Abstract
Partial cortical blindness is a visual deficit caused by unilateral damage to the primary visual cortex, a condition previously considered beyond hopes of rehabilitation. However, recent data demonstrate that patients may recover both simple and global motion discrimination following intensive training in their blind field. The present experiments characterized motion-induced neural activity of cortically blind (CB) subjects prior to the onset of visual rehabilitation. This was done to provide information about visual processing capabilities available to mediate training-induced visual improvements. Visual Evoked Potentials (VEPs) were recorded from two experimental groups consisting of 9 CB subjects and 9 age-matched, visually-intact controls. VEPs were collected following lateralized stimulus presentation to each of the 4 visual field quadrants. VEP waveforms were examined for both stimulus-onset (SO) and motion-onset (MO) related components in postero-lateral electrodes. While stimulus presentation to intact regions of the visual field elicited normal SO-P1, SO-N1, SO-P2 and MO-N2 amplitudes and latencies in contralateral brain regions of CB subjects, these components were not observed contralateral to stimulus presentation in blind quadrants of the visual field. In damaged brain hemispheres, SO-VEPs were only recorded following stimulus presentation to intact visual field quadrants, via inter-hemispheric transfer. MO-VEPs were only recorded from damaged left brain hemispheres, possibly reflecting a native left/right asymmetry in inter-hemispheric connections. The present findings suggest that damaged brain hemispheres contain areas capable of responding to visual stimulation. However, in the absence of training or rehabilitation, these areas only generate detectable VEPs in response to stimulation of the intact hemifield of vision.
Keywords: hemianopia, cortical blindness, visual cortex, inter-hemispheric transfer time, callosal connections
1. Introduction
Cortical blindness (CB) is a chronic loss of conscious vision usually caused by stroke damaging primary visual cortex (V1) and/or the optic radiations (Cowey & Stoerig, 1991, 1995; Fujino, Kigizawa, & Yamada, 1986; Holmes, 1918, 1919; Lawton Smith, 1962; Teuber, Battersby, & Bender, 1960; Trobe, Lorber, & Schlezinger, 1973; Weiskrantz, Warrington, Sanders, & Marshall, 1974; Zhang, Kedar, Lynn, Newman, & Biousse, 2006a, 2006b). CB is a severe impairment because V1 normally acts as a gateway of visual information transfer between sub-cortical centers (primarily the dorsal lateral geniculate nucleus, or dLGN) and multiple, extra-striate, visual cortical areas (Felleman & Van Essen, 1991; D C Van Essen, Anderson, & Felleman, 1992; D.C. Van Essen & Maunsell, 1983). Thus, V1 damage markedly reduces feed-forward visual input to extra-striate visual areas, affecting all visual perceptual modalities (perception of color, shape, motion, etc.). Moreover, because ischemic or hemorrhagic insults affecting V1 tend to have low mortality rates, patients are left with significant difficulties performing visually-guided activities of daily living, including reading, visual search, locomotion, navigation and driving (e.g. Bowers, Mandel, Goldstein, & Peli, 2009; Cole, 1999; Gutteridge & McDonald, 2004; Kerkhoff, 2000; McDonald, Spitsyna, Shillcock, Wise, & Leff, 2006; Pambakian & Kennard, 1997; Spitzyna et al., 2007; Vargas-Martin & Peli, 2002; Warren, 2009). Thus, developing strategies to mitigate visual deficits in CB is vital, though controversial (Bouwmeester, Heutink, & Lucas, 2007; Das & Huxlin, 2010; Horton, 2005; Kerkhoff, 2000; Pambakian & Kennard, 1997; Reinhard et al., 2005; Stoerig, 2008).
Building on our understanding of blindsight - the paradoxical ability of many CB subjects to unconsciously process visual stimuli in their blind fields (e.g. P. Azzopardi & Cowey, 1998; P. Azzopardi & Cowey, 2001; Holmes, 1918; Perenin & Jeannerod, 1975; Pöppel, Held, & Frost, 1973; Riddoch, 1917; Weiskrantz, 1996; Weiskrantz, Barbur, & Sahraie, 1995; Weiskrantz, et al., 1974; Zeki & ffytche, 1998) - we and others have shown that it is possible to re-train both simple and complex visual discriminations in portions of the blind field using moving or flickering stimuli (Huxlin et al., 2009; Raninen, Vanni, Hyvärinen, & Näsänen, 2006; Sahraie et al., 2010; Sahraie et al., 2006). However, these findings raise many questions. Chief among them is: what mediates recovery - spared visual areas in the damaged brain hemisphere, visual areas in the contralateral hemisphere, or a combination of both?
To answer this question, we must first understand the functional consequences of V1 damage for the remaining circuitry – in essence, establishing what neural substrates are available to be recruited by visual rehabilitation strategies. In the present study, we assessed visually-evoked potentials (VEPs) in naïve CB subjects (and age-matched controls) while they performed a global direction discrimination task in the visual periphery of their blind and intact visual fields (similar to that used by Huxlin and colleagues - Huxlin, et al., 2009). We analyzed two principal VEPs – those generated by stimulus onset (SO-VEPs) and by motion onset (MO-VEPs) – at lateral, posterior, electrode sites. In addition to measuring basic parameters (amplitudes, latencies, etc.), we also asked whether when VEPs are elicited in V1-damaged brain hemispheres, they arise through the canonical, retino-geniculo-cortical route, or only through an indirect (callosal) projection from visual areas of the intact brain hemisphere.
The use of VEPs in CB has a long history, starting with Bergman (Bergman, 1957), and followed by consistent reports until the 1990s (see Aldrich, Alessi, Beck, & Gilman, 1987 for review of earlier publications). In contrast with the present study, the majority of prior work used flash and checkerboard pattern reversals presented either to the full visual field (Brigell, Celesia, Salvi, & Clark-Bash, 1990; Celesia, Meredith, & Pluff, 1983; Onofrj, Bodis-Wollner, & Mylin, 1982) or separately to blind and intact hemi-fields (Biersdorf, Bell, & Beck, 1992; Brigell, et al., 1990; Celesia, et al., 1983; ffytche, Guy, & Zeki, 1996; Kuroiwa & Celesia, 1981; Onofrj, et al., 1982). All prior studies reported normal VEP responses from the intact brain hemisphere of CB subjects. Some also found reliable, though not always normal, VEPs over damaged brain hemispheres upon blind field stimulation (Anderson, Holliday, Singh, & Harding, 1996; Bodis-Wollner, Atkin, Raab, & Wolkstein, 1977; Celesia, Archer, Kuroiwa, & Goldfader, 1980; Celesia & Brigell, 1999; Ffytche, Guy, & Zeki, 1995; Holliday, Anderson, & Harding, 1997; Rossion, de Gelder, Pourtois, Guerit, & Weiskrantz, 2000), but most claimed an absence of VEPs under such conditions (Aldrich, et al., 1987; Biersdorf, et al., 1992; Blumhardt, Barrett, & Halliday, 1977; Blumhardt, Barrett, Kriss, & Halliday, 1982; Brigell, et al., 1990; Celesia & Brigell, 1999; Celesia, et al., 1983; Kuroiwa & Celesia, 1981; Onofrj, et al., 1982; Watanabe et al., 2007). This discrepancy is perplexing and could have arisen from a multiplicity of inter-study differences: stimulus and task conditions, fixation control, whether patients passively viewed stimuli or were engaged in a demanding task, variability in the age, shape, size and severity of the cortical damage and field defect, as well as in brain anatomy and connectivity between subjects. Furthermore, none of the prior studies investigated inter-hemispheric transfer (IHT) or its most common metric: inter-hemispheric transfer time (IHTT). IHTT is computed as the difference in latency of VEP components recorded from the brain hemisphere contralateral to the visual stimulus, from those recorded in the ipsilateral brain hemisphere, which obtains most of its visual input indirectly from the first brain hemisphere, via the corpus callosum (Brown, Larson, & Jeeves, 1994; Saron & Davidson, 1989). V1 and V2 of the two brain hemispheres are only callosally interconnected across their representation of the vertical meridian (Hof et al., 1997; Hubel & Wiesel, 1967; Kennedy, Dehay, & Bullier, 1986). In contrast, extra-striate visual areas are more extensively interconnected, over a larger proportion of the visual field, with corresponding areas in the contralateral brain hemisphere (Maunsell & Van Essen, 1987).
