Abstract
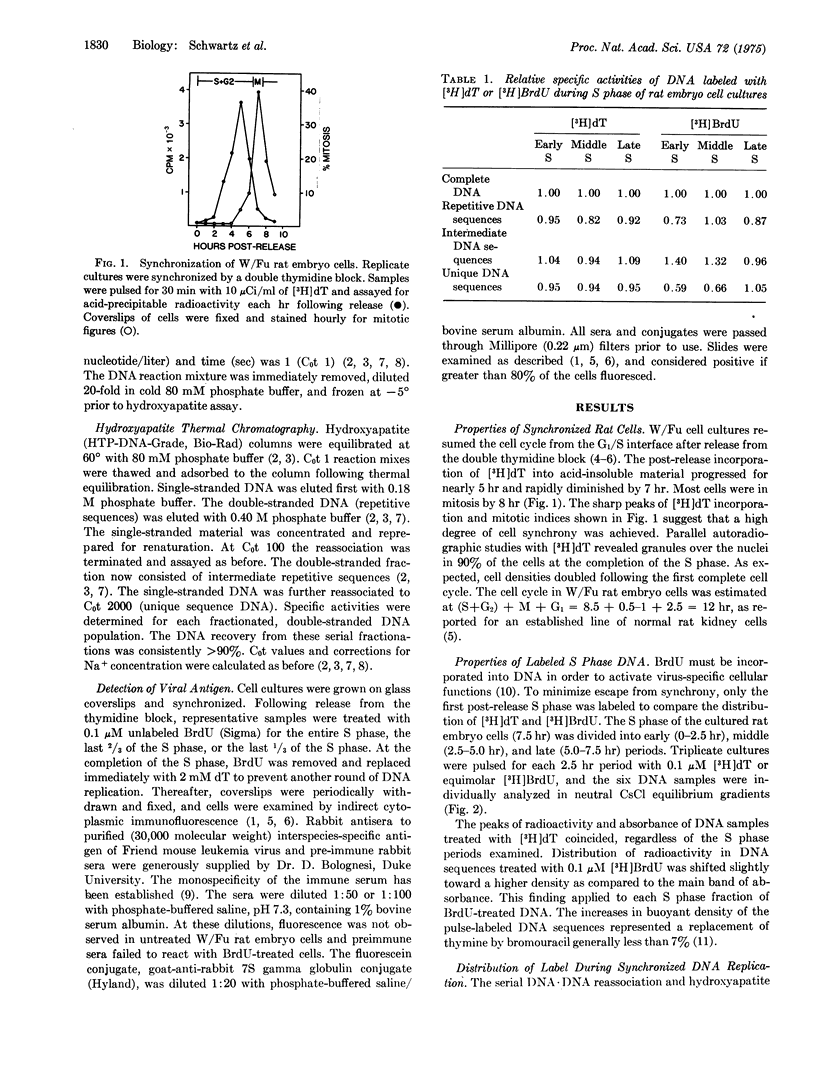
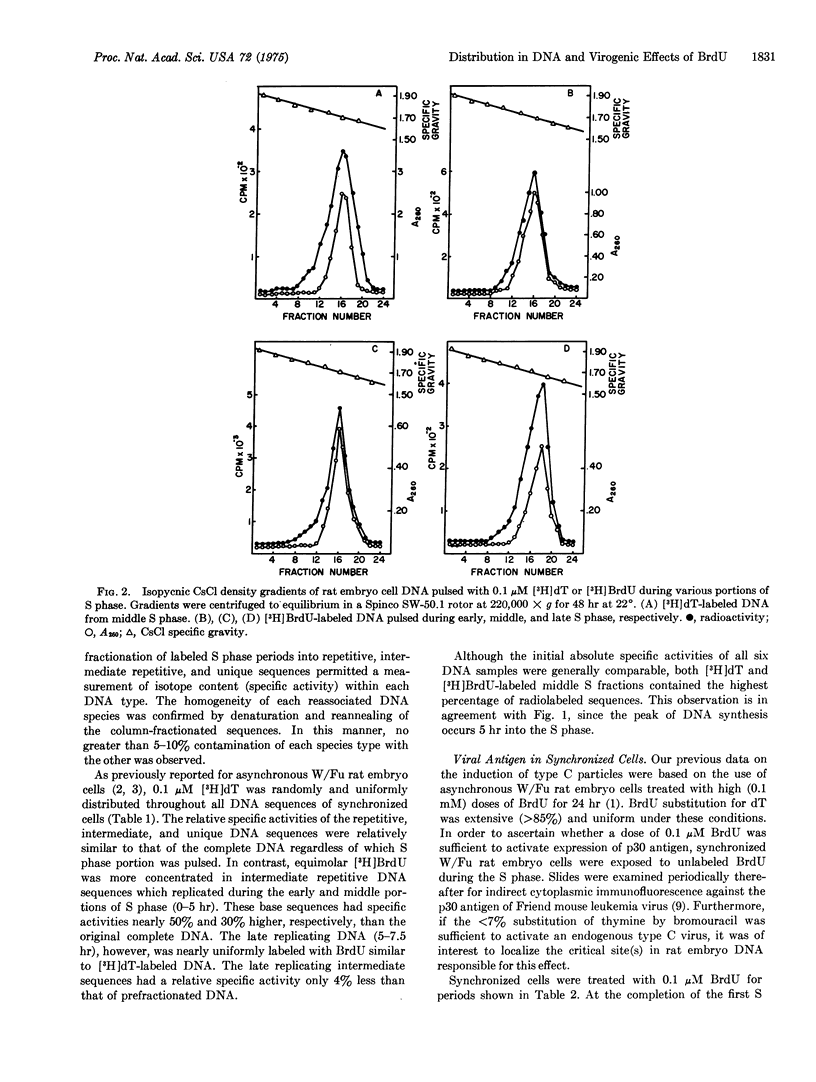
Rat embryo cell cultures were synchronized by a double thymidine block. The DNA replication phase (S) was divided into an early, middle, and late period. Cell cultures in the early, middle, or late S phase were pulsed with 0.1 muM 5-bromo[(3)H]deoxyuridine (BrdU) or equimolar [(3)H]dT. DNA-DNA reassociation experiments of each sample revealed that [(3)H]BrdU was more concentrated in the intermediate repetitive than the repetitive or unique DNA sequences of the early and middle S phase. In contrast, [(3)H]dT was nearly uniformly jistributed throughout all nucleotide sequences during the entire S phase. synchronized rat cells were pulsed during various portions of the S phase with unlabeled 0.1 mM or 0.1 muM BrdU and examined for sytoplasmic immumofluorescence against the 30,000 molecular weight group-specific antigen (p30) of Friend mouse leukemia virus. Equally strong fluorescence was detected 12 hr later in cells treated with each concentration of BrdU. Furthermore, incorporation of BrdU during late S phase was suffieient to elicit maximal antigen expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bick M. D., Davidson R. L. Total substitution of bromodeoxyuridine for thymidine in the DNA of a bromodeoxyuridine-dependent cell line. Proc Natl Acad Sci U S A. 1974 May;71(5):2082–2086. doi: 10.1073/pnas.71.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J., Garrard W. T., Gottesfeld J., Holmes D. S. Functional organization of the mammalian genome. Cold Spring Harb Symp Quant Biol. 1974;38:303–310. doi: 10.1101/sqb.1974.038.01.034. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Baluda M. A., Shoyab M. Differences between the integration of avian myeloblastosis virus DNA in leukemic cells and of endogenous viral DNA in normal chicken cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3152–3156. doi: 10.1073/pnas.71.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L. J., Campbell W. P. The distribution of 5-bromodeoxyuridine in the DNA of polyoma-transformed mouse cells and some apparent effects on transcription. Exp Cell Res. 1974 Jul;87(1):127–131. doi: 10.1016/0014-4827(74)90533-3. [DOI] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P., Schäfer W., Pister L., Hunsmann G., De Noronha F. Polypeptides of mammalian oncornaviruses. I. Isolation and serological analysis polypeptides from murine and feline C-type viruses. Virology. 1973 Dec;56(2):565–579. doi: 10.1016/0042-6822(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Nonoyama M., Chang S. Y., Tagamets A., Showalter S. D. Programming of events in Epstein-Barr virus-activated cells induced by 5-iododeoxyuridine. Virology. 