Abstract
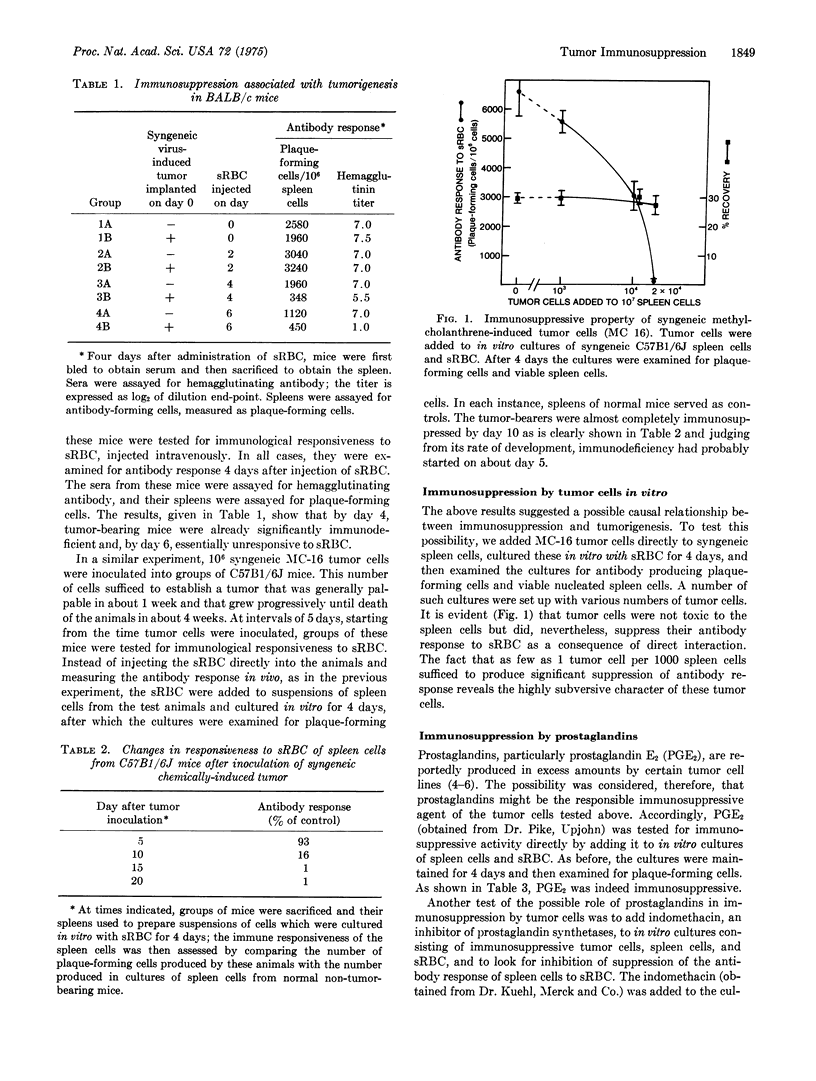
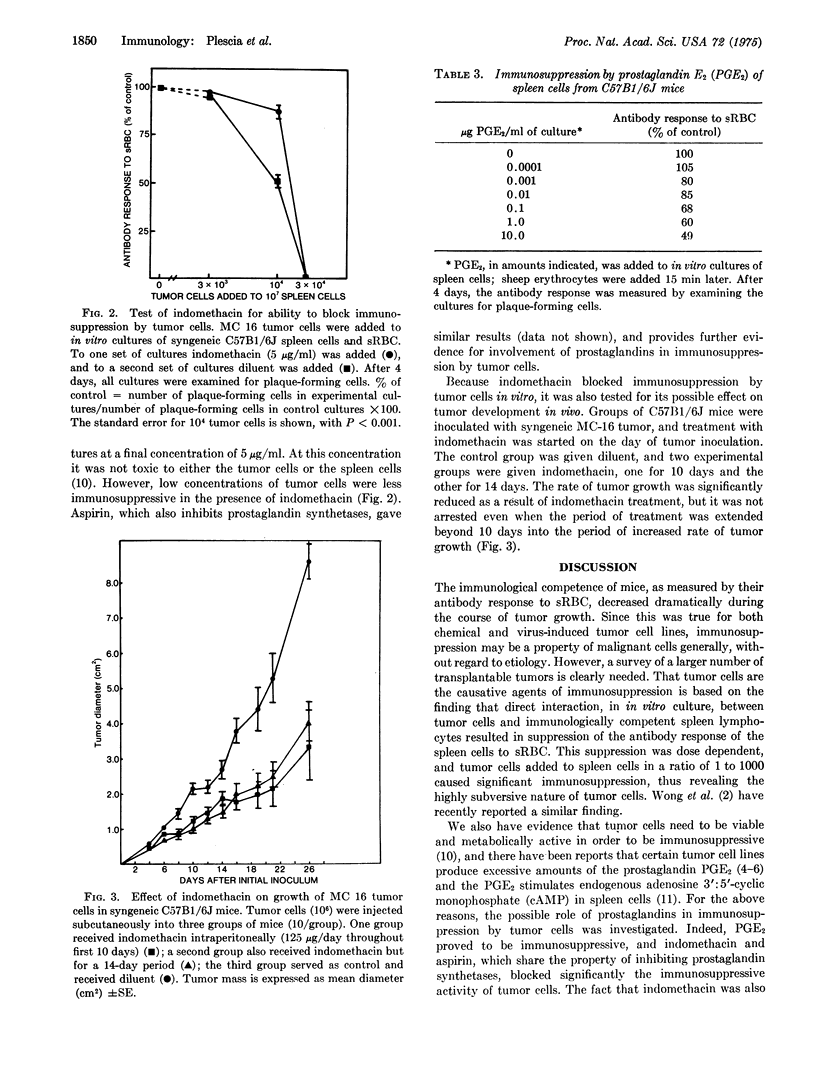
Mice bearing syngeneic tumors, chemical and virus-induced, became immunologically unresponsive to sheep erythrocytes. The increase in the degree of unresponsiveness with tumor growth suggested a causal relationship. Immunosuppression was in fact caused by the tumor cells because the addition of tumor cells to in vitro cultures of spleen cells and sheep erythrocytes resulted in suppression of antibody response. Suppression was dose dependent with a ratio of 1 to 1000 of tumor cells to spleen cells sufficient to produce significant suppression. Prostaglandins were found to have a role in immunosuppression by tumor cells in that PGE2 was itself immunosuppressive and in that indomethacin and aspirin, inhibitors of prostaglandin synthetases, blocked immunosuppression in vitro and retarded tumor growth in vivo. These findings suggest that tumors, although antigenic, may be able to escape immuno-sureillance by their host by means of subverting the immune system. Thus, success of immunotherapy may well depend on our ability to prevent or block the immunosuppressive activity of tumors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Click R. E., Benck L., Alter B. J. Immune responses in vitro. I. Culture conditions for antibody synthesis. Cell Immunol. 