Abstract
Background
Members of the bacterial genus Arthrobacter are both readily cultured and commonly identified in Antarctic soil communities. Currently, relatively little is known about the physiological traits that allow these bacteria to survive in the harsh Antarctic soil environment. The aim of this study is to investigate if Antarctic strains of Arthrobacter owe their resilience to substantial genomic changes compared to Arthrobacter spp. isolated from temperate soil environments.
Results
Quantitative PCR-based analysis revealed that up to 4% of the soil bacterial communities were comprised of Arthrobacter spp. at four locations in the Ross Sea Region. Genome analysis of the seven Antarctic Arthrobacter isolates revealed several features that are commonly observed in psychrophilic/psychrotolerant bacteria. These include genes primarily associated with sigma factors, signal transduction pathways, the carotenoid biosynthesis pathway and genes induced by cold-shock, oxidative and osmotic stresses. However, these genes were also identified in genomes of seven temperate Arthrobacter spp., suggesting that these mechanisms are beneficial for growth and survival in a range of soil environments. Phenotypic characterisation revealed that Antarctic Arthrobacter isolates demonstrate significantly lower metabolic versatility and a narrower salinity tolerance range compared to temperate Arthrobacter species. Comparative analyses also revealed fewer protein-coding sequences and a significant decrease in genes associated with transcription and carbohydrate transport and metabolism in four of the seven Antarctic Arthrobacter isolates. Notwithstanding genome incompleteness, these differences together with the decreased metabolic versatility are indicative of genome content scaling.
Conclusions
The genomes of the seven Antarctic Arthrobacter isolates contained several features that may be beneficial for growth and survival in the Antarctic soil environment, although these features were not unique to the Antarctic isolates. These genome sequences allow further investigations into the expression of physiological traits that enable survival under extreme conditions and, more importantly, into the ability of these bacteria to respond to future perturbations including climate change and human impacts.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-015-1220-2) contains supplementary material, which is available to authorized users.
Keywords: Antarctica, Comparative genomics, Arthrobacter
Background
Antarctica is largely covered by glacial ice sheets, with ice-free areas making up ~0.32% of the entire continental land mass [1]. Of these ice-free areas, 90% are located along the continental coastline and occur on the Antarctic Peninsula and the Ross Sea Region (RSR). Soils of the RSR are exposed to a wide range of environmental extremes including physical extremes of temperature and elevated ultraviolet (UV) radiation, as well as geochemical extremes of high salinity, low water and low nutrient availability [2]. Together these environmental conditions make Antarctic soils some of the harshest environments on Earth.
Prior to the advent of molecular ecology techniques, cultivation- and microscopy-based studies had reported that Antarctic soils are dominated by a few, cosmopolitan groups of bacteria [3,4]. However, modern molecular methods have allowed for a more accurate assessment of bacterial community composition. Pyrosequencing and clone libraries of the 16S rRNA gene from RSR soils have identified representatives of 15 phyla including Acidobacteria, Actinobacteria, Armatimonadetes, Bacteroidetes, Chloroflexi, Cyanobacteria, Deinococcus-Thermus, Firmicutes, Gemmatimonadetes, Nitrospira, Planctomycetes, Proteobacteria, Spirochaetes, Verrucomicrobia and Candidate ‘TM7’ [5-13].
While modern techniques have aided our understanding of bacterial diversity in Antarctic terrestrial environments, they have also revealed adaptive mechanisms of psychrophilic organisms through genomic data. At the time of writing, genomes of 46 psychrophilic/psychrotolerant bacteria and archaea were complete and published (as reviewed by De Maayer et al. [14]). Of these, just four studies have investigated psychrophilic/psychrotolerant organisms isolated from Antarctic environments including Methanococcoides burtonii from Ace Lake, Vestfold Hills [15,16], Exiguobacterium antarcticum from microbial mats, Lake Fryxell [17], Octadecabacter antarcticus from Antarctic sea ice [18], and Cellulophaga algicola from the surface of a sea-ice diatom Melosira, East Antarctica [19]. These studies have reported the presence of cold adaptation relating to membrane modification, compatible solute accumulation, reactive oxygen species (ROS) detoxification, and significant changes in bacterial protein sequences including reduction in charged residues, hydrophobic clusters and proline content. These genomes have provided an insight into the lifestyle of psychrophilic microorganisms in permanently cold environments (<5°C) such as the Antarctic marine and lacustrine environments. However, there remains a gap in our knowledge of the adaptive mechanisms of psychrotolerant bacteria isolated from Antarctic terrestrial environments wherein large temperature fluctuations are common. In soils of the RSR, members of the Acidobacteria, Actinobacteria, Bacteroidetes, Deinococcus-Thermus and Proteobacteria phyla represent dominant taxa [5-7,9-12]. Of these, Actinobacteria are of special note. The phylum Actinobacteria is composed of phylogenetically diverse organisms that have been primarily investigated for their ability to cause disease in plants and animals, to produce anti-microbial compounds and anti-tumour agents, and to degrade recalcitrant molecules in soil environments [20]. Within Actinobacteria, members of the genus Arthrobacter are of note as they are among the most frequently isolated bacteria, occurring most commonly in soils and environments contaminated with industrial chemicals and radioactive materials. Their ubiquity can be attributed to their nutritional versatility and their resistance to environmental stressors [21]. At the time of writing, Arthrobacter included 82 species with validly published names (http://www.bacterio.net/a/). Of these, complete and published genomes are available for just six species, namely A. aurescens TC1, A. chlorophenolicus A6, Arthrobacter sp. FB24, A. nitroguajacolicus Rue61a, A. phenanthrenivorans Sphe3, and A. arilaitensis re117 [22].
In soils of the RSR, Arthrobacter species are both readily cultured and commonly observed in 16S rRNA gene clone libraries. Furthermore, they can be dominant in the soil environment as observed in soils on the Hatherton Drift, Transantarctic Mountains [5]. Despite their prevalence in soils of the RSR, very little is known about the physiological traits that allow these organisms to survive, flourish and establish dominance in the harsh Antarctic soil environment. A key question is if Antarctic strains of Arthrobacter owe their resilience to substantial genomic changes compared to Arthrobacter spp. isolated from temperate soil environments. Therefore, the three objectives of this study were (1) to investigate the abundance and diversity of Arthrobacter species found in soil microbial communities at four locations in the RSR, (2) to compare genomes of seven Antarctic Arthrobacter isolates with seven temperate Arthrobacter spp., focusing on traits that may contribute to survival and growth in the Antarctic soil environment, and (3) to investigate the metabolic versatility and salinity tolerance range of Antarctic Arthrobacter isolates compared to three temperate, soil-dwelling Arthrobacter species. For this, a combination of genotypic and phenotypic techniques including quantitative PCR (qPCR), whole genome sequencing and BIOLOG’s Phenotype Microarray (PM) technologies were utilised. To our knowledge this is the first study to provide genomic and phenotypic insights into the metabolic potential and ecological role of Arthrobacter strains isolated from RSR soils.
Results and discussion
Abundance and diversity of Arthrobacter spp. in soils of RSR
A total of eight samples from two soil depths at four locations within the RSR was investigated by qPCR (Figure 1) to determine the relative abundance of members of the phylum Actinobacteria and genus Arthrobacter. Specificity of the qPCR assays were tested by clone library preparations with DNA from three Antarctic soil samples and a marine sponge sample. All sequenced clones belonged to the correct target group. The relative abundance of each bacterial taxon was calculated as a ratio of measured copy numbers for each taxon-specific qPCR assay to measured copy numbers for the ‘all Bacteria’ assay. Members of Actinobacteria and Arthrobacter were present at all sample sites. Actinobacteria represented approximately 10-40% of the bacterial community at all soil locations, consistent with published data based on 16S rRNA gene clone libraries (as observed in Figure 1) [5-9,23]. In this study, up to 4% of the bacterial community was comprised of Arthrobacter species, with the lowest relative abundance observed in soils of Minna Bluff and the highest in soils of Granite Harbour. These results were also broadly consistent with published data (as observed in Figure 1) [5-9,23].
Figure 1.

Relative abundance of bacteria belonging to the phylum Actinobacteria and genus Arthrobacter in soils of RSR determined by 16S rRNA gene clone libraries [ 5 - 7 , 9 , 23 ] and by qPCR data (this study). Black bar represents Actinobacteria, grey bar represents Arthrobacter. Bar represents standard deviation for qPCR data. Sample sites: SB, Scott Base; MP, Marble Point; MB, Minna Bluff; GH, Granite Harbour; LV, Luther Vale.
Phylogenetic analysis was performed on 16S rRNA gene sequences of clones and isolates associated with Arthrobacter from soils of the RSR. This analysis revealed that clones and isolates clustered together, clearly illustrating that the cultured isolates are representative of Arthrobacter spp. observed in soil bacterial communities (Additional file 1). Of these, seven isolates that represent the phylogenetic diversity of Arthrobacter spp. occurring in RSR soils were chosen for subsequent comparative analyses by whole genome sequencing and phenotypic characterisation.
