Abstract
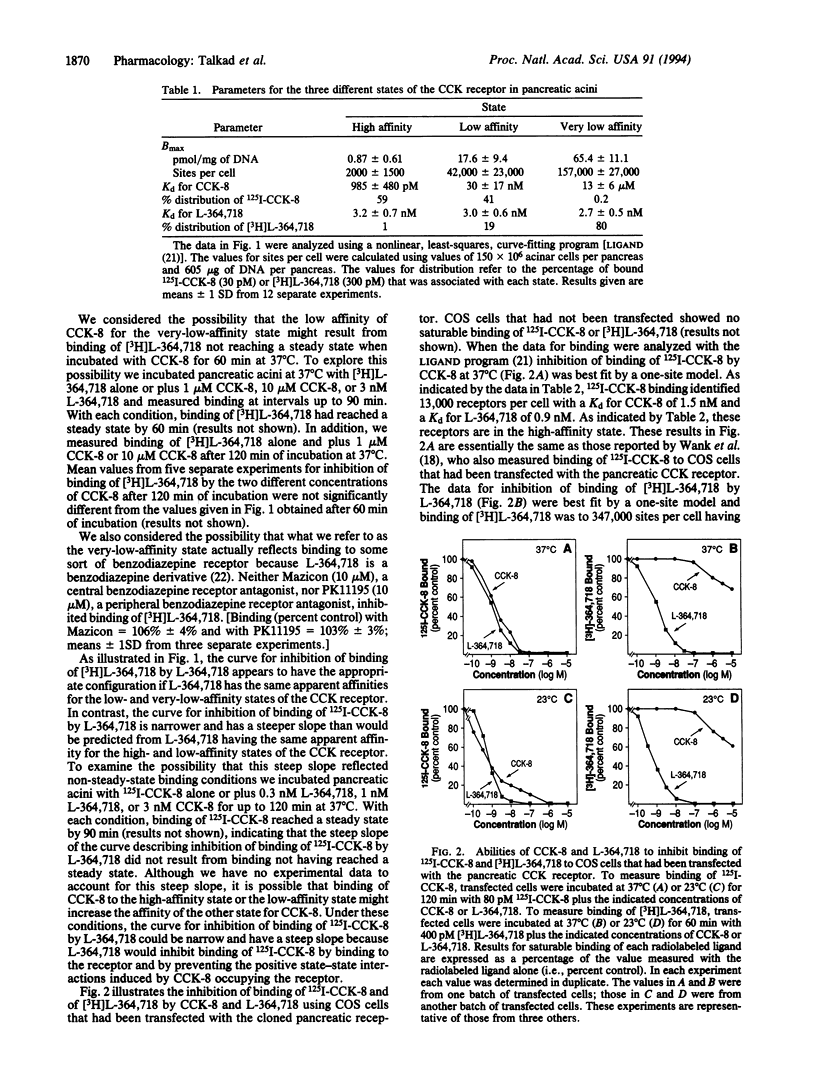
We used rat pancreatic acini as well as COS-7 cells transfected with the cloned pancreatic cholecystokinin (CCK) receptor and measured the abilities of CCK octapeptide (CCK-8) and L-364,718 (a CCK receptor antagonist) to inhibit binding of 125I-labeled CCK-8 (125I-CCK-8) and [3H]L-364,718. With pancreatic acini 125I-CCK-8 bound to two different states of the CCK receptor. The high-affinity state (1% of the receptors) had a Kd for CCK-8 of 985 pM and the low-affinity state (19% of the receptors) had a Kd for CCK-8 of 30 nM. [3H]L-364,718 bound to low-affinity receptors and to a previously unrecognized very-low-affinity state (80% of the receptors) having a Kd for CCK-8 of 13 microM. L-364,718 had the same affinity (Kd 3 nM) for each of the three different states of the CCK receptor. Similar measurements using transfected COS cells also identified three different states of the CCK receptor, with the very-low-affinity state being the most abundant. Thus, the ability of the CCK receptor to exist in three different states is an intrinsic property of the CCK receptor molecule itself.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang R. S., Chen T. B., Bock M. G., Freidinger R. M., Chen R., Rosegay A., Lotti V. J. Characterization of the binding of [3H]L-365,260: a new potent and selective brain cholecystokinin (CCK-B) and gastrin receptor antagonist radioligand. Mol Pharmacol. 1989 Jun;35(6):803–808. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Chen T. B., Kunkel K. A. Characterization of the binding of [3H]-(+/-)-L-364,718: a new potent, nonpeptide cholecystokinin antagonist radioligand selective for peripheral receptors. Mol Pharmacol. 1986 Sep;30(3):212–217. [PubMed] [Google Scholar]
- Delvalle J., Wang L., Gantz I., Yamada T. Characterization of H2 histamine receptor: linkage to both adenylate cyclase and [Ca2+]i signaling systems. Am J Physiol. 1992 Dec;263(6 Pt 1):G967–G972. doi: 10.1152/ajpgi.1992.263.6.G967. [DOI] [PubMed] [Google Scholar]
- Gaisano H. Y., Klueppelberg U. G., Pinon D. I., Pfenning M. A., Powers S. P., Miller L. J. Novel tool for the study of cholecystokinin-stimulated pancreatic enzyme secretion. J Clin Invest. 1989 Jan;83(1):321–325. doi: 10.1172/JCI113877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I., Schäffer M., DelValle J., Logsdon C., Campbell V., Uhler M., Yamada T. Molecular cloning of a gene encoding the histamine H2 receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):429–433. doi: 10.1073/pnas.88.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interactions of COOH-terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J Biol Chem. 1982 May 25;257(10):5554–5559. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lignon M. F., Galas M. C., Rodriguez M., Martinez J. Correlation between phospholipid breakdown, intracellular calcium mobilization and enzyme secretion in rat pancreatic acini treated with Boc-[Nle28, Nle31]-CCK-7 and JMV180, two cholecystokinin analogues. Cell Signal. 1990;2(4):339–346. doi: 10.1016/0898-6568(90)90063-g. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maton P. N., Pradhan T., Moore S. C-terminal peptides of calcitonin gene-related peptide act as agonists at the cholecystokinin receptor. Peptides. 1990 Nov-Dec;11(6):1163–1167. doi: 10.1016/0196-9781(90)90147-w. [DOI] [PubMed] [Google Scholar]
- Matozaki T., Martinez J., Williams J. A. A new CCK analogue differentiates two functionally distinct CCK receptors in rat and mouse pancreatic acini. Am J Physiol. 1989 Oct;257(4 Pt 1):G594–G600. doi: 10.1152/ajpgi.1989.257.4.G594. [DOI] [PubMed] [Google Scholar]