The degree to which visual areas in a V1-damaged brain hemisphere can generate VEPs either from canonical, dLGN-to-cortex feed-forward input, or via callosal input from the intact brain hemisphere has important implications for understanding visual processing abilities preserved in cortically-blind fields. It may also allow us to predict the extent of recovery that may occur following rehabilitation, and provide guidance as to the type of training that would most efficiently induce that recovery.
2. Materials and Methods
2.1. Subjects
Nine subjects with unilateral [partial] CB were recruited at least 6 months post-stroke (see Table 1 for subject demographics). They ranged in age from 34 to 75 years, with a mean ± SD of 57.1 ± 16.7 years and a median of 59 yrs. All CB subjects sustained unilateral damage to the occipital lobe, including primary visual cortex (V1) and/or optic radiations, as verified by examination of structural MRI scans obtained from their neurologist.
Table 1. CB Subject Demographics.
Individuals with cortical blindness are denoted CB1-9. M: male, F: female
| Subject | Gender | Age (yrs) | Time since stroke (mths) | Affected Hemifield |
|---|---|---|---|---|
| CB1 | F | 34 | 24 | Right |
| CB2 | F | 34 | 8 | Left |
| CB3 | M | 42 | 252 | Right |
| CB4 | M | 54 | 11 | Right |
| CB5 | F | 59 | 29 | Left |
| CB6 | F | 69 | 11 | Right |
| CB7 | M | 72 | 6 | Right |
| CB8 | M | 74 | 7 | Right |
| CB9 | F | 75 | 36 | Left |
In addition, nine age-matched individuals (2 females and 7 males) were recruited to serve as visually intact controls, including two of the authors (TM and VK). Control subjects had no history of neurological disorders (including visual defects) or cognitive problems. They ranged in age from 30 to 73 years, with a mean ± SD of 58.6 ± 14.9 years and a median of 66 yrs.
All subjects possessed normal or corrected-to-normal visual acuity. All experimental procedures were approved by the Institutional Review Board of the University of Rochester Medical Center and were carried out in accordance with the Declaration of Helsinki. These procedures were explained to subjects, and written, informed consent was obtained prior to participation.
2.2. Perimetric visual field testing
Before electroencephalographic (EEG) recording, Humphrey visual field tests (24-2 and 10-2) were performed monocularly, using a Humphrey Perimeter (HFA II 750), by the same ophthalmic technicians. This mapped the spatial extent of each subject's reported visual deficit and allowed us to obtain a rough measure of the patient's fixation accuracy before EEG recording. The Humphrey eye tracker was turned on for all visual field tests and adequate tracking was obtained for all subjects in all Humphrey fields. The percentage of fixation losses, false positives and false negative responses were computed for each visual field test and only patients with acceptable fixation control (fewer than 20% fixation losses) and fewer than 10% false positive and negative responses were accepted for the present study. The 24-2 Humphrey results were then averaged across the two eyes and presented as iso-sensitivity maps of visual detection performance (Figure 1). This gave us a better estimate of baseline visual performance for EEG testing, which was carried out binocularly, with stimuli presented at four locations, indicated by colored circles in Figure 1.
Figure 1.
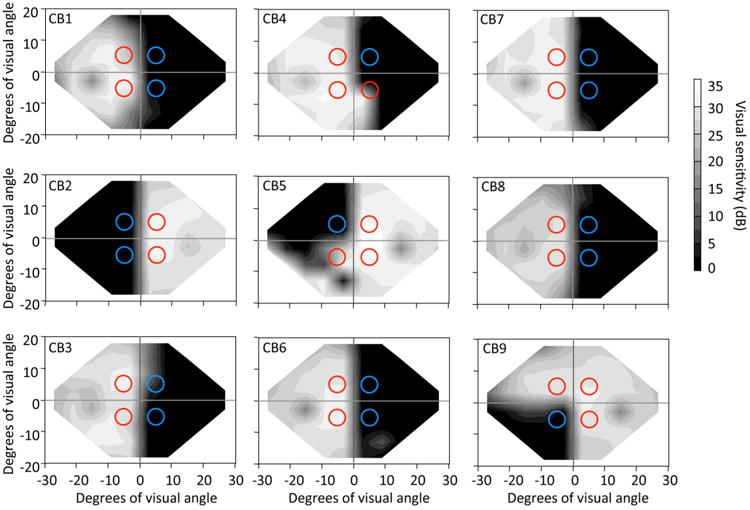
Humphrey visual field perimetry (24-2 test) assessed monocularly and averaged across the two eyes. The grey scale indicates average luminance detection sensitivity in dB. Circles represent the visual field locations and sizes of random dot stimuli the subjects were asked to discriminate: red circles indicate stimuli that were presented in quadrants of intact vision, while blue circles represent stimuli that were not discriminable because they were presented inside blind regions of the visual field.
2.3. Psychophysical procedure during EEG recordings
Subjects were seated 57 cm (distance maintained using a chin-rest and a forehead bar) from a 21-inch CRT monitor in a darkened room. Participants were asked to indicate left or right direction of motion of white, random dot stimuli on a uniform black background. Trials started when a fixation cross (0.5° of visual angle in length/width) appeared in the center of the computer screen, lasting 1000 ms, followed by static, random dots (Figure 2). Random dots were presented in a circular space 5 degrees in diameter, at a density of 2.6 dots/degree2. When they first appeared, the dots were stationary for a uniformly-distributed, randomly-chosen interval of between 1 and 2 s, after which they began moving at 10°/s (Figure 2). Motion was either “coherent” (all dots moving to the right or left) or “noisy” (320° range of dot directions, sampled from a uniform distribution centered around the right- or leftward vectors). Dot motion lasted for 500 ms or until subjects responded. Stimuli were presented in one of 4 visual field quadrants - Upper Left (UL), Upper Right (UR), Lower Left (LL), or Lower Right (LR) - so that the (x,y) coordinates in degrees of visual angle for the center of each stimulus were (5,5), (-5,5), (5, -5) and (-5,-5) – see colored circles in Figure 1. Subjects were instructed to press the left and right computer mouse buttons when they perceived left and right global directions of motion, respectively, for each stimulus presentation using their right hand. They were asked to answer as quickly as possible and to guess if they were uncertain or unable to perceive the stimulus direction. The dots disappeared after a response was made or after the 500 ms presentation. Each subject completed 10 practice trials before EEG recording began and then performed a total of 70 trials in each of the four visual quadrants for each of the two conditions (DR0 and DR320), with trial order randomized with respect to motion direction and quadrant. Thus, a total of 140 trials were collected per visual field quadrant. Experimental stimuli were controlled by custom software on a Windows XP computer and interfaced with a second PC, which recorded and analyzed the EEG activity. At the end of each EEG recording session, average reaction times (RT) and accuracy measures (% correct performance) were extracted at each of the four visual field locations where stimuli were presented. Performance was further separated according to whether the stimulus had been placed completely in the cortically blind field (blue circles in Figure 1), or whether it was fully or partially within intact or spared regions of the visual field (red circles in Figure 1).
Figure 2.
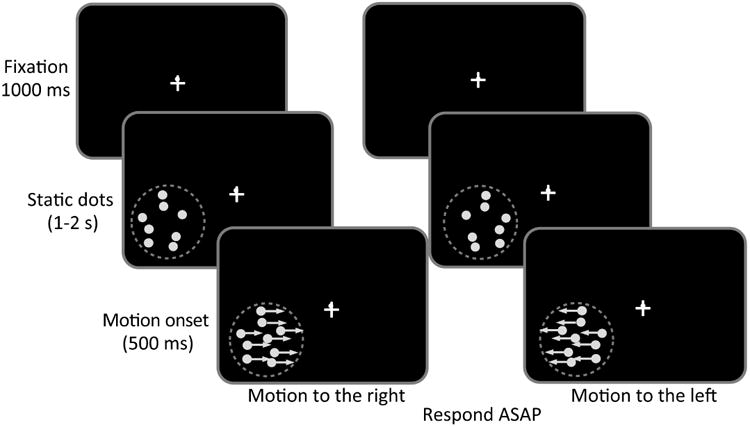
Schematic illustration of the global direction discrimination task performed during acquisition of Visual Evoked Potentials (VEPs). On each trial, after a random inter-trial interval of 1–2 s, a fixation cross appeared in the center of the computer screen for 1 s, followed by a cloud of stationary random dots presented inside a 5 deg diameter window in one of the 4 visual field quadrants. After an interval that varied randomly between 1–2 s, the dots began to move either to the right or the left for 500 ms. Subjects were asked to indicate direction of motion of the stimulus as soon as possible (Respond ASAP).