Genome analyses
Genome overview
Genomes of the seven Antarctic Arthrobacter isolates are composed of the chromosome, each constructed from varying numbers of DNA scaffolds ranging from 53 to 158. The completeness of the seven genomes ranged from 78-98%, assessed by the occurrence of essential, single-copy genes. Due to this incompleteness, one must regard with caution the apparent absence or low copy number of a given gene. General genome features of the seven Antarctic Arthrobacter strains, as compared with the seven temperate Arthrobacter spp., are listed in Table 1. The genome G + C content for the seven Antarctic strains ranges from approx. 61-65%, similar to that for the seven temperate Arthrobacter species. The genomes contain 3,429-4,772 open reading frames (ORFs) with an average coding density of 88%. While genomes of strains I3, H5 and H14 contain 4,703, 4,552 and 4,566 protein-coding sequences (CDSs) respectively, significantly fewer CDSs were observed in genomes of strains H20 (3,466), 35/47 (3,470), Br18 (3,575) and H41 (3,373) (P < 0.01). For each genome, of the total CDSs, approx. 74% of the CDSs were classified into clusters of orthologous groups (COG) categories. Notably, the highest percentage of genes was assigned to COG categories such as amino acid transport and metabolism [E], carbohydrate transport and metabolism [G], and transcription [K] (Figure 2). Putative horizontally transferred genes, identified by the Joint Genome Institute (JGI) annotation pipeline, constituted 1.53-3.79% of the total genes observed. All Antarctic strains harboured a high number of genes associated with mobile genetic elements (up to 2.4% of total genes) encoding for phage integrases, transposases, and other phage elements (Additional file 2). Two putative phage sequences were identified in the genome of strain Br18, and genomes of strains H20 and H14 contained one putative phage sequence each (Additional file 3) [24]. No phage sequences were identified in the remaining four Antarctic strains. Significantly fewer copies of the 16S rRNA gene were identified in Antarctic Arthrobacter genomes as compared to the temperate Arthrobacter spp. (P <0.05).
Table 1.
General genome features of seven Antarctic Arthrobacter strains (this study) vs. seven temperate Arthrobacter spp. [22]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome data | ||||||||||||||
| Genome size (bp) | 5,226,648 | 4,585,800 | 4,980,870 | 4,954,410 | 5,081,038 | 4,535,320 | 5,070,478 | 3,554,370 | 3,562,925 | 4,340,329 | 3,452,356 | 3,321,099 | 4,379,674 | 4,835,497 |
| DNA coding region (%) | 4,602,246 (88.05) | 4,096,643 (89.33) | 4,458,001 (89.50) | 4,420,17 (89.23) | 4,572,987 (90) | 4,024,613 (88.74) | 4,552,065 (89.78) | 3,169,499 (89.17) | 3,140,572 (88.15) | 3,765,602 (86.76) | 3,090,905 (89.53) | 2,903,224 (87.42) | 3,887,579 (88.76) | 4,164,528 (86.12) |
| G + C content (%) | 62.43 | 63.58 | 65.96 | 66.21 | 62.25 | 65.37 | 65.39 | 63.76 | 65.28 | 60.57 | 62.30 | 63.98 | 61.29 | 65.18 |
| Scaffold count | 3 | 49 | 3 | 125 | 3 | 3 | 4 | 53 | 120 | 158 | 88 | 148 | 104 | 124 |
| Total RNA genes | 94 | 64 | 103 | 53 | 71 | 64 | 86 | 58 | 68 | 67 | 63 | 56 | 64 | 69 |
| tRNA genes | 54 | 46 | 88 | 50 | 53 | 50 | 51 | 43 | 51 | 48 | 44 | 44 | 49 | 53 |
| 16S rRNA genes | 6 | 2 | 5 | 1 | 6 | 4 | 5 | 2 | 1 | 3 | 2 | 1 | 2 | 2 |
| Other RNA genes | 20 | 10 | - | - | - | 2 | 20 | 9 | 11 | 12 | 12 | 9 | 9 | 9 |
| Total number of genes | 4,793 | 4,516 | 4,744 | 4,592 | 4,655 | 4,273 | 4,622 | 3,528 | 3,643 | 4,633 | 3,529 | 3,429 | 4,616 | 4,772 |
| Total protein CDSs (%) | 4699 (98.04) | 4452 (98.58) | 4641 (97.83) | 452 (98.84) | 4584 (98.47) | 4209 (98.5) | 4536 (98.14) | 3470 (98.36) | 3575 (98.13) | 4566 (98.55) | 3466 (98.21) | 3373 (98.37) | 4552 (98.61) | 4703 (98.55) |
| With function prediction (%) | 3419 (71.33) | 3429 (75.93) | 3125 (65.87) | 2784 (60.76) | 3800 (81.63) | 3101 (72.57) | 3279 (70.94) | 2793 (79.17) | 2750 (75.49) | 3320 (71.66) | 2660 (75.38) | 2614 (76.23) | 3393 (73.51) | 3630 (76.07) |
| Without function prediction (%) | 1280 (26.71) | 1023 (22.65) | 1516 (31.96) | 1745 (38.08) | 784 (18.64) | 1108 (25.93) | 1257 (27.20) | 677 (19.19) | 825 (22.65) | 1246 (26.89) | 806 (22.84) | 759 (22.13) | 1159 (25.11) | 1073 (22.49) |
| With COGs (%) | 3307 (69) | 3339 (73.94) | 3124 (65.84) | 3546 (77.39) | 3625 (77.87) | 3297 (77.16) | 3361 (72.72) | 2743 (77.75) | 2697 (74.03) | 3221 (69.52) | 2588 (73.34) | 2584 (75.36) | 3307 (71.64) | 3591 (75.25) |
| Horizontally transferred genes (%) | 242 (5.05) | 188 (4.16) | 239 (5.04) | 83 (1.81) | - | 68 (1.59) | 149 (3.22) | 73 (2.07) | 97 (2.66) | 129 (2.78) | 117 (3.32) | 74 (2.16) | 175 (3.79) | 73 (1.53) |
| Genome Completeness (%) | - | - | - | - | - | - | - | 92.30 | 98 | 94.23 | 90.38 | 77.88 | 97.12 | 98 |
| Metadata | ||||||||||||||
| Isolation source | South Dakota | Mural painting Austria | Fort Collins, Colorado | Geneva, New York | Ruetgerswerke AG, Germany | Perivleptos, Greece | Seymour, Indiana | Scott Base, Ross Sea Region | Britannia, Darwin Mountains | Hatherton, Darwin Mountains | Hatherton, Darwin Mountains | Hatherton, Darwin Mountains | Hatherton, Darwin Mountains | Isca, Darwin Mountains |
| Habitat | Atrazine enriched soil | Biofilm | Soil | Soil | Sludge | Soil | Soil | Soil | Soil | Soil | Soil | Soil | Soil | Soil |
| Colony colour | NR | Light yellow on NA** | Pearl grey on PC# | Cream on SA$ | Yellow on NA% | Yellow-cream on LB$$ | NR | Yellow | Pink/red | cream | Yellow | Pink/red | Cream | cream |
| Temperature range (°C) | 30* | 15-37** | 3-37# | NR | 4-37% | 4-37$$ | 4-37%% | 5-37 | 5-25## | 5-25## | 5-25## | 5-25## | 5-25## | 5-25## |
1, A. aurescens TC1; 2, A. castelli DSM 16402; 3, A. chlorophenolicus A6; 4, A. globiformis NBRC 12137; 5, A. nitroguajacolicus Rue61a; 6, A. phenanthrenivorans Sphe3; 7, Arthrobacter sp. FB24; 8, 35/47; 9, Br18; 10, H14; 11, H20; 12, H41; 13, H5; 14, I3.
For Antarctic isolates, colony colour observed following incubation at 15°C for 3–4 d on R2A agar.
*, Data from [25]; **, Data from [26]; #, Data from [27]; $, Data from [28]; %, Data from [29]; $$, Data from [30]; %%, Data from [31]; ##, Data from [5].
NA, Nutrient Agar; LA, Luria-Bertani Agar; P-C, Peptone-Carbohydrate Agar; SA, Standard Agar; NR, not reported.
Figure 2.

Comparison of gene content in seven temperate Arthrobacter spp. and seven Antarctic Arthrobacter isolates by COG categories. Asterisks represent abundant COG categories. Letters K, G, and E represent COG categories transcription, carbohydrate and amino acid transport and metabolism, respectively.
General genome comparisons
General comparisons between genomes of seven temperate Arthrobacter spp. and seven Antarctic Arthrobacter strains were carried out using CMG-Biotools [32]. Firstly, amino acid usage was calculated for all 14 Arthrobacter isolates using their protein sequences. The amino acid usage tree (Figure 3) shows three main clusters. This clustering pattern is similar to the clustering observed in the maximum likelihood tree based on 16S rRNA gene sequences (Figure 4). This analysis revealed that Ala, Gly, Leu, and Val are the most frequently used amino acids across all Arthrobacter genomes. Predicted proteome comparisons and a pan- and core-genome plot analysis were also performed on all 14 Arthrobacter genomes using CMG-Biotools [32]. Proteomes were predicted for each isolate using Prodigal [33] and then BLAST algorithm (Basic Local Alignment Search Tool)-based proteome comparisons were performed to identify whether proteins are shared between predicted proteomes [34]. In Figure 5, the main part of the matrix (shaded green) consists of pairwise proteome comparisons and the bottom row (shaded red) represents a self-comparison where a hit within the proteome to a protein other than the query is identified as an internal homolog or a paralog. The BLAST matrix illustrates that conservation between Antarctic Arthrobacter genomes (24.4-53.6%) is low as compared to genomes of Arthrobacter spp. isolated from temperate soil environments (42.2-76.6%) (excluding A. castelli that was isolated from the biofilm of a mural). This observation was also supported by the pan- and core-genome plot analysis (Additional file 4), as the Antarctic core- and pan-genome comprised 1,285 and 10,873 gene families respectively and the temperate core- and pan-genome comprised 1,559 and 9,798 gene families respectively. Approximately 4.8% of the total CDSs were in paralogous clusters across all Arthrobacter genomes. The final Arthrobacter pan-genome comprised 14,902 sequences, indicative of a large diversity of accessory genes. The Arthrobacter core-genome is comprised of 1,153 gene families, representing approx. 27% of the total genes in Arthrobacter genomes. A large proportion of genes in the Antarctic and temperate core-genomes were assigned to COG categories including amino acid transport and metabolism [E], carbohydrate transport and metabolism [G] and translation, ribosomal structure and biogenesis [J].
Figure 3.