1974 Nov;62(1):71–89. doi: 10.1016/0042-6822(74)90304-3. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Interspersion of repetitive and single-copy sequences in nuclear ribonucleic acid of high molecular weight. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1108–1112. doi: 10.1073/pnas.71.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Matheson D. W., Beltz B., Rosenthal M. Inhibition of melanogenesis with 5-bromodeoxyuridine treatment in a single period of DNA synthesis. J Natl Cancer Inst. 1974 May;52(5):1537–1540. doi: 10.1093/jnci/52.5.1537. [DOI] [PubMed] [Google Scholar]
- Levitt D., Dorfman A. The irreversible inhibition of differentiation of limb-bud mesenchyme by bromodeoxyuridine. Proc Natl Acad Sci U S A. 1972 May;69(5):1253–1257. doi: 10.1073/pnas.69.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panem S., Kirsten W. H. Release of mouse leukemia-sarcoma virus from synchronized cells. J Natl Cancer Inst. 1973 Feb;50(2):563–565. doi: 10.1093/jnci/50.2.563. [DOI] [PubMed] [Google Scholar]
- Panem S., Schauf V. Cell-cycle dependent appearance of murine leukemia-sarcoma virus antigens. J Virol. 1974 Jun;13(6):1169–1175. doi: 10.1128/jvi.13.6.1169-1175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Horio D., Kirsten W. H. Non-random incorporation of 5-bromodeoxyuridine in rat cell DNA. Biochem Biophys Res Commun. 1974 Dec 11;61(3):927–933. doi: 10.1016/0006-291x(74)90244-7. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Kirsten W. H. Distribution of 5-bromodeoxyuridine in the DNA of rat embryo cells. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3570–3574. doi: 10.1073/pnas.71.9.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. A., Panem S., Stefanski E., Kirsten W. H. Endogenous type C particles from rat embryo cells treated with 5-bromodeoxyuridine. Cancer Res. 1974 Sep;34(9):2255–2259. [PubMed] [Google Scholar]
- Stellwagen R. H., Tomkins G. M. Differential effect of 5-bromodeoxyuridine on the concentrations of specific enzymes in hepatoma cells in culture. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1147–1150. doi: 10.1073/pnas.68.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich N., Lowy D. R., Hartley J. W., Rowe W. P. Studies of the mechanism of induction of infectious murine leukemia virus from AKR mouse embryo cell lines by 5-iododeoxyuridine and 5-bromodeoxyuridine. Virology. 1973 Jan;51(1):163–173. doi: 10.1016/0042-6822(73)90376-0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D. W., Sarma P. S. Induction of type C virus-related functions in normal rat embryo fibroblasts by treatment with 5-iododeoxyuridine. Int J Cancer. 1973 Nov 15;12(3):551–562. doi: 10.1002/ijc.2910120303. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