2.4. EEG Recording and Analysis
A 64-electrode Acti Cap scalp electroencephalogram was used to record EEG with the BrainVision Recorder (BrainVision, Inc., Durham, NC) while subjects performed the left-right, global direction discrimination task detailed above. Data were collected in a single experimental session for each control and CB subject. The EEG recording locations included an on-line and a ground at AFz midline electrodes (Figure 3A). Low and high pass filter settings were 70 Hz and 0.1 Hz, respectively. The cutoff frequencies for these filters were set at 3dB down; the roll off was 12dB per octave at both sides. Impedances were maintained below 10 kΩ for each channel and balanced across all channels within a 5 kΩ range. EEG sampling was set at 500 Hz with 32 bit resolution.
Figure 3.
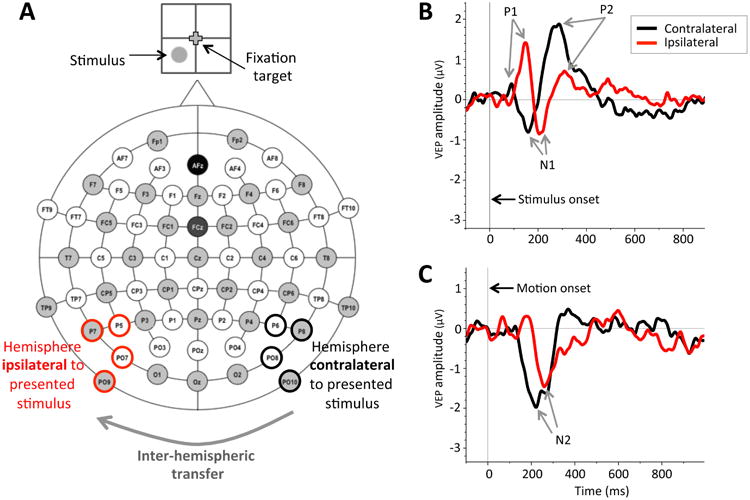
Recording of Visual Evoked Potentials (VEPs) during lateralized stimulus presentation. A: Schematic representation of 64 electrode locations used, with analyzed electrodes highlighted in black (contralateral to stimulus presentation, indicated in the cartoon on top) and red (ipsilateral to stimulus presentation). Ipsilateral responses are thought to arise because of inter-hemispheric transfer from the brain hemisphere contralateral to the presented stimulus. B: Grand average waveforms obtained from the contralateral and ipsilateral brain hemispheres in visually-intact control subjects for stimulus onset VEPs (SO-VEPs) during stimulus presentation in the lower left visual field quadrant (shown in A). Vertical line at time 0 ms indicates random dots onset. P1, N1, and P2 designate SO-VEP components analyzed. The black line shows SO-VEP responses from the brain hemisphere contralateral to the presented stimulus - i.e. from pooled P6, P8, PO8, and PO10 electrodes (outlined in black in A). Red line shows SO-VEP responses from the brain hemisphere ipsilateral to the presented stimulus - i.e., from pooled P5, P7, PO7, and PO9 electrodes (outlined in red in A). C: Grand average waveforms of control subjects for motion onset VEPs (MO-VEPs) illustrating the clear N2 component obtained for stimulus presentation in the lower left quadrant. Vertical line at time 0 indicates the onset of dot motion. As in B, the black waveform represents contralateral MO-VEP response, while the red waveform represents the ipsilateral MO-VEP response. In both B and C, the difference in latency between the red and back peaks/troughs denotes the inter-hemispheric transfer time.
ERP data were analyzed and VEP waveforms were generated using BrainVision Analyzer (BrainVision, Inc.). Eye-blink artifacts were identified, and the EEG was reconstructed without the blink using Independent Component Analysis. Further artifacts were rejected semi-automatically. Then, through DC Detrend and Baseline correction, the average amplitude of a 100 ms pre-stimulus interval was corrected to 0 μV. Averages for each electrode on each participant for each of the four stimulus conditions (four quadrants collapsed across motion direction) were calculated. These averages were then filtered using a Butterworth Zero Phase Filter with a low cutoff of 0.3305 Hz and a high cutoff of 20 Hz, a time constant of 0.4815s, and a roll off of 12 dB per octave. Activity was finally pooled across four posterior parietal-occipital electrodes in each brain hemisphere: for left hemispheres, we pooled across electrodes P7, P5, PO7 and PO9, whereas for right hemispheres, we pooled across electrodes P8, P6, PO8, and PO10 (bolded in Figure 3A). After pooling, we used a peak detection algorithm to determine SO and MO-VEPs components. Components were considered present if their peak was >0.5 μV in amplitude in the majority of subjects and located at approximately the correct latency. From the SO-VEPs, we reliably identified the P1 component (a positive peak amplitude between 80 and 150 ms following static stimulus onset – Figure 3B), the N1 component (a negative peak amplitude between 130 and 180 ms following static stimulus onset – Figure 3B), and the P2 component (a positive peak amplitude between 210 and 300 ms following static stimulus onset – Figure 3B). From MO-VEPs, we identified the N2 component (a negative peak amplitude between 100 and 250 ms following motion onset – Figure 3C). We also tried to identify the MO-P1 component (a positive peak amplitude between 80 and 150 ms following motion onset) as in our previous work (Kavcic, Martin, & Zalar, 2013; Martin, Huxlin, & Kavcic, 2010). However, the MO-P1 component was not sufficiently well-defined and did not reach 0.5μV in amplitude in the majority of our participants during lateralized stimulus presentation. As such, it was not included in the present analyses.
Grand averages were calculated across quadrants of the same hemifield for each control and CB participant, though in CB participants, we separated activity for stimulus placement in intact or blind quadrants of the visual field. We then computed the amplitude and latency of each peak of interest. Within the CB group, we analyzed VEPs across 22 intact visual quadrants (14 in the left visual hemifield, and 8 in the right visual hemifield – red circles in Figure 1), and across 14 impaired visual quadrants (4 in the left visual hemifield and 10 in the right visual hemifield – blue circles in Figure 1).
3. Results
3.1. Global direction discrimination performance is impaired in cortically blind fields
Performance in the intact visual field quadrants of CB participants was similar in terms of both accuracy (Figures 4C, E) and reaction time (RT - Figure 4D, F), to performance seen in visually-intact controls (Figures 4A, B). Since there was no significant difference in performance between intact visual field quadrants in either CB participants or controls, we used t-tests to evaluate differences between the DR0 and DR320 stimuli within and between the two participant groups.
Figure 4.