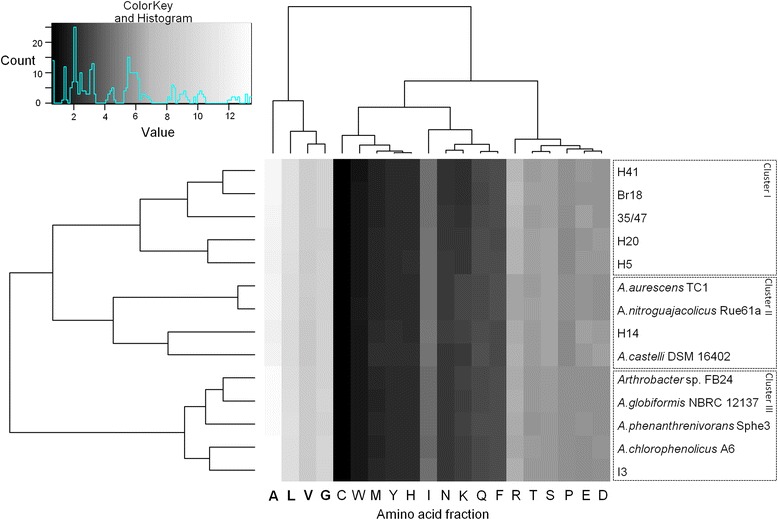
Amino acid usage heatmap of seven temperate Arthrobacter spp. and seven Antarctic Arthrobacter isolates based on their protein content. The percentage of amino acid usage was plotted in gplots using R. Amino acids highlighted in bold face represent abundant amino acids.
Figure 4.
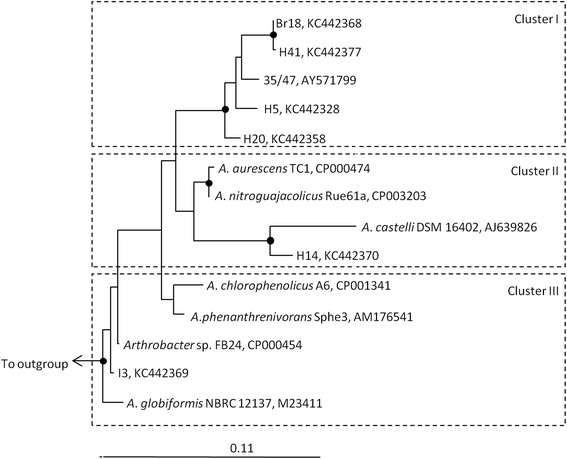
Maximum likelihood phylogenetic tree based on 16S rRNA gene sequences from seven Antarctic Arthrobacter isolates and seven temperate Arthrobacter species. Filled circles indicate bootstrap support of >90%, and open circles represent >75% support (maximum parsimony, 1000 resamplings). Bar, 0.11 substitutions per compared nucleotide site. Outgroup comprises Microbacterium maritypicum, AM 181506 and M. profundi, EF623999.
Figure 5.

BLAST matrix of an all against all protein comparison of 14 Arthrobacter genomes. The blue box contains genomes of seven temperate Arthrobacter spp. and the red box contains genomes of seven Antarctic Arthrobacter isolates. #, Note that proteins represent total CDSs and families represent CDSs not in paralogous clusters (unique CDSs). Red diamonds represent protein families in paralogous clusters.
Genomic traits linked to environmental stress-related adaptation
Several known adaptive mechanisms for growth and survival in cold terrestrial environments were identified in the genomes of all 14 Arthrobacter isolates and are summarised in Table 2 (Additional file 5), and in the following categorical descriptions.
Table 2.
List of genes linked to environmental stress response
| Product name | Gene symbol | Enzymes | COGs | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIGMA FACTORS | |||||||||||||||||
| DNA-directed RNA polymerase sigma 24 | rpoE | - | COG1595 | 15 | 11 | 9 | 6 | 14 | 8 | 9 | 7 | 7 | 13 | 9 | 7 | 12 | 9 |
| DNA-directed RNA polymerase sigma 28 | fliA | - | COG1191 | 1 | - | 2 | 1 | 1 | 1 | 1 | - | 1 | - | 1 | 2 | - | 2 |
| DNA-directed RNA polymerase, sigma 70 | rpoD | - | COG0568 | 1 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 1 | 1 | 1 | 1 |
| OXIDATIVE STRESS RESPONSE | |||||||||||||||||
| Superoxide dismutase | sodA | EC:1.15.1.1 | COG0605 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cu/Zn superoxide dismutase | sodC | EC:1.15.1.1 | COG2032 | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| Mn-containing catalase | - | - | COG3546 | 1 | - | - | - | 1 | - | - | - | 2 | - | 1 | 1 | - | - |
| Catalase | katE | EC:1.11.1.6 | COG0753 | 2 | 2 | 2 | 1 | 2 | 3 | 2 | 1 | 3 | 2 | 2 | 2 | 2 | 1 |
| Catalase (peroxidase I) | katG | EC:1.11.1.21 | COG0376 | - | - | 1 | 1 | - | 1 | - | - | - | - | - | - | - | - |
| Peroxiredoxin | bcp | EC:1.11.1.15 | COG1225 | 2 | 3 | 3 | 1 | 3 | 3 | 2 | 5 | 4 | 4 | 3 | 4 | 4 | 6 |
| Organic hydroperoxide reductase | osmC, ohr | - | COG1764 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 3 |
| Thioredoxin | trx | EC:1.8.1.8 | COG3118 | 4 | 3 | 5 | 3 | 6 | 5 | 5 | 3 | 7 | 5 | 4 | 3 | 5 | 4 |
| Thioredoxin reductase | trxB | EC:1.8.1.9 | COG0492 | 4 | 4 | 3 | 3 | 3 | 6 | 2 | 4 | 5 | 4 | 1 | 4 | 2 | 2 |
| Thiol-disulfide isomerase and thioredoxins | trxA | - | COG0526 | 2 | 3 | 2 | - | 1 | 2 | 5 | 4 | 3 | 2 | 2 | 1 | 3 | 1 |
| Redox-sensitive transcriptional activator | soxR | - | COG0789 | 1 | - | - | - | 1 | - | - | - | - | 2 | 1 | - | 1 | - |
| OSMOPROTECTION | |||||||||||||||||
| Glycogen metabolism | |||||||||||||||||
| Glycogen synthase | glgA | EC:2.4.1.21 | COG0438 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1,4-alpha-glucan branching enzyme | glgB | EC:2.4.1.18 | COG0296 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ADP-glucose pyrophosphorylase | glgC | EC:2.7.7.27 | COG0448 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 |
| Glucan phosphorylase | glgP | EC:2.4.1.1 | COG0058 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Glycogen debranching enzyme | glgX | EC:3.2.1.33 | COG1523 | 2 | 2 | 2 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Trehalose metabolism | |||||||||||||||||
| Trehalose-6-phosphate synthase | otsA | EC:2.4.1.15 | COG0380 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Trehalose-6-phosphatase | otsB | EC:3.1.3.12 | COG1877 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Trehalose synthase | treS | EC:3.2.1.1, EC:5.4.99.16 | COG0366 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Maltooligosyl trehalose synthase | treY | EC:5.4.99.15 | COG3280 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Malto-oligosyltrehalose trehalohydrolase | treZ | EC:3.2.1.141 | COG0296 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Glycine betaine/proline ABC transporter | |||||||||||||||||
| glycine betaine/proline ABC transporter - ATP binding subunit | proV | - | COG1125 | 2 | 3 | 1 | 1 | 3 | 1 | 1 | 2 | 3 | 3 | 2 | 3 | 3 | 1 |
| glycine betaine/proline ABC transporter - membrane subunit | proW | - | COG1174 | 2 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 6 | 6 | 4 | 6 | 6 | 2 |
| glycine betaine/proline ABC transporter - periplasmic binding protein | proX | - | COG1732 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 3 | 2 | 3 | 3 | 1 |
| COLD SHOCK RESPONSE | |||||||||||||||||
| Cold shock' DNA binding domain | csd | - | COG1278 | 3 | 4 | 5 | 4 | 3 | 4 | 4 | 3 | 3 | 4 | 3 | 3 | 2 | 4 |
| CspA-like cold acclimation protein | capA | - | COG1278 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | - | - | 1 | 1 |
| Transcription elongation factor | nusA | - | COG0195 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Polyribonucleotide nucleotidyltransferase | pnp | EC:2.7.7.8 | COG1185 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ribosome-binding factor A | rbfA | - | COG0858 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Translation initiation factor 1 | infA | - | COG0361 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 |
| Translation initiation factor 2 | infB | - | COG0532 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| MEMBRANE ADAPTATIONS | |||||||||||||||||
| Delta6-desaturase | desA | EC:1.14.19.3 | COG3239 | 3 | 1 | 1 | 2 | 3 | 1 | 2 | 2 | 1 | 5 | 3 | 6 | 4 | 2 |
| Carotenoid biosynthesis | |||||||||||||||||
| Isopentenyldiphosphate δ isomerase | idi | EC 5.3.3.2 | COG1443 | 1 | 1 | - | - | 1 | - | - | 1 | 1 | 1 | 1 | 1 | - | - |
| Geranylgeranyl pyrophosphate synthase | crtE | EC:2.5.1.1, EC:2.5.1.10, EC:2.5.1.29 | COG0142 | 1 | 2 | - | - | 1 | 1 | - | 2 | 2 | 1 | 2 | 4 | 2 | 1 |
| Phytoene synthase | crtB | - | COG1562 | 1 | 1 | - | - | 1 | - | - | 1 | 1 | 1 | 1 | 1 | - | - |
| Phytoene desaturase | crtI | EC:1.3.99.26, EC:1.3.99.28, EC:1.3.99.29, EC:1.3.99.31 | COG1233 | 1 | 2 | - | - | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 2 | 2 | 2 |
| Lycopene elongase | crtEB | - | COG0382 | 1 | 1 | - | - | 1 | - | - | 1 | 1 | 1 | 1 | 1 | - | - |
| Lycopene epsilon cyclase domain crtYe | crtYe | - | TIGR03462 | 1 | 1 | - | - | - | - | - | 1 | - | 1 | 1 | - | - | - |
| Lycopene epsilon cyclase domain crtYf | crtYf | - | TIGR03462 | - | 1 | - | - | - | - | - | 1 | - | 1 | 1 | - | - | - |
1, A. aurescens TC1; 2, A. castelli DSM 16402; 3, A. chlorophenolicus A6; 4, A. globiformis NBRC 12137; 5, A. nitroguajacolicus Rue61a; 6, A. phenanthrenivorans Sphe3; 7, Arthrobacter sp. FB24; 8, 35/47; 9, Br18; 10, H14; 11, H20; 12, H41; 13, H5; 14, I3.
