. Relationship between mesenchymal stem cells and liver cancer recurrence after liver transplantation in mice. Zhonghua yi xue za zhi. 2014;94:455–458. [PubMed] [Google Scholar]
- Wei H, Li Z, Hu S, Chen X, Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem. 2010;111:967–978. doi: 10.1002/jcb.22785. [DOI] [PubMed] [Google Scholar]
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whu SW, Tsai C-L, S-h H. Evaluation of human bone marrow mesenchymal stem cells seeded into composite scaffolds and cultured in a dynamic culture system for neocartilage regeneration in vitro. J Med Biol Eng. 2009;29:52–59. [Google Scholar]
- Willerth SM, Sakiyama-Elbert SE (2008) Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery [PubMed]
- Wong RS (2011) Mesenchymal stem cells: angels or demons? BioMed Research International 2011
- Wu Y-h, Wang J, Gong D-x G, H-y H, S-s ZH (2012) Effects of low-level laser irradiation on mesenchymal stem cell proliferation: a microarray analysis. Lasers Med Sci 27:509–519 [DOI] [PubMed]
- Xu J, et al. High density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via activation of the PI3K/Akt pathway and suppression of reactive oxygen species. Int J Mol Sci. 2012;13:17104–17120. doi: 10.3390/ijms131217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Wang J, Chen SC, Hsieh YL (2013) Synergistic effects of low‐level laser and mesenchymal stem cells on functional recovery in rats with crushed sciatic nerves Journal of tissue engineering and regenerative medicine [DOI] [PubMed]
- Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- Yoon D, Kim Y, Jung H, Paik S, Lee J. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low‐density culture. Cell Prolif. 2011;44:428–440. doi: 10.1111/j.1365-2184.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Hydrogen peroxide preconditioning enhances the therapeutic efficacy of Wharton’s jelly mesenchymal stem cells after myocardial infarction. Chin Med J (Engl) 2012;125:3472–3478. [PubMed] [Google Scholar]
- Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation‐induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–45. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]