Abstract
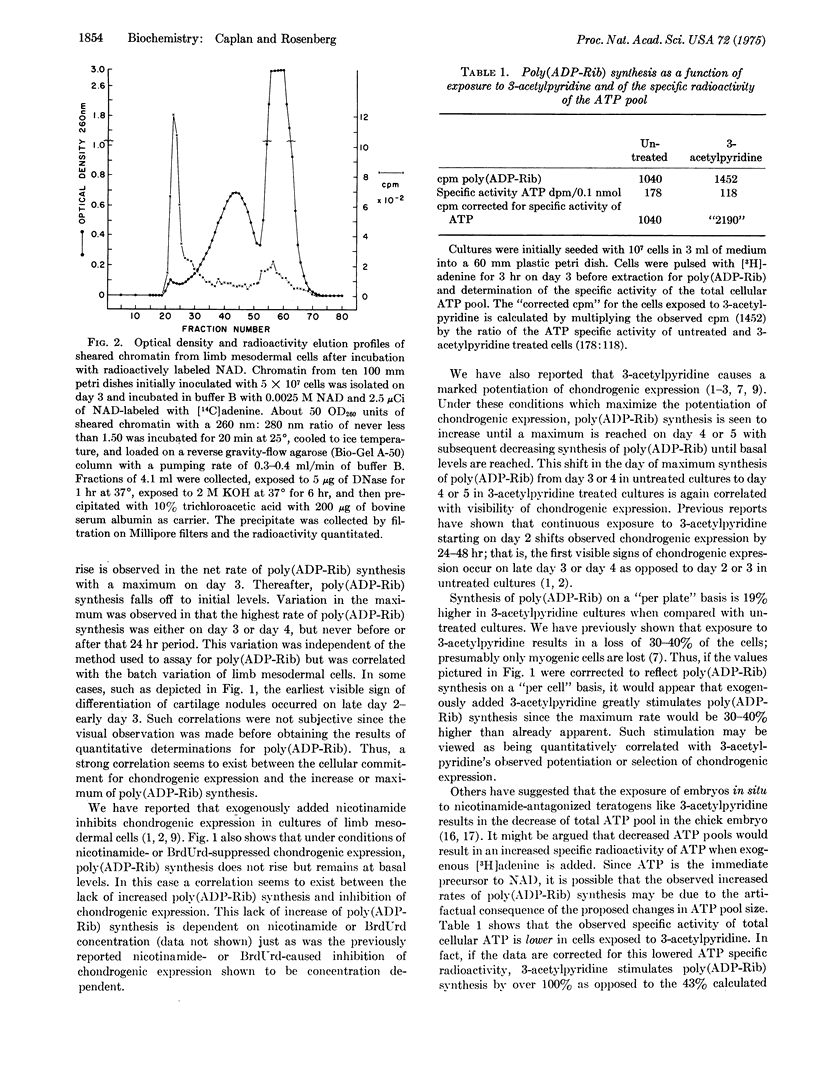
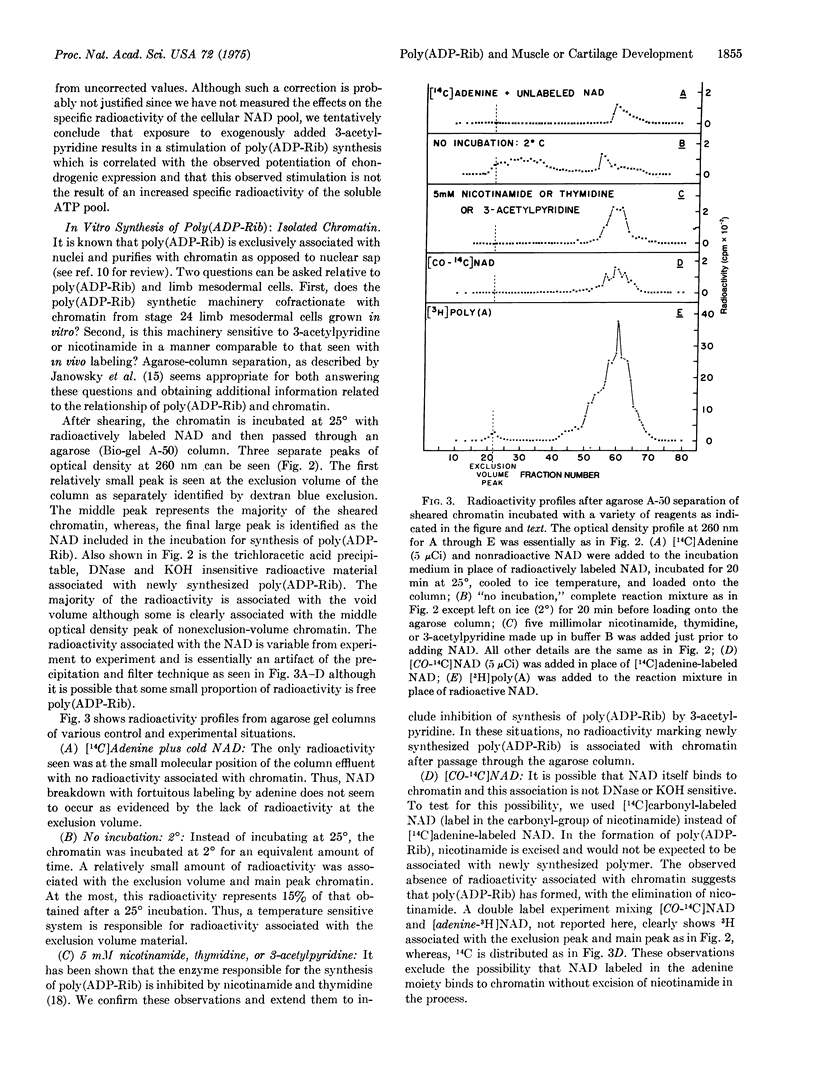
Mesodermal cells of embryonic chick limbs have the capacity to differentiate into either muscle or cartilage. Previous reports from this laboratory show a correlation between pyridine nucleotide levels and this differentiation, and thus suggest that fluctuations in the cellular NAD levels play a role in the control of muscle versus cartilage development. Poly(ADP-Rib) is chromatin-associated and forms from the polymerization of NAD with the excision of nicotinamide. The studies reported here show that: (A) the rate of net synthesis of poly(ADP-Rib) is correlated with the differentiation of chondrogenic cells from stage 24 limb mesodermal cells grown in vitro; (B) inhibition of chondrogenic expression caused by exposure to nicotinamide or BrdUrd is correlated with maintenance of basal levels of poly(ADP-Rib) synthesis, and this inhibition is dependent on the concentration of nicotinamide or BrdUrd exogenously supplied; (C) potentiation of chondrogenic expression caused by exposure of limb mesodermal cells in vitro to 3-acetylpyridine is correlated with stimulation of the rate of poly(ADP-Rib) synthesis if corrected for the specific activity of the ATP pool or compared to untreated cultures on a per cell basis; (D) isolated chromatin from mesodermal cells has the enzymatic machinery for synthesizing poly(ADP-Rib); (E) this machinery is inhibited by nicotinamide, thymidine, and 3-acetylpyridine; and (F) newly synthesized poly(ADP-Rib) is either associated with a discrete fraction of chromatin or is completely extracted from chromatin by the high column salts, which result in an aggregation with eventual elution at the exclusion volume position of the agarose column. Taken together, these observations provide a possible explanation for how fluctuations in cellular NAD levels can communicate with or be "sensed" by genomic related machinery and eventually result in differtial phenotypic expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caplan A. I. Comparison of the capacity of nicotinamide and nicotinic acid to relieve the effects of muscle and cartilage teratogens in developing chick embryos. Dev Biol. 1972 Jun;28(2):344–351. doi: 10.1016/0012-1606(72)90018-8. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. Effects of a nicotinamide-sensitive teratogen 6-aminonicotinamide on chick limb cells in culture. Exp Cell Res. 1972 Jan;70(1):185–195. doi: 10.1016/0014-4827(72)90196-6. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. Effects of the nicotinamide-sensitive teratogen3-acetylpyridine on chick limb cells in culture. Exp Cell Res. 1970 Oct;62(2):341–355. doi: 10.1016/0014-4827(70)90564-1. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Koutroupas S. The control of muscle and cartilage development in the chick limb: the role of differential vascularization. J Embryol Exp Morphol. 1973 Jun;29(3):571–583. [PubMed] [Google Scholar]
- Caplan A. I., Stoolmiller A. C. Control of chondrogenic expression in mesodermal cells of embryonic chick limb. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1713–1717. doi: 10.1073/pnas.70.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. I. The effects of the nicotinamide sensitive teratogen 3-acetylpyridine on chick limb mesodermal cells in culture: biochemical parameters. J Exp Zool. 1972 Jun;180(3):351–362. doi: 10.1002/jez.1401800306. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. The site and sequence of action of 6-aminonicotinamide in causing bone malformations of embryonic chick limb and its relationship to normal development. Dev Biol. 1972 May;28(1):71–83. doi: 10.1016/0012-1606(72)90127-3. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Zwilling E., Kaplan N. O. 3-acetylpyridine: effects in vitro related to teratogenic activity in chicken embryos. Science. 1968 May 31;160(3831):1009–1010. doi: 10.1126/science.160.3831.1009. [DOI] [PubMed] [Google Scholar]
- Colyer R. A., Burdette K. E., Kidwell W. R. Poly ADP-ribose synthesis and DNA replication in synchronized mouse L-cells. Biochem Biophys Res Commun. 1973 Aug 6;53(3):960–966. doi: 10.1016/0006-291x(73)90185-x. [DOI] [PubMed] [Google Scholar]