Numbers in each column represent copy numbers per genome. Locus tags for each copy number are listed in Additional file 5.
Sigma factors
Sigma factors are dissociable units of bacterial RNA polymerase that control the conditional expression of a specific set of genes in response to a particular stress or stimulus. Copies of genes of the σ70 factor, RpoD, more commonly referred to as the house-keeping or general stress response sigma factor, were identified in all Antarctic and temperate Arthrobacter genomes. The presence of multiple copies of genes for RpoD is a common feature in psychrophilic bacteria such as Planococcus halocryophilus [35] and Psychromonas ingrahamii [36]. Further analysis also revealed the presence of several copies of genes for the σ24 factor, RpoE, associated with regulating cellular responses to heat shock and other stresses on cell membrane and periplasmic proteins in all Antarctic and temperate Arthrobacter genomes. In Escherichia coli, RpoE also regulates cell lysis in a prolonged stationary phase, thus providing nutrients for the next generation of cells [37].
Oxidative stress response
Antarctic Arthrobacter genomes contain several copies of genes encoding (putative) oxidases that contribute to the abundance of endogenous H2O2 and other ROS (Additional file 6). Furthermore, ROS are formed at a higher abundance as a result of increased oxygen solubility at low temperatures [38]. Consequently, combating free radical damage alongside surviving exposure to UV radiation is crucial for survival in the Antarctic soil environment. Genomes of the Antarctic Arthrobacter isolates contain several genes that confer protection from free radical damage and allow for detoxification of ROS. This includes up to two copies of the superoxide dismutase gene, sodA and up to three copies of the catalase gene that were identified in all Antarctic Arthrobacter genomes. Additionally, several copies of the peroxiredoxin gene, bcp and thioredoxin genes, trx, trxB were identified in all Antarctic Arthrobacter genomes. Genes for antioxidant activity have also been identified in other cold-adapted bacteria including Colwellia psychrerythraea [39], Desulfotalea psychrophila [40], and P. halocryophilus [35]. These genes were identified in genomes of all temperate Arthrobacter species. Further analysis revealed the presence of up to two copies of the redox-sensitive transcriptional activator gene, soxR in isolates H14, H20 and H5. However, the regulatory gene, soxS was absent in all Antarctic and temperate Arthrobacter genomes. In E. coli, SoxR regulates the expression of transcription factor, SoxS in response to H2O2 and other superoxide compounds. SoxS in turn activates the expression of several superoxide stress response genes. SoxR is observed without SoxS in many organisms including Pseudomonas aeruginosa, P. putida and Streptomyces coelicolor. For these bacteria, it is hypothesized that the SoxR homolog regulates the redox-active secondary metabolite, pyocyanin, which is involved in redox homeostasis [41]. However, as genomes of the Antarctic Arthrobacter strains lack homologs of genes involved in phenazine biosynthesis, we hypothesize that the SoxR regulon may directly interact with proteins involved in the superoxide stress response.
Osmotic stress response
Metabolites including compatible solutes, cryoprotectants, exopolysaccharides (EPS) and other protective polysaccharides can confer resistance to environmental stressors including UV radiation, osmotic stress, oxidation, and desiccation, thus playing a crucial role in the Antarctic soil environment. Glycogen and trehalose protect the cell from desiccation, osmotic stress and cold shock, and under nutrient limiting conditions can also serve as a source of carbon [42]. The glycogen biosynthesis pathway (from ADP-D-glucose) comprises three steps catalyzed by three enzymes, glucose-1-phosphate adenylytransferase (glgC), ADP-glucose type (glycosyl-transferring) starch/glycogen synthase (glgA) and glycogen branching enzyme (glgB). Genes (glgA, glgB and glgC) for the entire glycogen biosynthesis pathway were identified in all Antarctic and temperate Arthrobacter genomes. Genes encoding glycogen degradation enzymes, glucan phosphorylase (glgP) and glycogen debranching enzyme (glgX), were also identified in all Antarctic and temperate Arthrobacter genomes [43]. Genes for trehalose biosynthesis, trehalose-6-phosphate synthase (otsA) and trehalose-6-phosphate phosphatase (otsB) were present in all Antarctic and temperate Arthrobacter genomes. In E. coli, trehalose synthesis by enzymes OtsA and OtsB is induced by cold shock and is essential for cell viability [44]. In the psychrotolerant bacterium Arthrobacter strain A3, trehalose serves as a source of carbon, allowing cells to maintain normal metabolism at prolonged low temperatures [45]. Additional pathways for trehalose biosynthesis from maltose by the enzyme trehalose synthase (treS), and from maltodextrin by malto-oligosyl trehalose synthase (treY) and malto-oligosyl trehalose trehalohydrolase (treZ), were also identified in all Antarctic and temperate Arthrobacter genomes. Genome analysis has also revealed the presence of genes encoding a number of ABC-type transporter systems for mediating cytoplasmic accumulation of organic compatible solutes including choline, glycine betaine and proline (proV, proW, proX) [46] in all Antarctic and temperate Arthrobacter genomes. Genome surveys of psychrophilic bacteria and archaea also revealed the presence of multiple genes for the uptake and/or synthesis of compatible solutes, illustrating the importance of these compounds for osmoprotection and cryoprotection in cold environments [47,48].
A large number of genes involved in several sugar biosynthesis pathways were identified in all Antarctic and temperate Arthrobacter genomes (Additional file 7). EPS produced by members of the genera Pseudoalteromonas, Shewanella, Polaribacter, and Flavobacterium isolated from the Antarctic marine environment was largely composed of neutral sugars including glucose, fucose, and mannose, amino sugars including N-acetyl galactosamine and N-acetyl glucosamine and uronic acids including galacturonic and glucuronic acids. In these bacteria, EPS may protect cells from the extremes of low temperature and high salinity in the marine environment [49]. EPS production has also been reported in psychrophilic bacteria such as C. psychrerythraea [39] and Psychromonas ingrahamii [36]. The presence of a wide range of osmoprotection systems suggests that the Antarctic Arthrobacter isolates are well equipped to cope with desiccation and osmotic stress.
Cold shock response
Cold shock can result in the inhibition of bacterial cell growth and proliferation as a result of stabilization of DNA and RNA secondary structures, reduction in membrane fluidity and solute uptake. Therefore, upon exposure to a sudden temperature downshift, bacteria respond with a specific pattern of transient gene expression of members of a family of small, nucleic acid-binding cold-shock proteins (CSPs). CSPs are of note as they regulate messenger RNA (mRNA) translation, rate of mRNA degradation and transcription termination, all of which are dependent on the ribosome that is targeted by cold shock [50-52]. Several copies of the DNA-binding cold-shock proteins were identified in all Antarctic and temperate Arthrobacter genomes similar to the multiple copies observed in C. psychrerythraea [39], Psychrobacter arcticus [53] and Shewanella oneidensis [54]. Additionally, genes encoding cold-shock-inducible proteins: ribosome-binding factor A (rbfA), translation initiation factors, IF-1 and IF-2 (infA, infB), polynucleotide phosphorylase (pnp), RNA-binding cold-shock domain A (csdA) and NusA, N-using substance protein A (nusA) were identified in the Antarctic and temperate Arthrobacter genomes [55]. Homologs of the CspA-like cold acclimation protein, CapA, were identified in genomes of four Antarctic Arthrobacter isolates, 35/47, H14, H5 and I3. Unlike CSPs that are transiently expressed upon cold shock, in A. globiformis S155, CapA was over-expressed following cold shock and during prolonged growth at low temperatures [51]. The presence of the CapA homolog in Antarctic Arthrobacter genomes may explain the survival of these organisms at prolonged low temperatures. Genes for CapA were also identified in genomes of all temperate Arthrobacter species.
Cell membrane adaptations
In the Antarctic soil environment, maintaining membrane permeability and fluidity at sub-zero temperatures is crucial for continued cell viability. Genome analyses of Antarctic Arthrobacter isolates have revealed the presence of several genes for fatty acid desaturases (des), which are important in this context. Upon cold shock, the expression of des genes is regulated by a two-component signal transduction pathway, comprising a membrane-integrated histidine kinase that senses change in membrane fluidity and a response regulator that binds the promoter region of des genes. Together they increase the production of desaturases that add double bonds into pre-existing fatty acid tails within the membrane, thus restoring membrane fluidity. Genes for desaturases were also observed in genomes of all temperate Arthrobacter spp., similar to the copies observed in the psychrotolerant bacterium, Exiguobacterium sibiricum [56]. In Bacillus subtilis, the DesKR system regulates the expression of the des gene coding for ∆5 desaturase [57,58]. All Antarctic Arthrobacter isolates contained several copies of genes associated with signal transduction mechanisms (approx. 3% of the total CDSs) (Additional file 8). A similar proportion of genes (approx. 4% of the total CDSs) were attributed to signal transduction mechanisms in genomes of the temperate Arthrobacter species.