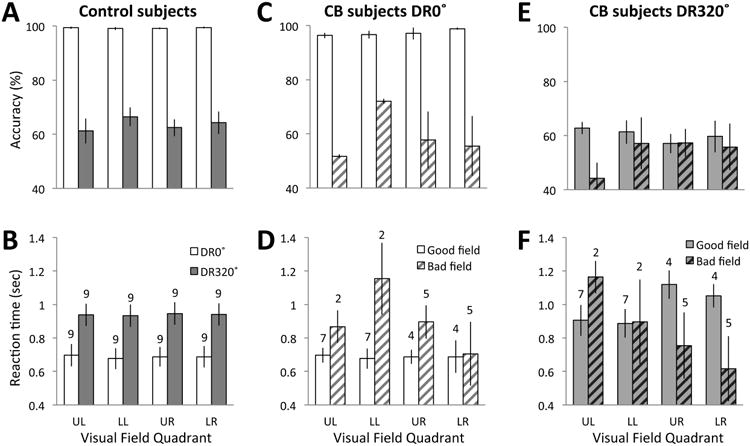
Global direction discrimination performance in each quadrant of the visual field. A: Mean accuracies of control subjects for motion direction discrimination in the DR0 (open bars) and DR320 (grey shaded bars) conditions for all 4 visual field quadrants. B: Average response times of control subjects for motion direction discrimination with respect to DR0 (open bars) and DR320 (grey shaded bars) stimuli presented to each visual field quadrant. C: Plot of average accuracy exhibited by CB subjects when discriminating direction of motion of DR0 motion stimuli, indicated separately for stimulus presentation to intact (open bars) and blind (shaded bars) visual field quadrants. D: Average response times for CB subjects performing the global direction discrimination task using DR0 stimuli, plotted separately for stimulus presentation to intact (open bars) and blind (striped bars) visual field quadrants. E: Average accuracy of CB subjects while performing the direction discrimination task using DR320 stimuli, plotted separately for intact (solid grey bars) and blind (striped grey bars) visual field quadrants. F: Average response times of CB subjects performing the global direction discrimination task using DR320 motion stimuli, plotted separately for intact (solid grey bars) and blind (striped grey bars) visual field quadrants. See text for statistical results. UL = upper left quadrant, LL = lower left quadrant, UR = upper right quadrant, LR = lower right quadrant. Values plotted are means ± SEM. Numbers above bars in lower panels represent the number of participants in the mean for each particular quadrant.
Accuracy
Control participants performed the global direction discrimination task in all four visual field quadrants at nearly 99% correct accuracy when stimuli consisted of coherently-moving random dots (DR0 – white bars in Figure 4A). Similarly, CB participants performed at 97 % correct in their intact visual field quadrants (white bars in Figure 4C). Student's t-tests comparing performance between all quadrants of controls and the intact quadrants of CB participants showed that controls had a slight but significantly higher accuracy for discriminating DR0 stimuli [t(16)=2.301, p=0.035]. When discriminating DR320 random dot stimuli, performance fell to ∼64% correct in control participants and 61% correct in intact quadrants of CB participants (grey bars in Figure 4A and E). Student's t-tests comparing performance between all quadrants of controls and the intact quadrants of CB participants showed that there were no significant difference between the two groups of participants [t(16)=0.673, p=0.51]. When testing the differences between the two task a 2X4 repeated measures ANOVA with stimulus type (DR0, DR320) and visual field quadrant (UL, LL, UR, LR) as within-participant variables showed only a statistically significant main effect of stimulus type [F(1,8)=120.40, p<0.0005], with greater accuracy with DR0 than with DR320 stimuli (mean ± SEM = 99.2 ± 0.1% correct for DR0; 63.6 ± 1.1% correct for DR320). For CB participants, paired-sample t-tests confirmed that in intact visual quadrants, accuracy was significantly higher for DR0 than DR320 stimuli [t(8)=14.439, p=0.002].
For CB participants, we also tested differences in performance between intact and blind quadrants of the visual field. As expected, there was significantly higher accuracy for motion discrimination of DR0 stimuli in intact quadrants relative to blind quadrants [t(7)=6.36; p<0.0005] (Figure 4C). For DR320 stimuli, accuracy for stimulus motion direction discrimination in intact versus blind quadrants was not significantly different, [t(7)=1.256, p=0.249] (Figure 4E).
Reaction times
As expected, control participants discriminated the global direction of motion of DR0 faster than the motion of DR320 random dot stimuli (Figure 4B). A 2X4 repeated measures ANOVA with stimulus (DR0, DR320) and visual field quadrant (UL, LL, UR, LR) as within-participant variables showed only a main effect of stimulus type [F(1,8)=32.00, p<0.0005]. As in controls, when pooling across quadrants, paired-sample t-tests showed that on average, RTs were significantly faster for DR0 stimuli (mean ± SEM = 754 ± 36ms, white bars in Figure 4D) than DR320 stimuli (957 ± 47ms, plain grey bars in Figure 4F; t(20)=6.65, p<0.0005). When comparing control and CB participants, independent-sample t-tests showed no significant differences between the intact quadrants of CB participants and controls [DR0: t(16)=0.644, p=0.528; DR320: t(16)=-0.095, p=0.926].
Within CB participants, paired samples t-tests indicated significant differences in RTs between intact and blind quadrants for DR0 stimuli [t(7)=6.36, p<0.0005, Figure 4D] but not for DR320 stimuli [t(7)=1.256, p=0.25, Figure 4F]. Paired sample t-test showed no significant differences in RTs in blind quadrants between DR0 and DR320 stimuli [t(7)=-1.128, p=0.296].
3.2. VEPs are significantly altered in CB participants
SO-P1, N1, and P2 and MO-N2 components were reliably obtained from pooled left and right posterior electrodes in both control (Figure 5) and CB participants (Figures 6, 7). Our most remarkable finding, however, was that in CB participants, VEP components were only identifiable upon stimulation of the intact visual hemifield (Figure 6). When visual stimuli were presented to blind visual field quadrants (i.e. contralateral to the damaged V1), CB participants did not exhibit recognizable SO- and MO-VEPs in either damaged or intact brain hemispheres (grey traces in Figure 7). Inter-hemispheric transfer results are summarized schematically in Figure 8E.
Figure 5.
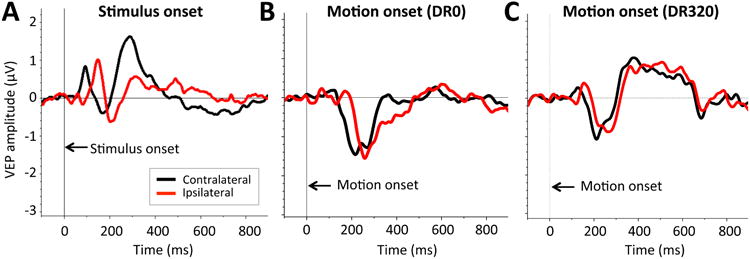
Grand average waveforms in control subjects. A: Mean grand average SO-VEPs averaged across all 4 visual field quadrants. B: Mean grand average MO-VEPs for DR0 motion stimuli. C: Mean grand average MO-VEPs for DR320 motion stimuli. Black traces represent MO-VEP responses from the brain hemisphere contralateral to stimulus presentation. Red traces represent MO-VEP responses from the brain hemisphere ipsilateral to stimulus presentation.
Figure 6.
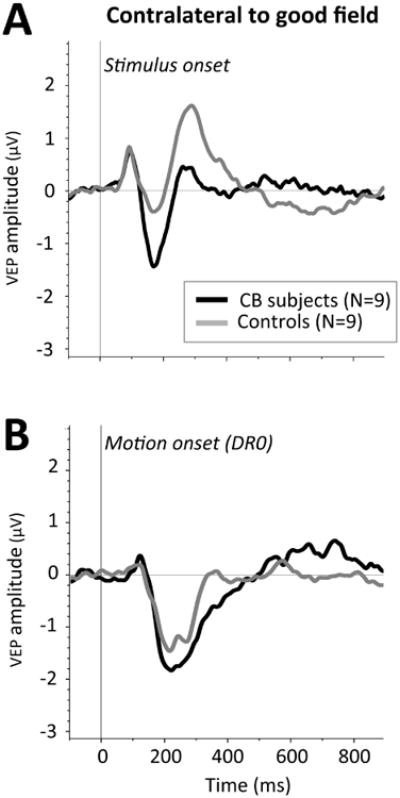
Comparison of grand average waveforms following stimulus presentation to intact visual quadrants of CB subjects (black traces) and corresponding locations in visually-intact controls (grey traces). A: Grand average waveforms for SO-VEPs. B: Grand average waveforms for MO-VEPs.
Figure 7.
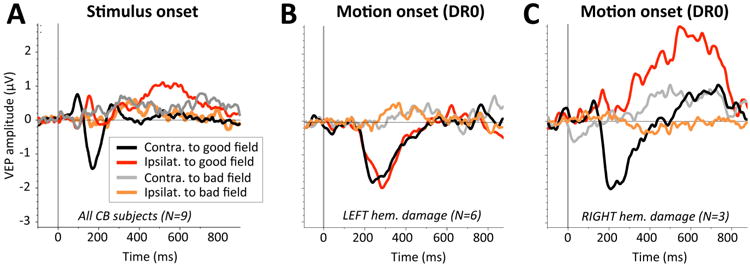
Grand average waveforms obtained in response to stimulus onset (SO-VEPS) and motion onset (MO-VEPs) in CB subjects. A: Grand average SO-VEPs obtained contralateral (black trace) or ipsilateral (red trace) to intact visual field quadrants, and contralateral (gray trace) or ipsilateral (orange trace) to blind field quadrants. B: Grand average MO-VEP waveforms for DR0 motion stimuli obtained in CB subjects with left brain hemisphere damage only. Color conventions as in A. C: Grand average MO-VEP waveforms for DR0 motion stimuli for CB subjects with right brain hemisphere damage only. Color conventions as in A.