- Menozzi D., Vinayek R., Jensen R. T., Gardner J. D. Down-regulation and recycling of high affinity cholecystokinin receptors on pancreatic acinar cells. J Biol Chem. 1991 Jun 5;266(16):10385–10391. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Presti M. E., Gardner J. D. Receptor antagonists for gastrointestinal peptides. Am J Physiol. 1993 Mar;264(3 Pt 1):G399–G406. doi: 10.1152/ajpgi.1993.264.3.G399. [DOI] [PubMed] [Google Scholar]
- Sankaran H., Goldfine I. D., Bailey A., Licko V., Williams J. A. Relationship of cholecystokinin receptor binding to regulation of biological functions in pancreatic acini. Am J Physiol. 1982 Mar;242(3):G250–G257. doi: 10.1152/ajpgi.1982.242.3.G250. [DOI] [PubMed] [Google Scholar]
- Sankaran H., Goldfine I. D., Deveney C. W., Wong K. Y., Williams J. A. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J Biol Chem. 1980 Mar 10;255(5):1849–1853. [PubMed] [Google Scholar]
- Sato S., Stark H. A., Martinez J., Beaven M. A., Jensen R. T., Gardner J. D. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 1989 Aug;257(2 Pt 1):G202–G209. doi: 10.1152/ajpgi.1989.257.2.G202. [DOI] [PubMed] [Google Scholar]
- Stark H. A., Sharp C. M., Sutliff V. E., Martinez J., Jensen R. T., Gardner J. D. CCK-JMV-180: a peptide that distinguishes high-affinity cholecystokinin receptors from low-affinity cholecystokinin receptors. Biochim Biophys Acta. 1989 Feb 9;1010(2):145–150. doi: 10.1016/0167-4889(89)90154-7. [DOI] [PubMed] [Google Scholar]
- Tahiri-Jouti N., Dufresne M., Viguerie N., Fourmy D., Estève J. P., Rivier J., Moroder L., Susini C., Vaysse N. Somatostatin 28 interacts with CCK receptor in brain and pancreas. Neuropeptides. 1991 Jun;19(2):65–71. doi: 10.1016/0143-4179(91)90134-5. [DOI] [PubMed] [Google Scholar]
- Vinayek R., Patto R. J., Menozzi D., Gregory J., Mrozinski J. E., Jensen R. T., Gardner J. D. Occupation of low-affinity cholecystokinin (CCK) receptors by CCK activates signal transduction and stimulates amylase secretion in pancreatic acinar cells. Biochim Biophys Acta. 1993 Mar 10;1176(1-2):183–191. doi: 10.1016/0167-4889(93)90195-u. [DOI] [PubMed] [Google Scholar]
- Wank S. A., Harkins R., Jensen R. T., Shapira H., de Weerth A., Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank S. A., Jensen R. T., Gardner J. D. Kinetics of binding of cholecystokinin to pancreatic acini. Am J Physiol. 1988 Jul;255(1 Pt 1):G106–G112. doi: 10.1152/ajpgi.1988.255.1.G106. [DOI] [PubMed] [Google Scholar]
- Wank S. A., Pisegna J. R., de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8691–8695. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Bailey A. C., Roach E. Temperature dependence of high-affinity CCK receptor binding and CCK internalization in rat pancreatic acini. Am J Physiol. 1988 Apr;254(4 Pt 1):G513–G521. doi: 10.1152/ajpgi.1988.254.4.G513. [DOI] [PubMed] [Google Scholar]
- Williams J. A., McChesney D. J. Cholecystokinin induces the interaction of its receptor with a guanine nucleotide binding protein. Regul Pept. 1987 Aug 3;18(2):109–117. doi: 10.1016/0167-0115(87)90041-3. [DOI] [PubMed] [Google Scholar]
- Yu D. H., Huang S. C., Wank S. A., Mantey S., Gardner J. D., Jensen R. T. Pancreatic receptors for cholecystokinin: evidence for three receptor classes. Am J Physiol. 1990 Jan;258(1 Pt 1):G86–G95. doi: 10.1152/ajpgi.1990.258.1.G86. [DOI] [PubMed] [Google Scholar]
- Yuan D. S., Wank S. A., Gardner J. D. Cibacron blue-induced enhancement of agonist binding to cholecystokinin (CCK) receptors in solubilized pancreatic membranes. Biochim Biophys Acta. 1993 Feb 23;1146(1):52–58. doi: 10.1016/0005-2736(93)90337-y. [DOI] [PubMed] [Google Scholar]
- Zhang L., Mantey S., Jensen R. T., Gardner J. D. An analogue of substance P with broad receptor antagonist activity. Biochim Biophys Acta. 1988 Oct 28;972(1):37–44. doi: 10.1016/0167-4889(88)90100-0. [DOI] [PubMed] [Google Scholar]