Carotenoid biosynthesis also contributes to cold adaptation by stabilizing the cell membrane, maintaining proton permeability and by promoting oxidative stress resistance [59,60]. Pigmentation is a common feature of Antarctic Arthrobacter isolates. Genes involved in the synthesis of lycopene (red-coloured) have been identified in Antarctic Arthrobacter strains H41 and Br18. Decaprenoxanthin (yellow-coloured) biosynthesis genes have been identified in Antarctic Arthrobacter strains H14, H20, and 35/47 [61]. These findings are in agreement with the coloured pigments produced by these isolates (data not shown). Genome analysis of P. halocryophilus also revealed the presence of genes for lycopene biosynthesis. It is hypothesized that these genes are responsible for the bright orange colouration observed in P. halocryophilus cultures [35]. The decaprenoxanthin biosynthesis pathway includes genes, crtE (geranylgeranyl pyrophosphate synthase), crtI (phytoene desaturase) and crtB (phytoene synthase) for the production of lycopene and genes, crtEB (lycopene elongase) and crtYef (lycopene epsilon cyclise) for the production of decaprenoxanthin. Antarctic Arthrobacter isolates I3 and H5 only contained copies of genes crtE and crtI. In contrast, just two of the seven temperate Arthrobacter spp., A. aurescens TC1 and A. castelli DSM 16402, contained genes involved in decaprenoxanthin biosynthesis.
Phenotypic characterisation of Antarctic Arthrobacter strains
Seven Antarctic Arthrobacter isolates and three temperate, soil-dwelling Arthrobacter species were selected for phenotypic characterisation and comparative analysis by BIOLOG PM1 and PM2A plates assessing carbon utilisation and plate PM9 assessing salinity tolerance.
Carbon utilisation profiles
Antarctic Arthrobacter isolates demonstrated significantly lower metabolic versatility as compared to temperate Arthrobacter isolates (P <0.05), as the temperate species were able to utilise 123-140 different C sources and the Antarctic strains were only able to utilise 98-121 different C sources (Additional file 9). The diverse patterns of C utilisation were further reflected in the MDS plot as four separate clusters were observed (Figure 6a).
Figure 6.

MDS plots of BIOLOG substrate usage data of seven Antarctic Arthrobacter isolates and three closely related temperate Arthrobacter species. a. Carbon utilisation profile determined by BIOLOG PM1 and PM2A assay plates. b. Salinity tolerance profile determined by BIOLOG PM9 assay plate. Antarctic strains: I3, H14, H5, H20, 35/47, Br18, H41. Temperate strains: A. chlorophenolicus A6, A. phenathrenivorans Sphe3, Arthrobacter sp. FB24.
Antarctic Arthrobacter isolates were able to utilise C substrates that form components of bacterial and fungal cell walls [62,63] including carbohydrates, N-acetyl glucosamine, glucose, mannose, xylose, arabinose, rhamnose and amino acids, Ala, Glu, Gly, and Lys, glycogen and trehalose that serve as microbial carbon and energy storage molecules [43,64] and sucrose, mannitol and arabitol that serve as storage molecules in lichens [65]. With the exception of ornithogenic soils formed under penguin rookeries, soils of the RSR typically contain low concentrations of organic C, ranging from 0.01 to 0.96 mg C g−1 soil [66]. Recalcitrant compounds including pectin and lignin are limited in Antarctic soil environments due to the absence of higher plants [67]. Instead, Antarctic soil environments largely contain C compounds as a result of aeolian distribution of organic C derived predominantly from: (i) cyanobacterial mats and mosses from lacustrine and marine ecosystems, (ii) endolithic microbial communities, and (iii) soil-dwelling mosses, lichens and microbial communities [66]. In addition, the Antarctic isolates lacked the ability to utilise plant cell wall components including carbohydrate, allose; carboxylic acids, citric acid, 4-hydroxybenzoic acid and galacturonic acid; and polymer, pectin, all of which are typically absent in RSR soils [68]. In contrast, all three temperate Arthrobacter spp. were able to utilise these C sources.
Salinity tolerance patterns
Antarctic Arthrobacter isolates showed a significantly narrower salinity tolerance range as compared to temperate Arthrobacter species (P <0.01) (Additional file 10). Differences in salinity tolerance are clearly illustrated in the MDS plot where two separate clusters of temperate and Antarctic Arthrobacter spp. are observed (Figure 6b). A positive phenotype for all Antarctic Arthrobacter isolates was observed with up to 7% NaCl, 4% KCl and 5% sodium sulphate and up to 100 mM ammonium sulphate (pH 8) and 100 mM sodium nitrate. Salinity is a key feature of soils in the RSR with some soils, such as those at lower Taylor Valley, containing 62.04 kg of salts m-2 [69]. The salt composition of RSR soils is largely dependent on geographic location as coastal soils are largely comprised of chloride and nitrate salts while inland soils are dominated by sulphate and nitrate salts [67]. As a result, an ability to withstand high concentrations of these salts is essential for survival in the soil environment. As compared to temperate Arthrobacter spp., all Antarctic strains showed significantly reduced tolerance to organic salts, Na formate (<1%) and Na lactate (<1%), which are likely to be absent in Antarctic soil environments.
Conclusions
We have undertaken a comparative genomic study of seven Antarctic and seven temperate Arthrobacter spp. to identify genomic features that may be essential for growth and survival in the Antarctic terrestrial environment. Genomes of all Antarctic Arthrobacter isolates contained several features that are also observed in psychrophilic/psychrotolerant bacteria and archaea. These included genes for sigma factors, ROS detoxification, osmoprotection systems, cold shock response and a carotenoid biosynthesis pathway. However, a large proportion of these genes were also identified in temperate Arthrobacter spp., suggesting that these genes may be important for growth and survival in a range of soil habitats. Further investigations by transcriptomic- and proteomic-based techniques, as previously reported for A. chlorophenolicus [70,71] and A. phenanthrenivorans [72], are essential to reveal the expression profiles of these genes as well as identify novel traits or genes that are crucial for cold adaptation.
It should be noted that relative to temperate species, and notwithstanding the incompleteness of the genome assemblies, four Antarctic Arthrobacter isolates (Br18, H20, H41 and 35/47) contained significantly fewer CDSs. Phenotypic characterisation assessing carbon utilisation profiles of these isolates revealed lowered metabolic versatility. In addition, of the total CDSs identified in the genomes of these isolates significantly fewer CDSs were assigned to COG categories, transcription [K] and carbohydrate transport and metabolism [G] (P < 0.01). The fewer CDSs, the lowered metabolic versatility, and the significant reduction in CDSs associated with transcription and carbohydrate transport and metabolism, suggest the occurrence of genome content scaling in four of the seven Antarctic Arthrobacter isolates. This occurrence was also reported in three strains of Paenibacillus darwinianus that were isolated from gamma-irradiated soils of the RSR [73,74]. In bacteria, an increase in genome size is often linked with an increase in metabolic versatility, allowing bacteria to produce new enzymes that exploit a wide range of environmental conditions [75]. However, an increase in versatility is linked with a four-fold increase in regulatory proteins associated with transcription and two-component signal transduction systems [76,77]. In environments such as soil, efficient regulation of enzyme expression, enabling exploitation of scarce yet diverse, complex nutrients can offer a selective advantage, thus lowering the penalty of slow growth. This growth strategy is common amongst dominant bacteria in soil environments [78]. In the Antarctic soil environment, organic residues are scarce yet labile, with C and N being mineralisable within a relatively short period of time (90 days) under optimal conditions [79]. In the harsh Antarctic soil environment, maintenance of metabolic versatility comes at a higher cost and, more importantly, reproductive efficiency (promoted by smaller genomes containing fewer CDSs) is beneficial for survival and growth. Finally, these genome sequences allow further investigations into the expression of physiological traits that enable survival under extreme conditions and, more importantly, into the ability of these bacteria to respond to future perturbations including climate change and human impacts.
Materials and methods
qPCR analysis
Site descriptions and sampling strategy
Soil samples were collected from four sites: Scott Base (77°55’S, 166°45’E), Granite Harbour (77°24’S, 162°31’E), Minna Bluff (78°31’S, 166°46’E) and Marble Point (77°25’S, 163°41’E). To the best of our knowledge all sites sampled were free of recent anthropogenic disturbances, with a possible exception of foot traffic. Soil classification and descriptions are as previously described [80]. At each site, three pits (~ 50 m from each other) representing biological replicates were dug. Following removal of the desert pavement, four soil samples (~ 15 g each), two samples at depths 0-5 cm and two at 5-10 cm, representing technical replicates were collected. Soil samples were stored in LifeGuard™ Soil Preservation Solution (MoBio), 2 ml of solution per g soil, frozen at -20°C and transported back to New Zealand for processing. For long-term storage, samples were frozen at -80°C.
DNA extraction
DNA was extracted in duplicate (0.5 g soil/tube) from one technical replicate at each depth at all sample sites by mechanical cell disruption (bead-beating) as previously described [81]. DNA extracts were quantified by Qubit® dsDNA BR assay kit (Life Technologies) and their purity (A260/A280) was assessed on a NanoDrop™ ND-1000 Spectrophotometer (Biolab).
qPCR validation and protocol
qPCR assays were designed for the selective amplification of: (1) all Bacteria, (2) phylum Actinobacteria, (3) genus Arthrobacter. Primer sequences for each reaction are listed in Additional file 11. Specificity of all primer sequences was tested in silico using the Probe Match tool in the RDP-Release 10 (Ribosomal Database Project) classifier program [82]. Primer reactions were optimised with genomic DNA from the following pure cultures: Arthrobacter spp. H5, H14, H20 and 35/47, Nocardioides sp. D26, Pontibacter sp. D8, Modestobacter sp. Br44, Paenibacillus darwinianus BrT, and Escherichia coli NZRM 916. Finally, primer specificity was tested by clone library preparations as described by Aislabie et al. [8] with DNA from: three Antarctic soil samples (Scott Base, Minna Bluff and Marble Point) and a marine sponge sample (Rhopaloeides odorabile). Twenty clones per DNA sample were selected for identification by 16S rRNA gene sequencing.