Figure 8.
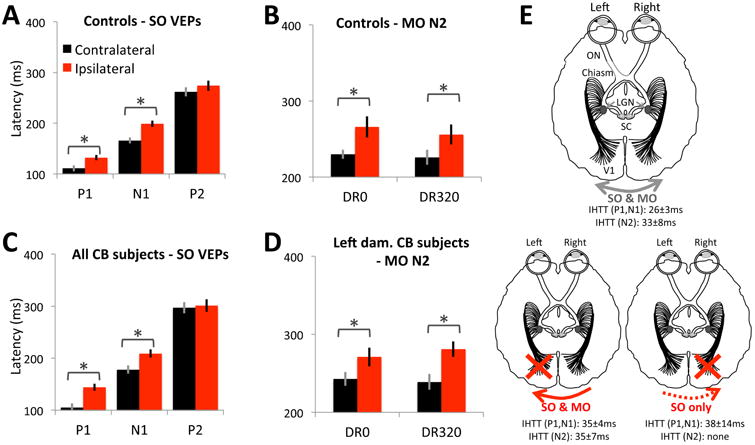
Latencies of visually-evoked components and inter-hemispheric transfer patterns in controls and CB subjects. A. Mean latencies for SO-P1, N1 and P2 components obtained from brain hemispheres contralateral (black bars) and ipsilateral (red bars) to visual stimuli presented to control subjects. A significant delay is observed between contra- versus ipsilaterally evoked responses for all components except for P2. B. Mean latencies for the MO-N2 component elicited from brain hemispheres contralateral and ipsilateral to visual stimuli (either DR0 – left bars, or DR320 – right bars) presented to control subjects. Note the significant delay for information reaching the ipsilateral brain hemisphere. C. Mean latencies for SO-P1, N1 and P2 components obtained from brain hemispheres contralateral and ipsilateral to visual stimuli presented to CB subjects. Data were averaged across all subjects. D. Mean latencies for the MO-N2 component elicited from brain hemispheres contralateral and ipsilateral to visual stimuli (either DR0 or DR320) presented to CB subjects with left brain hemisphere damage. Right-damaged subjects did not exhibit interhemispheric transfer for motion-evoked responses. Error bars = SEM. * = significant differences (see text for statistics). E. Schematic representation of interhemispheric transfer in visually intact controls (top diagram), as well as CB subjects with either left or right brain hemisphere V damage (bottom two diagrams). Mean ± SEM interhemispheric transfer times (IHTT) are provided for SO and MO-VEPs which exhibited them. LGN: lateral geniculate nucleus, SC: superior colliculus.
In control participants, since there were no significant differences in amplitudes and latencies for the VEP components of interest when stimuli were presented to different visual field quadrants, responses were averaged across all 4 quadrants in each participant, then averaged across control participants. Components were then analyzed with a 2 (group) X 2 (brain hemisphere, contralateral vs. ipsilateral to stimulation) mixed factorial ANOVA, except for the MO-N2, in which dot direction range (DR0 and DR320) was added as a repeated measure.
3.2.1. SO-VEPs
SO-P1
P1 responses were clearly discernible in all control participants, and in the brain hemisphere contralateral to stimulation in all CB participants. Ipsilateral to the visual stimulus, the SO-P1 was identifiable in only 8/9 CB participants. SO-P1 amplitudes (Table 2) showed no significant effects of brain hemisphere [F(1,12)=1.218, p=0.291], group [F(1,12)=0.02, p=0.889], nor interaction [F(1,12)=0.186, p=0.674]. For P1 latency (Figures 8A, C, E), there was no significant main effect of group [F(1,12)=0.13, p=0.725] but, there was a significant main effect of brain hemisphere [F(1,12)=108.11, p<0.0005], and an interaction between group and brain hemisphere [F(1,12)=9.171, p=0.01]. Evoked response latency was indeed shorter on the contralateral side for both groups, but more so for CB than control participants (P1 IHTTs in controls were 20±3ms, versus 43±7ms in CB participants; two-tailed, independent t-test, t15=3.25, p=0.0054).
Table 2. Amplitude of stimulus onset (SO-) components following stimulus presentation to intact visual field quadrants in control and CB subjects.
Values are mean ± SEM for SO-P1, SO-N1, and SO-P2, computed separately for electrodes contralateral and ipsilateral to stimuli presentation. Data were averaged across quadrants in each subject group. Shaded pairs of values denote significant differences between contralateral and ipsilateral stimulus presentations.
| SO-P1 (μV) | SO-N1 (μV) | SO-P2 (μV) | ||
|---|---|---|---|---|
| Controls | ||||
| Contra. | 1.2 ± 0.3 | -1.1 ± 0.3 | 1.5 ± 0.4 | |
| Ipsi. | 1.0 ± 0.2 | -0.8 ± 0.3 | 0.8 ± 0.2 | |
|
| ||||
| CB subjects | ||||
| Contra. | 0.9 ± 0.3 | -2.8 ± 0.4 | 1.4 ± 0.4 | |
| Ipsi. | 1.3 ± 0.3 | -1.4 ± 0.4 | 0.9 ± 0.3 | |
SO-N1
As with P1, a reliable N1 > -1μV in peak amplitude was identifiable in all controls, in all CB participants' contralateral brain hemispheres, and in 8/9 CB participants over the ipsilateral brain hemisphere. N1 amplitudes were significantly greater over contralateral than ipsilateral brain hemispheres (Table 2) - the main effect of brain hemisphere was significant [F(1,12)=30.699, p<0.0005], as was the interaction between brain hemisphere and group [F(1,12)=18.59, p=0.001]. However, the effect of group was only marginal [F(1,12)=4.507, p=0.055]. The SO-N1 latencies (Figures 8A, C, E) showed a significant main effect of brain hemisphere [F(1,12)=65.296, p<0.0005], with earlier peaks over contralateral than ipsilateral sides. Since average IHTTs were similar in CB and control participants (IHTTCB=28±8ms; IHTTcontrols=33±4ms), there was no main effect of group [F(1,12)=1.22, p= 0.291], and no interaction between brain hemisphere and group on latency [F(1,12)=0.075, p=0.788].
SO-P2
The P2 component was clearly identifiable in all control participants, in all CB participants' contralateral brain hemispheres, and in 8/9 CB participants' ipsilateral brain hemispheres. P2 amplitudes were significantly greater over contralateral than ipsilateral brain hemispheres (Table 2; F(1,13)=9.461, p=0.009). However, there was no main effect of group [F(1,13)=0.001, p=0.982], and brain hemisphere did not interact with group [F(1,13)=0.141, p=0.713]. P2 latencies (Figures 8A, C, E) were not significantly different between brain hemispheres [F(1,15)=3.469, p=0.082] or groups [F(1,15)=1.03, p=0.326], and there was no significant interaction between brain hemisphere and group [F(1,13)=0.289, p=0.6].
3.2.2. MO-VEPs
The MO-P1 component did not reach 0.5μV in amplitude in a sufficient proportion of participants (whether controls or CB) to warrant inclusion in subsequent analyses. In contrast, when motion stimuli were presented to controls or the intact visual field quadrants of CB participants, reliable MO-N2 components were obtained in at least one brain hemisphere. In participants with left brain hemisphere damage (N=6), MO-VEPs could be recorded in both brain hemispheres – i.e. in the one contralateral to the stimulus (intact right hemisphere – see black traces in Figure 7B), as well as in the brain hemisphere ipsilateral to the stimulus (left hemisphere containing the V1 damage – red traces in Figure 7B). Given the anatomical organization of the early visual system, the visually-evoked activity in the brain hemisphere ipsilateral to the stimulus presentation likely arose via callosal input from the intact, right brain hemisphere. In contrast, participants with right brain hemisphere damage (N=3) showed no measurable, indirect [callosal] information transfer to the damaged brain hemisphere following motion onset in their intact hemifield of vision (red trace in Figure 7C). This occured in spite of a strongly elicited MO-N2 in the intact brain hemisphere contralateral to stimulus presentation (black trace in Figure 7C).
