Abstract
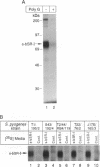
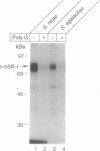
Macrophage scavenger receptors exhibit unusually broad binding specificity for polyanionic ligands and have been implicated in atherosclerosis and various host defense functions. Using a radiolabeled, secreted form of the type I bovine macrophage scavenger receptor in an in vitro binding assay, we have found that this receptor binds to intact Gram-positive bacteria, including Streptococcus pyogenes, Streptococcus agalactiae, Staphylococcus aureus, Enterococcus hirae, and Listeria monocytogenes. Competition binding studies using purified lipoteichoic acid, an anionic polymer expressed on the surface of most Gram-positive bacteria, show that lipoteichoic acids are scavenger receptor ligands and probably mediate binding of the receptor to Gram-positive bacteria. Lipoteichoic acids, for which no host cell receptors have previously been identified, are implicated in the pathogenesis of septic shock due to Gram-positive bacteria. Scavenger receptors may participate in host defense by clearing lipoteichoic acid and/or intact bacteria from tissues and the circulation during Gram-positive sepsis. Since scavenger receptors have been previously shown to bind to and facilitate bloodstream clearance of Gram-negative bacterial endotoxin (lipopolysaccharide), these receptors may provide a general mechanism for macrophage recognition and internalization of pathogens and their cell surface components.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton S., Resnick D., Freeman M., Ekkel Y., Ashkenas J., Krieger M. The collagenous domains of macrophage scavenger receptors and complement component C1q mediate their similar, but not identical, binding specificities for polyanionic ligands. J Biol Chem. 1993 Feb 15;268(5):3530–3537. [PubMed] [Google Scholar]
- Albus A., Arbeit R. D., Lee J. C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991 Mar;59(3):1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenas J., Penman M., Vasile E., Acton S., Freeman M., Krieger M. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res. 1993 Jun;34(6):983–1000. [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem. 1980 Jul 10;255(13):6284–6289. [PubMed] [Google Scholar]
- Behr T., Fischer W., Peter-Katalinić J., Egge H. The structure of pneumococcal lipoteichoic acid. Improved preparation, chemical and mass spectrometric studies. Eur J Biochem. 1992 Aug 1;207(3):1063–1075. doi: 10.1111/j.1432-1033.1992.tb17143.x. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Stephens D. S., Olsén A., Scott J. R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991 May;59(5):1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Higashino K., Kurihara Y., Wada Y., Miyazaki T., Nakamura H., Uesugi S., Imanishi T., Kawabe Y., Itakura H. Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J Biol Chem. 1993 Jan 25;268(3):2126–2133. [PubMed] [Google Scholar]
- Fischer W., Mannsfeld T., Hagen G. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem Cell Biol. 1990 Jan;68(1):33–43. doi: 10.1139/o90-005. [DOI] [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Penman M., Krieger M., Raetz C. R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991 Jul 25;352(6333):342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Hosein B., McCarty M., Fischetti V. A. Amino acid sequence and physicochemical similarities between streptococcal M protein and mammalian tropomyosin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3765–3768. doi: 10.1073/pnas.76.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I., McCarty M. Characterization and localization of the enzymatic deacylation of lipoteichoic acid in group A streptococci. J Exp Med. 1979 Dec 1;150(6):1498–1509. doi: 10.1084/jem.150.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Krieger M., Acton S., Ashkenas J., Pearson A., Penman M., Resnick D. Molecular flypaper, host defense, and atherosclerosis. Structure, binding properties, and functions of macrophage scavenger receptors. J Biol Chem. 1993 Mar 5;268(7):4569–4572. [PubMed] [Google Scholar]
- Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem Sci. 1992 Apr;17(4):141–146. doi: 10.1016/0968-0004(92)90322-z. [DOI] [PubMed] [Google Scholar]
- McCARTY M. The occurrence of polyglycerophosphate as an antigenic component of various gram-positive bacterial species. J Exp Med. 1959 Apr 1;109(4):361–378. doi: 10.1084/jem.109.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick D., Freedman N. J., Xu S., Krieger M. Secreted extracellular domains of macrophage scavenger receptors form elongated trimers which specifically bind crocidolite asbestos. J Biol Chem. 1993 Feb 15;268(5):3538–3545. [PubMed] [Google Scholar]
- Rohrer L., Freeman M., Kodama T., Penman M., Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990 Feb 8;343(6258):570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry K., Ezekowitz R. A. Collectins: pattern recognition molecules involved in first line host defense. Curr Opin Immunol. 1993 Feb;5(1):59–66. doi: 10.1016/0952-7915(93)90082-4. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Pulliam W. M., Hollingshead S. K., Fischetti V. A. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Beachey E. H. Binding of streptococcal lipoteichoic acid to the fatty acid binding sites on serum albumin. J Biol Chem. 1980 Jul 10;255(13):6092–6097. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Sutcliffe I. C., Shaw N. Atypical lipoteichoic acids of gram-positive bacteria. J Bacteriol. 1991 Nov;173(22):7065–7069. doi: 10.1128/jb.173.22.7065-7069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Moses A. E., Goldberg J. B., DiCesare T. J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Bisno A. L., Beachey E. H. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect Immun. 1981 Mar;31(3):985–991. doi: 10.1128/iai.31.3.985-991.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]