All qPCR reactions were performed in 10 μl reaction volumes in 384-well clear optical reaction plates (Applied Biosystems) on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Each reaction contained: 5 μl of Platinum® Green qPCR SuperMix-UDG with ROX (Life Technologies), 0.2 μM forward and reverse primers for all Bacteria and Actinobacteria-specific assays; 0.5 μM forward and reverse primers for Arthrobacter-specific assays, 1 μl template DNA (1 ng/μl) and ddH2O up to 10 μl. General qPCR cycling conditions were 2 min at 50°C, 2 min at 95°C, followed by 40 cycles of 95°C for 15 s and 1 min at the respective annealing temperature. Annealing temperatures were individually optimized for each assay. Each plate included triplicate reactions for all DNA samples, DNA standards and no-template controls. Melting curve analysis was performed at the end of each reaction to confirm the attribution of the fluorescence signal to a specific PCR product and not primer-dimers or other artifacts. DNA standards were prepared from a linear plasmid containing the entire 16S rRNA gene, amplified and cloned as described by Aislabie et al. [8] from genomic DNA of Arthrobacter sp. FB24 (NR_074590).
Standard curves and amplification efficiencies were calculated using 7900HT SDS software version 2.4 as described elsewhere [83]. Correlation coefficient (R2) and amplification efficiency (E) across all qPCR assays were >0.99 and 1.80-1.86, respectively.
Phylogenetic analyses of Arthrobacter from soils of the RSR
Phylogenetic analysis
Nearly full-length 16S rRNA gene sequences of Arthrobacter spp. from soils of the RSR were obtained from GenBank, aligned via the SINA web aligner and imported into the ARB phylogenetic package using the SILVA 108 database for analysis by the maximum likelihood, RAxML method. The topology of the tree was tested in MEGA v6.0 by bootstrap analysis based on 1000 resamplings [84,85].
Selection of isolates
Seven Arthrobacter isolates from RSR soils including: I3, H5, H14, H20, 35/47, Br18 and H41 were obtained from the Antarctic culture collection of Dr Jackie Aislabie (Landcare Research, Hamilton). These isolates were routinely cultured on R2A agar (Difco™, BD) at 15°C. Temperature range data for isolate 35/47 were determined by growth on R2A plates for two weeks at a range of temperatures, namely 5, 10, 15, 18, 20, 25, and 37°C.
DNA extraction and sequencing
High molecular weight DNA was extracted from seven Arthrobacter isolates: I3, H5, H14, H20, 35/47, Br18 and H41 using a modified CTAB (hexadecyltrimethylammonium bromide) and protein lysis method [86]. Briefly, cells were scraped off R2A agar plates and re-suspended in 740 μl TE buffer containing 20 μl lysozyme (100 mg/ml), 40 μl 10% SDS and 8 μl proteinase K (20 mg/ml). These cells were incubated overnight at 37°C. Following overnight incubation, 100 μl of 5 M NaCl and CTAB/NaCl solutions were added to the reaction and incubated at 65°C for 10 min. Subsequently 0.5 ml chloroform:isoamyl alcohol (24:1) was added, and the entire reaction was centrifuged at 16,000 g for 15 min. The aqueous phase was transferred to a clean microcentrifuge tube containing 0.5 ml phenol:chloroform:isoamyl alcohol (25:24:1) and the reaction was centrifuged at 16,000 g for 15 min. The aqueous phase was transferred to a clean microcentrifuge tube containing 0.6 vol isopropanol. To allow for DNA precipitation, all reactions were incubated at room temperature for 60 min, then centrifuged at 16,000 g for 30 min. The resulting DNA pellet was washed with 70% ethanol and re-suspended in TE buffer containing RNAse (99 μl TE buffer + 1 μl RNAse (10 mg/ml)) and incubated at 37°C for 20 min. DNA extracts were quantified by Quant-iT™ PicoGreen® dsDNA assay kit (Life Technologies) and their purity (A260/A280) was assessed on a NanoDrop™ ND-1000 Spectrophotometer (Biolab). Additionally, quality of each DNA extract was tested by electrophoresis on a 1% agarose gel.
Following extraction, high molecular weight DNA was sent to Macrogen (Seoul, South Korea) for sequencing on the Illumina HiSeq 2000 platform using 100 bp paired end libraries. With a sequencing output of 35 Gb, estimated coverage was 700X per genome.
De novo assembly, annotation and comparative analyses
FASTQ files for each genome were trimmed and quality filtered using the FASTQ Trimmer tool of the FASTX-toolkit v0.0.13 [87] and Sickle (https://github.com/ucdavis-bioinformatics/sickle) respectively. High-quality reads (Q > 30) were assembled into contigs using Velvet v1.2.10 [88]. Following initial assembly, PAGIT (post assembly genome improvement toolkit) tools including IMAGE (iterative mapping and assembly for gap elimination) and iCORN (iterative correction of reference nucleotides) were utilized for gap elimination and sequencing error correction [89]. Finally, SSPACE basic version 1.0 (stand-alone scaffolder of pre-assembled contigs using paired-read data) was utilized to build scaffolds from assembled contigs [90]. Genome completeness was assessed as previously described [91]. Gene prediction and genome annotation was performed using the automated JGI pipeline (Joint Genome Institute) [92].
For comparative analyses, A. aurescens TC1, A. castelli DSM 16402, A. chlorophenolicus A6, A. globiformis NBRC 12137, A. nitroguajacolicus Rue61a, A. phenanthrenivorans Sphe3 and Arthrobacter sp. FB24 were selected based on their genome completeness and habitat. These analyses were performed using JGI-IMG (Integrated Microbial Genomes)-Expert Review [85]. CMG biotools was utilised for pan- and core-genome plot analysis and predicted proteome comparisons [32]. Putative phage sequences were identified by the Phage Search Tool (PHAST) [24].
Availability of supporting data
This whole genome shotgun project has been deposited at DDBJ/EMBL/GenBank under the following accession numbers: 35/47-AZHY00000000, H14-AZRX00000000, H20-AZRY00000000, H41-AZRZ00000000, Br18-AZSA00000000, I3-AZSB00000000 and H5-AZSC00000000.
Phenotypic characterisation by BIOLOG
Salinity tolerance and carbon substrate utilisation
Carbon utilisation (PM1, PM2A) and salinity tolerance (PM9) were tested by Phenotype Microarray (PM) technology (BIOLOG). Ten Arthrobacter isolates, including seven Antarctic isolates: I3, H5, H14, H20, 35/47, Br18 and H41 and three temperate isolates: A. phenanthrenivorans DSM 18606 T, A. chlorophenolicus DSM 12829 T and Arthrobacter sp. FB24 DSM 22572T, were included for characterisation. Type strains of the temperate bacteria were obtained from DSMZ, Germany. Antarctic isolates were routinely cultured on R2A agar plates at 15°C and temperate isolates were routinely cultured on TSB (tryptic soy broth) agar (Bacto™, BD) plates at 28°C. Prior to inoculation of PM plates, Antarctic isolates were grown at 15°C for three days on R2A agar plates and temperate isolates were grown at 28°C for 12 h on TSB agar plates. PM plates were inoculated with 150 μl of cell suspension, prepared to the appropriate cell density as per the manufacturer’s instructions. PM plates inoculated with Antarctic isolates were incubated at 15°C and PM plates with temperate isolates were incubated at 28°C. Colour development (OD600nm) on all plates was recorded at 24 h intervals on an EnSpire® multimode plate reader over 10 days for Antarctic isolates and over four days for temperate isolates.
Data analysis
Isolate response profiles were built based on the response of each isolate to each substrate, determined as either positive or negative (binary data – usage/non-usage). Response profiles were analysed with the multivariate statistics package, Primer-E v6 (UK) by non-metric multi-dimensional scaling (MDS) plots on Bray Curtis similarity matrices constructed from transformed data (presence/absence).
Acknowledgements
This research was supported by Core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment’s Science and Innovation Group, New Zealand. We thank Antarctica New Zealand for providing logistic support during sample collection.
Abbreviations
- RSR
Ross sea region
- qPCR
quantitative polymerase chain reaction
- OTU
Operational taxonomic unit
- CDS
Protein coding sequence
- COG
Clusters of orthologous groups
- TCS
Two-component systems
- ROS
Reactive oxygen species
- ABC
ATP-binding cassette
Additional files
Phylogenetic tree based on nearly complete 16S rRNA gene sequences of Arthrobacter isolates and clones from RSR soils, constructed by the maximum likelihood, RAxML method.
List of mobile genetic element associated genes in Antarctic and temperate Arthrobacter genomes.
Putative phage sequences identified in Antarctic Arthrobacter strains Br18 (a, b); H20 (c); H14 (d) by phage search tool, PHAST [24].
The core- and pan- genome of 14 Arthrobacter genomes calculated using BLAST in CMG Biotools.
List of genes linked to environmental stress response in Antarctic and temperate Arthrobacter genomes.
Putative oxidases identified in seven temperate and seven Antartic Arthrobacter genomes.
Number of genes associated with sugar biosynthesis pathways in temperate and Antarctic Arthrobacter genomes.
Genes associated with histidine kinase signal transduction systems identified in temperate and Antarctic Arthrobacter genomes.
Carbon utilisation profiles of three temperate Arthrobacter spp. and seven Antarctic Arthrobacter isolates determined by BIOLOG PM1 and PM2A carbon plates.
Salinity tolerance profiles of three temperate Arthrobacter spp. and seven Antarctic Arthrobacter isolates determined by the BIOLOG PM9 Osmolyte plate.
A description of taxon-specific primers used for qPCR assays.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MD, MT, ST, and JA were involved in study design. MD performed the experiment and analysed the results. MT and JA contributed reagents, materials and analysis tools. MD wrote the paper. All authors read and approved the final manuscript.
Contributor Information
Melissa Dsouza, Email: melissa33in@gmail.com.
Michael W Taylor, Email: mw.taylor@auckland.ac.nz.
Susan J Turner, Email: sturner@biodiscovery.co.nz.
Jackie Aislabie, Email: aislabiej@landcareresearch.co.nz.