For MO-N2 amplitudes (Table 3), there was a main effect of direction range [F(1,12)=27.232, p<0.0005], with greater amplitude following lateralized presentation of coherently-moving dot stimuli. However, there were no significant effects of brain hemisphere [F(1,12)=0.001, p=0.97] or group [F(1,12)=0.526, p=0.482]. Nor were the interactions between brain hemisphere and group [F(1,12)=0.231, p=0.64], direction range and brain hemisphere [F(1,12)=0.114, p=0.742], or the three-way interaction [F(1,12)=0.084, p=0.777] significant. However, the interaction between group and direction range was significant [F(1,12)=16.575, p=0.002]. Coherent motion (DR0 stimuli) generated larger VEP amplitudes than DR320 stimuli, and this effect was much larger for CB than control participants (Table 3).
Table 3. Motion-onset N2 component amplitudes following stimulus presentation to intact visual field quadrants in control and CB subjects (left hemisphere damage only).
Values are means ± SEM, averaged across visual field quadrants for contralateral and ipsilateral global motion onsets in random dot stimuli. See text for statistical comparisons.
| MO-N2 (μV) | ||
|---|---|---|
| Controls | DR0 | |
| Contralateral | -2.0 ± 0.4 | |
| Ipsilateral | -2.0 ± 0.3 | |
| DR320 | ||
| Contralateral | -1.6 ± 0.4 | |
| Ipsilateral | -1.4 ± 0.2 | |
|
| ||
| CB subjects | DR0 | |
| Contralateral | -2.2 ± 0.3 | |
| Ipsilateral | -2.0 ± 0.3 | |
| DR320 | ||
| Contralateral | -1.5 ± 0.2 | |
| Ipsilateral | -1.4 ± 0.2 | |
For N2 latency (Figures 8B, D, E), there was a main effect of brain hemisphere [F(1,13)=29.031, p<0.0005], with earlier peaks over contralateral than ipsilateral brain hemispheres. The main effect of direction range was not significant [F(1,13)=1.71, p=0.214], but the main effect of group was significant [F(1,13)=7.718, p=0.016], with longer latencies for CB than control participants. The interaction between group and direction range was also significant [F(1,13)=4.991, p=0.044]. No other interactions were significant - whether between brain hemisphere and group [F(1,13)=0.377, p=0.55], brain hemisphere and direction range [F(1,13)=0.002, p=0.968], or the 3-way interaction [F(1,13)=0.763, p=0.398]. IHTTs for presentation to intact regions of the visual field of CB participants were similar for DR0 and DR320 (37±12ms and 32±8ms, respectively), and neither mean was significantly different from that obtained in visually-intact controls [t(16) = 0.445, p = 0.663].
4. Discussion
The present study allowed us to make two important determinations: first, there was a close correlation between visual perception and motion onset VEPs obtained from contralateral, occipito-temporal electrodes in all participants tested. Second, lateralized stimulus presentations inside CB fields elicited no measurable VEPs in contralateral [damaged] brain hemispheres. However, the same, damaged brain hemispheres exhibited reliable, albeit delayed SO-VEPs [though not MO-VEPs] following intact hemifield stimulation, suggesting that they could be activated via callosal input from the intact brain hemisphere.
4.1. VEPs from lateralized stimulus presentation reflect visual discrimination performance
Consistent with prior reports of poor direction discrimination of complex motion stimuli in cortically blind fields (P. Azzopardi & Cowey, 2001; Huxlin, et al., 2009), the nine CB participants in the present study were unable to discriminate the global direction of motion of coherently-moving or noisy, random dot stimuli in their blind visual quadrants. This was not due to misunderstanding of the task demands, since participants exhibited completely normal performance - both in terms of accuracy and RTs - in intact regions of their visual field.
As predicted from the existing literature (Ceponiene, et al., 2008; Di Russo, et al., 2002; Muller & Knight, 2002; Schendan & Kutas, 2007; Talsma & Kok, 2002), appearance of stimuli in intact visual field quadrants generated well defined SO-P1, SO-N1, and SO-P2 components in all participants. While the cerebral origins of SO-VEP components are still debated, the general consensus is that SO-P1 and N1 components are likely mediated by extra-striate visual cortex and that they represent initial sensory-perceptual encoding (Di Russo, Martinez, Sereno, Pitzalis, & Hillyard, 2002; Hillyard, Vogel, & Luck, 1998; Mangun, Hillyard, & Luck, 1990; Natale, Marzi, Girelli, Pavone, & Pollmann, 2006; Schechter et al., 2005; Woldorff et al., 1997). In contrast, the later P2 component has been proposed to index some aspect of visual working memory (Ceponiene, Westerfield, Torki, & Townsend, 2008; Lefebvre, Marchand, Eskes, & Connolly, 2005; Wolach & Pratt, 2001). MO-VEPs from occipito-parietal electrodes include P1 and N2 components, which are thought to originate in the human MT complex (hMT+) (Huk, Dougherty, & Heeger, 2002; Kau et al., 2013; Tootell et al., 1995), with the MO-P1 component reflecting local pattern processing, and the MO-N2 reflecting processing of visual motion (Bach & Ullrich, 1997; Kuba, Kremlacek, & Kubova, 1998).
In contrast to stimulation of intact quadrants of the visual field, stimulus presentation to blind quadrants in our CB participants elicited no detectable SO-P1, SO-N1, SO-P2 or MO-N2. This is generally consistent with prior EEG observations in this population (Aldrich, et al., 1987; Biersdorf, et al., 1992; Blumhardt, et al., 1977; Blumhardt, et al., 1982; Brigell, et al., 1990; Celesia & Brigell, 1999; Celesia, et al., 1983; Kuroiwa & Celesia, 1981; Onofrj, et al., 1982; Watanabe, et al., 2007). Previous studies that reported partial VEPs in CB patients used different tasks and electrode selections than the present experiments, and importantly, they did not examine responses to lateralized stimuli (Anderson, et al., 1996; Bodis-Wollner, et al., 1977; Celesia, et al., 1980; Celesia & Brigell, 1999; Ffytche, et al., 1995; Holliday, et al., 1997). Two prior reports, which used lateralized presentations in CB fields (Benson, Guo, & Hardiman, 1999; Rossion, et al., 2000) were both done in the famous patient GY. One of them (Benson, et al., 1999) contrasted VEPs in the intact and blind visual hemifields, and found decreased amplitudes and increased latencies for the SO-C1 and MO-N2 components in the blind hemifield compared to those elicited by stimulus presentation to the patient's intact visual hemifield. However, GY suffered his occipital damage at a relatively early age (8 years old) and he possesses abnormal (enhanced) inter-hemispheric connectivity as early in his visual system as the dLGN (Bridge, Thomas, Jbabdi, & Cowey, 2008; Ptito, Johannsen, Faubert, & Gjedde, 1999; Silvanto, Walsh, & Cowey, 2009). This abnormal connectivity may explain the presence of VEPs in his damaged brain hemisphere upon blind field stimulation and suggests that GY does not represent a valid comparison for the adult-onset, V1-damaged participants who form the majority of the cortically blind patient population, and were examined in the present study.