- Dienstman S. R., Biehl J., Holtzer S., Holtzer H. Myogenic and chondrogenic lineages in developing limb buds grown in vitro. Dev Biol. 1974 Jul;39(1):83–95. doi: 10.1016/s0012-1606(74)80010-2. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Humphreys T. A simple and sensitive method for quantitative measurement of cellular RNA synthesis. Anal Biochem. 1971 Apr;40(2):254–266. doi: 10.1016/0003-2697(71)90384-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Honjo T., Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968 Jul 10;243(13):3765–3767. [PubMed] [Google Scholar]
- Otake H., Miwa M., Fujimura S., Sugimura T. Binding of ADP-ribose polymer with histone. J Biochem. 1969 Jan;65(1):145–146. [PubMed] [Google Scholar]
- Overman D. O., Seegmiller R. E., Runner M. N. Coenzyme competition and precursor specificity during teratogenesis induced by 6-aminonicotinamide. Dev Biol. 1972 Aug;28(4):573–582. doi: 10.1016/0012-1606(72)90004-8. [DOI] [PubMed] [Google Scholar]
- Preiss J., Schlaeger R., Hilz H. Specific inhibition of poly adpribose polymerase by thymidine and nicotinamide in HeLa cells. FEBS Lett. 1971 Dec 15;19(3):244–246. doi: 10.1016/0014-5793(71)80524-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. J., Caplan A. I. Nicotinamide adenine dinucleotide levels in cells of developing chick limbs: possible control of muscle and cartilage development. Dev Biol. 1974 May;38(1):157–164. doi: 10.1016/0012-1606(74)90266-8. [DOI] [PubMed] [Google Scholar]
- Searls R. L., Janners M. Y. The stabilization of cartilage properties in the cartilage-forming mesenchyme of the embryonic chick limb. J Exp Zool. 1969 Mar;170(3):365–375. doi: 10.1002/jez.1401700313. [DOI] [PubMed] [Google Scholar]
- Seegmiller R. E., Overman D. O., Runner M. N. Histological and fine structural changes during chondrogenesis in micromelia induced by 6-aminonicotinamide. Dev Biol. 1972 Aug;28(4):555–572. doi: 10.1016/0012-1606(72)90003-6. [DOI] [PubMed] [Google Scholar]