References
- 1.Ugolini FC, Bockheim JG. Antarctic soils and soil formation in a changing environment: a review. Geoderma. 2008;144(1–2):1–8. doi: 10.1016/j.geoderma.2007.10.005. [DOI] [Google Scholar]
- 2.Adlam LS, Balks MR, Seybold CA, Campbell DI. Temporal and spatial variation in active layer depth in the McMurdo Sound Region, Antarctica. Antarct Sci. 2010;22(01):45–52. doi: 10.1017/S0954102009990460. [DOI] [Google Scholar]
- 3.Boyd WL, Boyd JW. Soil microorganisms of the McMurdo Sound area, Antarctica. Appl Microbiol. 1963;11(2):116–21. doi: 10.1128/am.11.2.116-121.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron RE, Kink J, David CN, Holdgate M. Microbiology, ecology and microclimatology of soil sites in Dry valleys of Southern Victoria Land, Antarctica. Antarctic Ecol. 1970;2:702–16. [Google Scholar]
- 5.Aislabie J, Lau A, Dsouza M, Shepherd C, Rhodes P, Turner S. Bacterial composition of soils of the Lake Wellman area, Darwin Mountains, Antarctica. Extremophiles. 2013;17(5):775–86. doi: 10.1007/s00792-013-0560-6. [DOI] [PubMed] [Google Scholar]
- 6.Niederberger TD, McDonald IR, Hacker AL, Soo RM, Barrett JE, Wall DH, et al. Microbial community composition in soils of Northern Victoria Land, Antarctica. Environ Microbiol. 2008;10(7):1713–24. doi: 10.1111/j.1462-2920.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 7.Aislabie JM, Jordan S, Barker GM. Relation between soil classification and bacterial diversity in soils of the Ross Sea region, Antarctica. Geoderma. 2008;144(1–2):9–20. doi: 10.1016/j.geoderma.2007.10.006. [DOI] [Google Scholar]
- 8.Aislabie JM, Chhour KL, Saul DJ, Miyauchi S, Ayton J, Paetzold RF, et al. Dominant bacteria in soils of Marble Point and Wright Valley, Victoria Land, Antarctica. Soil Biology Biochem. 2006;38(10):3041–56. doi: 10.1016/j.soilbio.2006.02.018. [DOI] [Google Scholar]
- 9.Saul DJ, Aislabie JM, Brown CE, Harris L, Foght JM. Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiol Ecol. 2005;53(1):141–55. doi: 10.1016/j.femsec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Van Horn DJ, Van Horn ML, Barrett JE, Gooseff MN, Altrichter AE, Geyer KM, et al. Factors controlling soil microbial biomass and bacterial diversity and community composition in a cold desert ecosystem: role of geographic scale. PLoS ONE. 2013;8(6):e66103. doi: 10.1371/journal.pone.0066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiao G, Lee CK, McDonald IR, Cowan DA, Cary SC. Rapid microbial response to the presence of an ancient relic in the Antarctic Dry Valleys. Nat Commun. 2012;3:660. doi: 10.1038/ncomms1645. [DOI] [PubMed] [Google Scholar]
- 12.Lee CK, Barbier BA, Bottos EM, McDonald IR, Cary SC. The inter-valley soil comparative survey: the ecology of Dry Valley edaphic microbial communities. ISME J. 2012;6(5):1046–57. doi: 10.1038/ismej.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pointing SB, Chan Y, Lacap DC, Lau MCY, Jurgens JA, Farrell RL. Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci U S A. 2009;106(47):19964–9. doi: 10.1073/pnas.0908274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maayer P, Anderson D, Cary C, Cowan DA. Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep. 2014;15(5):508–17. doi: 10.1002/embr.201338170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen MA, Lauro FM, Williams TJ, Burg D, Siddiqui KS, De Francisci D, et al. The genome sequence of the psychrophilic archaeon, Methanococcoides burtonii: the role of genome evolution in cold adaptation. ISME J. 2009;3(9):1012–35. doi: 10.1038/ismej.2009.45. [DOI] [PubMed] [Google Scholar]
- 16.Krin E, Pascal G, Barbe V, Bernsel A, Bertin PN, Cheung F, et al. Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 2005;15(10):1325–35. doi: 10.1101/gr.4126905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carneiro AR, Ramos RTJ, Dall'Agnol H, Pinto AC, de Castro SS, Santos AR, et al. Genome sequence of Exiguobacterium antarcticum B7, isolated from a biofilm in Ginger Lake, King George Island, Antarctica. J Bacteriol. 2012;194(23):6689–90. doi: 10.1128/JB.01791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollmers J, Voget S, Dietrich S, Gollnow K, Smits M, Meyer K, et al. Poles apart: arctic and antarctic Octadecabacter strains share high genome plasticity and a new type of xanthorhodopsin. PLoS ONE. 2013;8(5):e63422. doi: 10.1371/journal.pone.0063422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abt B, Lu M, Misra M, Han C, Nolan M, Lucas S, et al. Complete genome sequence of Cellulophaga algicola type strain (IC166T) Stand Genomic Sci. 2011;4(1):72. doi: 10.4056/sigs.1543845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodfellow M. Bergey’s manual® of systematic bacteriology. New York: Springer; 2012. Phylum XXVI. Actinobacteria phyl. nov; pp. 33–2028. [Google Scholar]
- 21.Stackebrandt E, Schumann P. Introduction to the taxonomy of actinobacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes. New York: Springer; 2006. pp. 297–321. [Google Scholar]
- 22.Pagani I, Liolios K, Jansson J, Chen I-MA, Smirnova T, Nosrat B, et al. The Genomes OnLine Database (GOLD) v. 4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res. 2012;40(D1):D571–9. doi: 10.1093/nar/gkr1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayton J. Microbial diversity in soils from the Ross Sea Region of Antarctica. Auckland: University of Auckland; 2009. [Google Scholar]
- 24.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39:W347–52. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mongodin EF, Shapir N, Daugherty SC, DeBoy RT, Emerson JB, Shvartzbeyn A, et al. Secrets of soil survival revealed by the genome sequence of Arthrobacter aurescens TC1. PLoS Genet. 2006;2(12):2094–106. doi: 10.1371/journal.pgen.0020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyrman J, Verbeeren J, Schumann P, Swings J, De Vos P. Six novel Arthrobacter species isolated from deteriorated mural paintings. Int J Syst Evol Microbiol. 2005;55(4):1457–64. doi: 10.1099/ijs.0.63358-0. [DOI] [PubMed] [Google Scholar]
- 27.Westerberg K, Elv̈ang AM, Stackebrandt E, Jansson JK. Arthrobacter chlorophenolicus sp. nov., a new species capable of degrading high concentrations of 4-chlorophenol. Int J Syst Evol Microbiol. 2000;50(6):2083–92. doi: 10.1099/00207713-50-6-2083. [DOI] [PubMed] [Google Scholar]
- 28.Conn H, Dimmick I. Soil bacteria similar in morphology to Mycobacterium and Corynebacterium. J Bacteriol. 1947;54(3):291. doi: 10.1128/jb.54.3.291-303.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotoučková L, Schumann P, Durnová E, Spröer C, Sedláček I, Neča J, et al. Arthrobacter nitroguajacolicus sp. nov., a novel 4-nitroguaiacol-degrading actinobacterium. Int J Syst Evol Microbiol. 2004;54(3):773–7. doi: 10.1099/ijs.0.02923-0. [DOI] [PubMed] [Google Scholar]
- 30.Kallimanis A, Kavakiotis K, Perisynakis A, Spröer C, Pukall R, Drainas C, et al. Arthrobacter phenanthrenivorans sp. nov., to accommodate the phenanthrene-degrading bacterium Arthrobacter sp. strain Sphe3. Int J Syst Evol Microbiol. 2009;59(2):275–9. doi: 10.1099/ijs.0.000984-0. [DOI] [PubMed] [Google Scholar]
- 31.Nakatsu CH, Barabote R, Thompson S, Bruce D, Detter C, Brettin T, et al. Complete genome sequence of Arthrobacter sp. strain FB24. Stand Genomic Sci. 2013;9(1):106–16. doi: 10.4056/sigs.4438185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vesth T, Lagesen K, Acar Ö, Ussery D. CMG-Biotools, a free workbench for basic comparative microbial genomics. PLoS ONE. 2013;8(4):e60120. doi: 10.1371/journal.pone.0060120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11(1):119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mykytczuk NC, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG. Bacterial growth at − 15 C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013;7(6):1211–26. doi: 10.1038/ismej.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley M, Staley JT, Danchin A, Wang TZ, Brettin TS, Hauser LJ, et al. Genomics of an extreme psychrophile, Psychromonas ingrahamii. BMC Genomics. 2008;9(1):210. doi: 10.1186/1471-2164-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitta T, Nagamitsu H, Murata M, Izu H, Yamada M. Function of the ςE regulon in dead-cell lysis in stationary-phase Escherichia coli. J Bacteriol. 2000;182(18):5231–7. doi: 10.1128/JB.182.18.5231-5237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chattopadhyay M. Low temperature and oxidative stress. Curr Sci. 2002;83(2):109. [Google Scholar]
- 39.Methé BA, Nelson KE, Deming JW, Momen B, Melamud E, Zhang X, et al. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci U S A. 2005;102(31):10913–8. doi: 10.1073/pnas.0504766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabus R, Ruepp A, Frickey T, Rattei T, Fartmann B, Stark M, et al. The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environ Microbiol. 2004;6(9):887–902. doi: 10.1111/j.1462-2920.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61(5):1308–21. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 42.Zevenhuizen LPTM. Levels of trehalose and glycogen in Arthrobacter globiformis under conditions of nutrient starvation and osmotic stress. Antonie Van Leeuwenhoek. 1992;61(1):61–8. doi: 10.1007/BF00572124. [DOI] [PubMed] [Google Scholar]
- 43.Wilson WA, Roach PJ, Montero M, Baroja-Fernández E, Muñoz FJ, Eydallin G, et al. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol Rev. 2010;34(6):952–85. doi: 10.1111/j.1574-6976.2010.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandror O, DeLeon A, Goldberg AL. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc Natl Acad Sci U S A. 2002;99(15):9727–32. doi: 10.1073/pnas.142314099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X-M, Jiang Y, Li Y-T, Zhang H-H, Li J, Chen X, et al. Regulation of expression of trehalose-6-phosphate synthase during cold shock in Arthrobacter strain A3. Extremophiles. 2011;15(4):499–508. doi: 10.1007/s00792-011-0380-5. [DOI] [PubMed] [Google Scholar]