The lack of SO- and MO-VEPs from stimuli presented to cortically blind fields, while consistent with the patients' lack of global discrimination abilities and much of the prior literature, is nevertheless puzzling. Indeed, area MT, which is critical for complex motion discrimination in primates (Lu, Qian, & Liu, 2004; Newsome & Paré, 1988; Pasternak & Merigan, 1994; Thompson & Liu, 2006), is usually intact and responsive in many CB participants (Barbur, Watson, Frackowiak, & Zeki, 1993; Bridge et al., 2010; Goebel, Muckli, Zanella, Singer, & Stoerig, 2001; Martin, Das, & Huxlin, 2012; Ptito, et al., 1999), and is suggested to play an important role in mediating blindsight (Alexander & Cowey, 2009). Thus, it was surprising to see no discernable waveforms elicited by a complex motion task that should have elicited responses from hMT+. In fact, we know that two of the present CB participants (CB1 and 9, who were used in a prior fMRI study (Martin, et al., 2012)), exhibited significant BOLD responses in hMT+ and extra-striate cortex in their damaged brain hemispheres upon presentation of global motion stimuli in their blind field (Martin, et al., 2012). There are several reasons why VEPs may fail to reflect activity in hMT+ of CB participants, even if such activity is present during fMRI. First, the direct projection from dLGN to hMT+ is primarily koniocellular (Sincich, Park, Wohlgemuth, & Horton, 2004) and consists of far fewer fibers than the projection from V1 or V2 to MT. As such, it may not be sufficient to adequately synchronize dendritic currents in MT even if it changes firing rates in many neurons. A change in firing rates may be sufficient to alter BOLD signal, but not to be observable at scalp electrodes. Another possibility is that without a feedback loop between MT and V1, there is insufficient, sustained activity in MT to generate a detectable VEP signal with the small, random dot stimuli used here.
4.2. Inter-hemispheric transfer generates VEPs in V1-damaged brain hemispheres
Overall, our results showed two unexpected finding: 1) a significant difference between left and right CB participants in terms of neuro-electric transfer of MO-VEPs, with left blind participants showing an apparent failure of IHT; and 2) greater IHTTs in blind participants compared to controls for SO-P1. Thus, while both brain hemispheres can transfer SO-related activity to each other, it appears that only intact right brain hemispheres can transfer motion-related activity to damaged, left brain hemispheres - not the other way around - and that for SO-P1, transfer is slower when it does happen (summarized in Figure 8E).
Is there something special about right brain hemispheres in terms of their ability to transfer visual motion information to left brain hemispheres? Marzi and colleagues (Marzi, Bisiacchi, & Nicoletti, 1991) argued that in normal participants, there may be an asymmetry in callosal connections, with more neurons projecting from right to left brain hemispheres than vice versa. Alternatively, there may be a relative abundance of fast-conducting, myelinated axons originating in the right brain hemisphere, relative to the left. This would result in both increased activation and faster transfer of information from right-to-left brain hemispheres (Barnett & Corballis, 2005). Partial support for this model was provided by Miller (Miller, 1996), who reviewed 15 studies and found that 14 showed the right brain hemisphere to be larger than the left, likely due to a larger number of neurons and myelinated axons. This is consistent with experimental observations of faster transfer of visual information from right to left brain hemisphere than vice-versa, based on behavioral studies (Brown, et al., 1994; Larson & Brown, 1997), as well as electrophysiological ones (Barnett & Corballis, 2005; Patston, Kirk, Rolfe, Corballis, & Tippett, 2007). However, none of these models predict our observed absence of IHT from intact left occipital cortex to damaged right brain hemispheres. Possible explanations for this may include different brain lesions in our cohort of left- and right-hemisphere-damaged participants, or that damage in the corona radiata and striate cortex affected hemispheric interactions differentially in the left versus right brain hemispheres. As for why SO-P1 IHTTs were longer in blind participants compared to controls, this also remains a matter of speculation. Our controls exhibited IHTTs within the normal range of values previously reported (Barnett & Corballis, 2005; Brown, et al., 1994; Saron & Davidson, 1989). The most likely hypothesis for greater IHTTs in patients with V1 damage is that such lesions could desynchronize the hemispheric interplay, thus slowing component latencies with respect to stimulus onset.
5. Conclusions
VEPs could be reliably elicited from both intact and damaged brain hemispheres in stroke patients with cortical blindness. However, in damaged brain hemispheres, SO-VEPs could only be recorded following stimulus presentation to intact visual field quadrants, via inter-hemispheric transfer. MO-VEPs were even more limited, and could only be recorded from damaged left brain hemispheres (i.e. when the right brain hemispheres were intact and able to transmit the information), possibly reflecting a native asymmetry in inter-hemispheric connections. Finally, the generation of VEPs by canonical, contralateral visual processing appeared to most closely reflect the presence of normal global motion perception. When such VEPs were absent, so was global motion perception. As such, MO-VEPS may serve as a useful diagnostic tool for evaluating visual capacities in CB participants, especially if conscious vision returns in parts of the blind field following visual rehabilitation.
Acknowledgments
The authors thank Dana Iorizzo, Emily Foley, and Melissa Skevington for assisting with EEG data collection and patient histories. We thank Terry Schaeffer and Dorothea Castillo from the Flaum Eye Institute for performing Humphrey perimetry on all patients. We also thank Dr. Antoine Barbot for constructive feedback on the manuscript. This work was supported by a grant from the NEI to KRH (EY021209), a Collaborative Grant from the Schmitt Program on Integrative Brain Research (to KRH), a Center for Visual Science (CVS) summer undergraduate fellowship to RLT (sponsored by NEI training grant T32 EY007125 to the CVS), an NEI Center Core grant to the CVS (P30 EY001319), an unrestricted grant from the Research to Prevent Blindness Foundation (RPB) to the Flaum Eye Institute and by a grant from the Alzheimer's Association to VK (HAT-07-60437). KRH is an RPB Lew R. Wasserman Merit Award recipient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldrich MS, Alessi AG, Beck RW, Gilman S. Cortical blindness: etiology, diagnosis, and prognosis. Annals of Neurology. 1987;21(2):149–158. doi: 10.1002/ana.410210207. [DOI] [PubMed] [Google Scholar]
- Alexander I, Cowey A. The cortical basis of global motion detection in blindsight. Experimental Brain Research. 2009;192:407–411. doi: 10.1007/s00221-008-1508-4. [DOI] [PubMed] [Google Scholar]
- Anderson SJ, Holliday IE, Singh KD, Harding GFA. Localization and functional analysis of human cortical area V5 using magneto-encephalography. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1996;263(1369):423–431. doi: 10.1098/rspb.1996.0064. [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A. Blindsight and visual awareness. Consciousness and Cognition. 1998;7:292–311. doi: 10.1006/ccog.1998.0358. [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A. Motion discrimination in cortically blind patients. Brain. 2001;124:30–46. doi: 10.1093/brain/124.1.30. [DOI] [PubMed] [Google Scholar]
- Bach M, Ullrich D. Contrast dependency of motion-onset and pattern-reversal VEPs: Interaction of stimulus type, recording site and response component. Vision Research. 1997;37:1845–1849. doi: 10.1016/s0042-6989(96)00317-3. [DOI] [PubMed] [Google Scholar]
- Barbur JL, Watson JDG, Frackowiak RSJ, Zeki S. Conscious visual perception without V1. Brain. 1993;116(Part 6):1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Corballis MC. Speeded right-to-left information transfer: the result of speeded transmission in right-hemisphere axons? Neuroscience Letters. 2005;380(1-2):88–92. doi: 10.1016/j.neulet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Benson PJ, Guo K, Hardiman MJ. Cortical evoked potentials due to motion contrast in the blind hemifield. NeuroReport. 1999;10(17):3595–3600. doi: 10.1097/00001756-199911260-00024. [DOI] [PubMed] [Google Scholar]
- Bergman PS. Cerebral blindness: an analysis of twelve cases, with especial reference to the electroencephalogram and patterns of recovery. Archives of Neurology and Psychiatry. 1957;78:568–684. [PubMed] [Google Scholar]
- Biersdorf WR, Bell RA, Beck RW. Pattern flash visual evoked potentials in patients with homonymous hemianopia. Documenta Ophthalmologica: Advances in Ophthalmology. 1992;80(1):51–61. doi: 10.1007/BF00161231. [DOI] [PubMed] [Google Scholar]
