Abstract
Cells isolated from testes of 20-day-old rats, maintained in primary culture in a defined medium, are shown to respond to follicle-stimulating hormone or 3:5-cyclic AMP with characteristic morphological changes. No response is observed in cells treated with luteinizing hormone or 5-?AMP. The cells form a mono-layer, and have been identified as presumptive Sertoli cells structurally by identification of unique tight junctions in electron micrographs of the preparations, along with other ultrastructural properties characteristic of Sertoli cells in situ. These cells do not undergo mitosis. The presumptive Sertoli cells are shown to be morphologically and functionally different from peritubular fibroblasts grown in parallel cultures. Fibroblasts have high rates of mitosis, do not respond to follicle-stimulating hormone, and frequently form multilayers. Other information on the biochemical responses of the cells is cited, which supports the conclusion that the cultured cell preparations consist primarily of Sertoli cells.
Full text
PDF
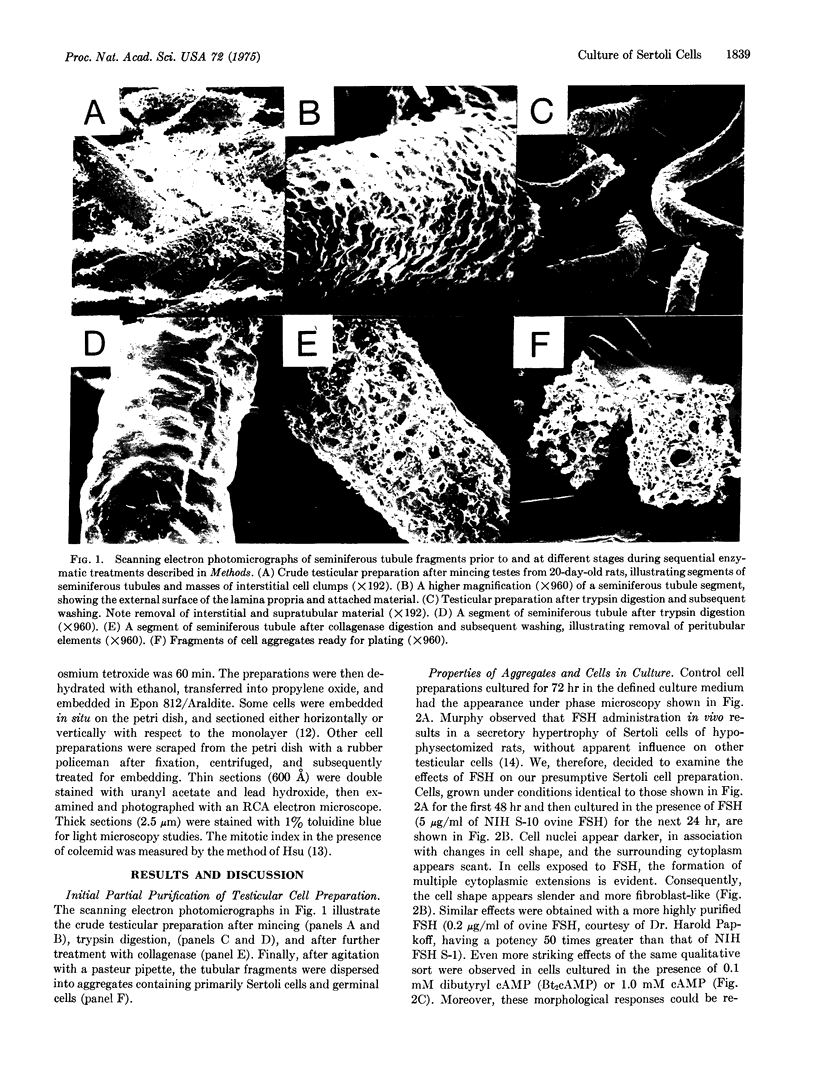
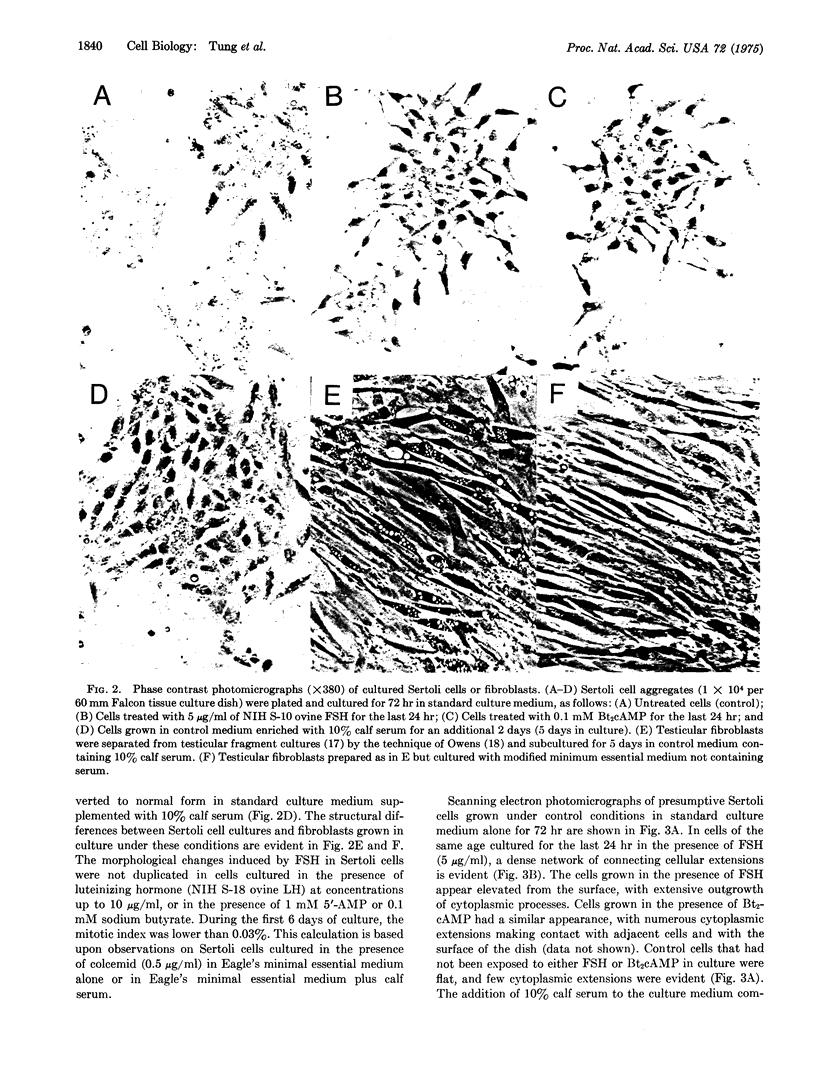
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bressler R. S., Ross M. H. Differentiation of peritubular myoid cells of the testis: effects of intratesticular implantation of newborn mouse testes into normal and hypophysectomized adults. Biol Reprod. 1972 Feb;6(1):148–159. doi: 10.1093/biolreprod/6.1.148. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Fritz I. B. Effects of gonadotropins on cyclic AMP production by isolated seminiferous tubule and interstitial cell preparations. Endocrinology. 1974 Feb;94(2):395–403. doi: 10.1210/endo-94-2-395. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Vernon R. G., Fritz I. B. The effect of gonadotrophins on the 3',5'-AMP levels of seminiferous tubules. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1523–1528. doi: 10.1016/0006-291x(72)90780-2. [DOI] [PubMed] [Google Scholar]
- Douglas W. H., Elser J. E. A method for in situ embedding of cultured cells grown on plastic surfaces. In Vitro. 1972 Jul-Aug;8(1):26–29. doi: 10.1007/BF02617940. [DOI] [PubMed] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973 Apr;175(4):639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- Flickinger C. J. The postnatal development of the Sertoli cells of the mouse. Z Zellforsch Mikrosk Anat. 1967;78(1):92–113. doi: 10.1007/BF00344405. [DOI] [PubMed] [Google Scholar]