1972 Feb;3(2):264–276. doi: 10.1016/0008-8749(72)90165-7. [DOI] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B., Jacob H., Gaillard J. A., Jacob F. Antiinflammatory effects of murine malignant cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4052–4056. doi: 10.1073/pnas.71.10.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Strausser H. R. Prostaglandins and cyclic nucleotides in Moloney sarcoma tumors. Prostaglandins. 1974 Jan 25;5(2):183–196. doi: 10.1016/s0090-6980(74)80112-7. [DOI] [PubMed] [Google Scholar]
- Kersey J. H., Spector B. D., Good R. A. Immunodeficiency and cancer. Adv Cancer Res. 1973;18:211–230. doi: 10.1016/s0065-230x(08)60753-8. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein Y., Shearer G. M., Kram J., Bauminger S. Hemolytic plaque formation by leukocytes in vitro. Control by vasoactive hormones. J Clin Invest. 1974 Jan;53(1):13–21. doi: 10.1172/JCI107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L., Goldhaber P. Evidence that the bone resorption-stimulating factor produced by mouse fibrosarcoma cells is prostaglandin E 2 . A new model for the hypercalcemia of cancer. J Exp Med. 1972 Dec 1;136(6):1329–1343. doi: 10.1084/jem.136.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Mankovitz R., Kennedy J. C. Immunosuppressive and immunostimulatory factors produced by malignant cells in vitro. Int J Cancer. 1974 Apr 15;13(4):530–542. doi: 10.1002/ijc.2910130413. [DOI] [PubMed] [Google Scholar]