- 46.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol. 1998;170(5):319–30. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 47.Qin Q-L, Xie B-B, Yu Y, Shu Y-L, Rong J-C, Zhang Y-J, et al. Comparative genomics of the marine bacterial genus Glaciecola reveals the high degree of genomic diversity and genomic characteristic for cold adaptation. Environ Microbiol. 2014;16(6):1642–53. doi: 10.1111/1462-2920.12318. [DOI] [PubMed] [Google Scholar]
- 48.Bowman JP. Psychrophiles: from biodiversity to biotechnology. Berlin: Springer; 2008. Genomic analysis of psychrophilic prokaryotes; pp. 265–84. [Google Scholar]
- 49.Nichols C, Lardière S, Bowman J, Nichols P, A.E. Gibson J, Guézennec J. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb Ecol. 2005;49(4):578–89. doi: 10.1007/s00248-004-0093-8. [DOI] [PubMed] [Google Scholar]
- 50.Berger F, Morellet N, Menu F, Potier P. Cold shock and cold acclimation proteins in the psychrotrophic bacterium Arthrobacter globiformis SI55. J Bacteriol. 1996;178(11):2999–3007. doi: 10.1128/jb.178.11.2999-3007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger F, Normand P, Potier P. capA, a cspA-like gene that encodes a cold acclimation protein in the psychrotrophic bacterium Arthrobacter globiformis SI55. J Bacteriol. 1997;179(18):5670–6. doi: 10.1128/jb.179.18.5670-5676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol. 1999;2(2):175–80. doi: 10.1016/S1369-5274(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 53.Ayala-del-Río HL, Chain PS, Grzymski JJ, Ponder MA, Ivanova N, Bergholz PW, et al. The genome sequence of Psychrobacter arcticus 273-4, a psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Environ Microbiol. 2010;76(7):2304–12. doi: 10.1128/AEM.02101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao H, Yang ZK, Wu L, Thompson DK, Zhou J. Global transcriptome analysis of the cold shock response of Shewanella oneidensis MR-1 and mutational analysis of its classical cold shock proteins. J Bacteriol. 2006;188(12):4560–9. doi: 10.1128/JB.01908-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phadtare S. Recent developments in bacterial cold-shock response. Curr Issues Mol Biol. 2004;6(2):125–36. [PubMed] [Google Scholar]
- 56.Rodrigues DF, Ivanova N, He Z, Huebner M, Zhou J, Tiedje JM. Architecture of thermal adaptation in an Exiguobacterium sibiricum strain isolated from 3 million year old permafrost: a genome and transcriptome approach. BMC Genomics. 2008;9(1):547. doi: 10.1186/1471-2164-9-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber MHW, Klein W, Müller L, Niess Ulf M, Marahiel MA. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol Microbiol. 2001;39(5):1321–9. doi: 10.1111/j.1365-2958.2001.02322.x. [DOI] [PubMed] [Google Scholar]
- 58.Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 2001;20(7):1681–91. doi: 10.1093/emboj/20.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fong N, Burgess M, Barrow K, Glenn D. Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol. 2001;56(5–6):750–6. doi: 10.1007/s002530100739. [DOI] [PubMed] [Google Scholar]
- 60.Dieser M, Greenwood M, Foreman CM. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. FEMS Microbiol Ecol. 2010;42(4):396–405. [Google Scholar]
- 61.Heider S, Peters-Wendisch P, Wendisch V. Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol. 2012;12(1):1–11. doi: 10.1186/1471-2180-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salton M. Studies of the bacterial cell wall: IV. The composition of the cell walls of some Gram-Positive and Gram-Negative bacteria. Biochim Biophys Acta. 1953;10:512–23. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- 63.Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of Fungi. Annu Rev Microbiol. 1968;22(1):87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- 64.Argüelles JC. Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch Microbiol. 2000;174(4):217–24. doi: 10.1007/s002030000192. [DOI] [PubMed] [Google Scholar]
- 65.Richardson DHS, Smith DC. Lichen physiology. IX. Carbohydrate movement from the Trebouxia symbiont of Xanthoria aureola to the fungus. New Phytologist. 1968;67(1):61–8. doi: 10.1111/j.1469-8137.1968.tb05454.x. [DOI] [Google Scholar]
- 66.Hopkins DW, Sparrow AD, Gregorich EG, Elberling B, Novis P, Fraser F, et al. Isotopic evidence for the provenance and turnover of organic carbon by soil microorganisms in the Antarctic dry valleys. Environ Microbiol. 2009;11(3):597–608. doi: 10.1111/j.1462-2920.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 67.Campbell I, Claridge G. Antarctica: soils, weathering processes and environment. The Netherlands: Elsevier Science; 1987. [Google Scholar]
- 68.Strobel BW. Influence of vegetation on low-molecular-weight carboxylic acids in soil solution—a review. Geoderma. 2001;99(3–4):169–98. doi: 10.1016/S0016-7061(00)00102-6. [DOI] [Google Scholar]
- 69.Bockheim J. Properties and classification of cold desert soils from Antarctica. Soil Sci Soc Am J. 1997;61(1):224–31. doi: 10.2136/sssaj1997.03615995006100010031x. [DOI] [Google Scholar]
- 70.Unell M, Abraham PE, Shah M, Zhang B, Ruckert C, VerBerkmoes NC, et al. Impact of phenolic substrate and growth temperature on the Arthrobacter chlorophenolicus proteome. J Proteome Res. 2009;8(4):1953–64. doi: 10.1021/pr800897c. [DOI] [PubMed] [Google Scholar]
- 71.Scheublin TR, Deusch S, Moreno-Forero SK, Müller JA, Meer JR, Leveau JH. Transcriptional profiling of Gram-positive Arthrobacter in the phyllosphere: induction of pollutant degradation genes by natural plant phenolic compounds. Environ Microbiol. 2014;16(7):2212–25. doi: 10.1111/1462-2920.12375. [DOI] [PubMed] [Google Scholar]
- 72.Vandera E, Samiotaki M, Parapouli M, Panayotou G, Koukkou AI. Comparative proteomic analysis of Arthrobacter phenanthrenivorans Sphe3 on phenanthrene, phthalate and glucose. J Proteomics. 2015;113:73–89. doi: 10.1016/j.jprot.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 73.Dsouza M, Taylor MW, Turner SJ, Aislabie J. Genome-based comparative analyses of Antarctic and temperate species of Paenibacillus. PLoS ONE. 2014;9(10):e108009. doi: 10.1371/journal.pone.0108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dsouza M, Taylor MW, Ryan J, MacKenzie A, Lagutin K, Anderson RF, et al. Paenibacillus darwinianus sp. nov., isolated from gamma-irradiated Antarctic soil. Int J Syst Evol Microbiol. 2014;64(Pt 4):1406–11. doi: 10.1099/ijs.0.056697-0. [DOI] [PubMed] [Google Scholar]
- 75.Ranea JAG, Grant A, Thornton JM, Orengo CA. Microeconomic principles explain an optimal genome size in bacteria. Trends Genet. 2005;21(1):21–5. doi: 10.1016/j.tig.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 76.van Nimwegen E. Scaling laws in the functional content of genomes. Trends Genet. 2003;19(9):479–84. doi: 10.1016/S0168-9525(03)00203-8. [DOI] [PubMed] [Google Scholar]
- 77.Ranea JAG, Buchan DWA, Thornton JM, Orengo CA. Evolution of protein superfamilies and bacterial genome size. J Mol Biol. 2004;336(4):871–87. doi: 10.1016/j.jmb.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 78.Klappenbach JA, Dunbar JM, Schmidt TM. rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol. 2000;66(4):1328–33. doi: 10.1128/AEM.66.4.1328-1333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barrett JE, Virginia RA, Parsons AN, Wall DH. Potential soil organic matter turnover in Taylor Valley, Antarctica. Arct Antarct Alp Res. 2005;37(1):108–17. doi: 10.1657/1523-0430(2005)037[0108:PSOMTI]2.0.CO;2. [DOI] [Google Scholar]
- 80.Ayton J, Aislabie J, Barker GM, Saul D, Turner S. Crenarchaeota affiliated with group 1.1b are prevalent in coastal mineral soils of the Ross Sea region of Antarctica. Environ Microbiol. 2010;12(3):689–703. doi: 10.1111/j.1462-2920.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 81.Foght J, Aislabie J, Turner S, Brown CE, Ryburn J, Saul DJ, et al. Culturable bacteria in subglacial sediments and ice from two Southern Hemisphere glaciers. Microbial Ecology. 2004;47(4):329–40. doi: 10.1007/s00248-003-1036-5. [DOI] [PubMed] [Google Scholar]
- 82.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37(suppl 1):D141–5. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fierer N, Jackson JA, Vilgalys R, Jackson RB. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol. 2005;71(7):4117–20. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Buchner A, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32(4):1363–71. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson K. Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. 1987;2.4:1–2.4. 5. doi: 10.1002/0471142727.mb0204s56. [DOI] [PubMed] [Google Scholar]
- 87.Pearson WR, Wood T, Zhang Z, Miller W. Comparison of DNA sequences with protein sequences. Genomics. 1997;46(1):24–36. doi: 10.1006/geno.1997.4995. [DOI] [PubMed] [Google Scholar]
- 88.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–9. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swain MT, Tsai IJ, Assefa SA, Newbold C, Berriman M, Otto TD. A post-assembly genome-improvement toolkit (PAGIT) to obtain annotated genomes from contigs. Nat Protoc. 2012;7(7):1260–84. doi: 10.1038/nprot.2012.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–9. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- 91.Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, Nielsen PH. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol. 2013;31(6):533–8. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- 92.Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Grechkin Y, et al. IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 2012;40(D1):D115–22. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