- French F. S., McLean W. S., Smith A. A., Tindall D. J., Weddington S. C., Petrusz P., Nayfeh S. N., Ritzén E. M., Hansson V., Trystad O. Androgen transport and receptor mechanisms in testis and epididymis. Nature. 1974 Aug 2;250(465):387–391. doi: 10.1038/250387a0. [DOI] [PubMed] [Google Scholar]
- Fritz I. B., Kopec B., Lam K., Vernon R. G. Effects of FSH on levels of androgen binding protein in the testis. Curr Top Mol Endocrinol. 1974;1:311–327. doi: 10.1007/978-1-4684-2595-6_19. [DOI] [PubMed] [Google Scholar]
- Kodani M., Kodani K. The in vitro cultivation of mammalian Sertoli cells. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1200–1206. doi: 10.1073/pnas.56.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY H. D. SERTOLI CELL STIMULATION FOLLOWING INTRATESTICULAR INJECTIONS OF FSH IN THE HYPOPHYSECTOMIZED RAT. Proc Soc Exp Biol Med. 1965 Apr;118:1202–1205. doi: 10.3181/00379727-118-30080. [DOI] [PubMed] [Google Scholar]
- Means A. R. Early sequence of biochemical events in the action of follicle stimulating hormone on the testis. Life Sci. 1974 Aug 1;15(3):371–389. doi: 10.1016/0024-3205(74)90337-3. [DOI] [PubMed] [Google Scholar]
- Owens R. B. Glandular epithelial cells from mice: a method for selective cultivation. J Natl Cancer Inst. 1974 Apr;52(4):1375–1378. doi: 10.1093/jnci/52.4.1375. [DOI] [PubMed] [Google Scholar]
- Setchell B. P. Secretions of the testis and epididymis. J Reprod Fertil. 1974 Mar;37(1):165–177. doi: 10.1530/jrf.0.0370165. [DOI] [PubMed] [Google Scholar]
- Steinberger A., Steinberger E. In vitro culture of rat testicular cells. Exp Cell Res. 1966 Nov-Dec;44(2):443–452. doi: 10.1016/0014-4827(66)90451-4. [DOI] [PubMed] [Google Scholar]
- Vernon R. G., Go V. L., Fritz I. B. Hormonal requirements of the different cycles of the seminiferous epithelium during reinitiation of spermatogenesis in long-term hypophysectomized rats. J Reprod Fertil. 1975 Jan;42(1):77–94. doi: 10.1530/jrf.0.0420077. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Wiebe J. P. Rat sertoli cells: a rapid method for obtaining viable cells. Endocrinology. 1975 Mar;96(3):618–624. doi: 10.1210/endo-96-3-618. [DOI] [PubMed] [Google Scholar]