- Blumhardt LD, Barrett GD, Halliday AM. The asymmetrical visual evoked potential to pattern reversal in one half field and its significance for the analysis of visual field defects. British Journal of Ophthalmology. 1977;61(7):454–461. doi: 10.1136/bjo.61.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumhardt LD, Barrett GD, Kriss A, Halliday AM. The pattern-evoked potential in lesions of the posterior visual pathways. Annals of the New York Academy of Sciences. 1982;388:264–289. doi: 10.1111/j.1749-6632.1982.tb50796.x. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Atkin A, Raab E, Wolkstein M. Visual association cortex and vision in man: pattern-evoked occipital potentials in a blind boy. science. 1977;198(4317):629–631. doi: 10.1126/science.918658. [DOI] [PubMed] [Google Scholar]
- Bouwmeester L, Heutink J, Lucas C. The effect of visual training for patients with visual field defects due to brain damage: a systematic review. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78:555–564. doi: 10.1136/jnnp.2006.103853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia, I: Detection performance in a driving simulator. Invest Ophthalmol Vis Sci. 2009;50(11):5137–5147. doi: 10.1167/iovs.09-3799. doi:iovs.09- 3799 [pii] 10.1167/iovs.09-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Hicks SL, Xie J, Okell TW, Mannan SK, Alexander I, et al. Kennard C. Visual activation of extra-striate cortex in the absence of V1 activation. Neuropsychologia. 2010;48:4148–4154. doi: 10.1016/j.neuropsychologia.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Thomas O, Jbabdi S, Cowey A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain. 2008;131(Pt 6):1433–1444. doi: 10.1093/brain/awn063. [DOI] [PubMed] [Google Scholar]
- Brigell MG, Celesia GG, Salvi F, Clark-Bash R. Topographic mapping of electrophysiologic measures in patients with homonymous hemianopia. Neurology. 1990;40:1566–1570. doi: 10.1212/wnl.40.10.1566. [DOI] [PubMed] [Google Scholar]
- Brown WS, Larson EB, Jeeves MA. Directional asymmetries in interhemispheric transmission time: evidence from visual evoked potentials. Neuropsychologia. 1994;32:439–448. doi: 10.1016/0028-3932(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Archer CR, Kuroiwa Y, Goldfader PR. Visual function of the extrageniculo-calcarine system in man: relationship to cortical blindness. Archives of Neurology. 1980;37(11):704–706. doi: 10.1001/archneur.1980.00500600052010. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Brigell MG. Cortical blindness and visual processing. Electroencephalography and Clinical Neurophysiology Supplement. 1999;49:133–141. [PubMed] [Google Scholar]
- Celesia GG, Meredith JT, Pluff K. Perimetry, visual evoked potentials and visual evoked spectrum array in homonymous hemianopsia. Electroencephalography and Clinical Neurophysiology. 1983;56(1):16–30. doi: 10.1016/0013-4694(83)90003-2. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Westerfield M, Torki M, Townsend J. Modality-specificity of sensory aging in vision and audition: evidence from event-related potentials. Brain Research. 2008;1215:53–68. doi: 10.1016/j.brainres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Cole C. When the left brain is not right the right brain may be left: report of personal experience of occipital hemianopia. J Neurol Neurosurg Psychiatr. 1999;67:169–173. doi: 10.1136/jnnp.67.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Stoerig P. The neurobiology of blindsight. Trends in Neurosciences. 1991;14(4):140–145. doi: 10.1016/0166-2236(91)90085-9. [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoerig P. Blindsight in monkeys. Nature. 1995;373:247–249. doi: 10.1038/373247a0. [DOI] [PubMed] [Google Scholar]
- Das A, Huxlin KH. New Approaches to Visual Rehabilitation for Cortical Blindness: Outcomes and Putative Mechanisms. The Neuroscientist. 2010;16(4):374–387. doi: 10.1177/1073858409356112. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez O, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human Brain Mapping. 2002;15(2):95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Guy CN, Zeki S. The Parallel Visual Motion Inputs Into Areas V1 and V5 Of Human Cerebral Cortex. Brain. 1995;118(Part 6):1375–1394. doi: 10.1093/brain/118.6.1375. [DOI] [PubMed] [Google Scholar]
- ffytche DH, Guy CN, Zeki S. Motion specific responses from a blind hemifield. Brain. 1996;119(6):1971–1982. doi: 10.1093/brain/119.6.1971. [DOI] [PubMed] [Google Scholar]
- Fujino T, Kigizawa K, Yamada R. Homonymous hemianopia: a retrospective study of 140 cases. Neuro-Ophthalmology. 1986;6:17–21. [Google Scholar]
- Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies in hemianopic patients. Vision Research. 2001;41:1459–1474. doi: 10.1016/s0042-6989(01)00069-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge IF, McDonald RA. Hemianopic visual field loss as the first clinical evidence of occipital arteriovenous malformation. Clin Exp Optom. 2004;87(6):394–399. doi: 10.1111/j.1444-0938.2004.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical Transactions of The Royal Society B. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Ungerleider LG, Adams MM, Webster MJ, Gattass R, Blumberg DM, Morrison JH. Callosally Projecting Neurons In the Macaque Monkey V1/V2 Border Are Enriched In Nonphosphorylated Neurofilament Protein. Visual Neuroscience. 1997;14(5):981–987. doi: 10.1017/s0952523800011688. [DOI] [PubMed] [Google Scholar]
- Holliday IE, Anderson SJ, Harding GFA. Magnetoencephalographic evidence for non-geniculostriate visual input to human cortical area V5. Neuropsychologia. 1997;35(8):1139–1146. doi: 10.1016/s0028-3932(97)00033-x. [DOI] [PubMed] [Google Scholar]
- Holmes G. Disturbances of vision by cerebral lesions. British Journal of Ophthalmology. 1918;2:353–384. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The cortical localization of vision. British Medical Journal. 1919;2:193–199. doi: 10.1136/bmj.2.3059.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC. Disappointing results from Nova Vision's visual restoration therapy. Br J Ophthalmol. 2005;89(1):1–2. doi: 10.1136/bjo.2004.058214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. Journal of Neurophysiology. 1967;30(6):1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. Journal of Neuroscience. 2002;22(16):7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxlin KR, Riley ME, Martin T, Kelly KN, Friedman DI, Burgin WS, Hayhoe M. Perceptual re-learning of complex visual motion after V1 damage in humans. Journal of Neuroscience. 2009;29(13):3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau S, Strumpf H, MErkel C, Stoppel CM, Heinze HJ, Hopf JM, Schoenfeld MA. Distinct neural correlates of attending speed vs. coherence of motion. NeuroImage. 2013;64:299–307. doi: 10.1016/j.neuroimage.2012.08.080. [DOI] [PubMed] [Google Scholar]
- Kavcic V, Martin T, Zalar B. Aging effects on visual evoked potentials (VEPs) for motion direction discrimination. International Journal of Psychophysiology. 2013;89:78–87. doi: 10.1016/j.ijpsycho.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Dehay C, Bullier J. Organization of the callosal connections of visual areas V1 and V2 in the macaque monkey. Journal of Comparative Neurology. 1986;247(3):398–415. doi: 10.1002/cne.902470309. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Neurovisual rehabiliation: recent developments and future directions. Journal of Neurology, Neurosurgery and Psychiatry. 2000;68:691–706. doi: 10.1136/jnnp.68.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba M, Kremlacek J, Kubova Z. Cognitive evoked potentials related to visual perception of motion in human subjects. Physiological Research - Academia Scientiarum Bohemoslovaca. 1998;47(4):265–270. [PubMed] [Google Scholar]
- Kuroiwa Y, Celesia GG. Visual evoked potentials with hemifield pattern stimulation. Their use in the diagnosis of retrochiasmatic lesions. Archives of Neurology. 1981;38(2):86–90. doi: 10.1001/archneur.1981.00510020044005. [DOI] [PubMed] [Google Scholar]
- Larson EB, Brown WS. Bilateral field interactions, hemispheric specialization and evoked potential interhemispheric transmission time. Neuropsychologia. 1997;35:573–581. doi: 10.1016/s0028-3932(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Lawton Smith J. Homonymous hemianopia. American Journal of Ophthalmology. 1962;54:616–623. [PubMed] [Google Scholar]
- Lefebvre CD, Marchand Y, Eskes GA, Connolly JF. Assessment of working memory abilities using an event-related brain potential (ERP)-compatible digit span backward task. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2005;116(7):1665–1680. doi: 10.1016/j.clinph.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lu H, Qian N, Liu Z. Learning motion discrimination with suppressed MT. Vision Research. 2004;44:1817–1825. doi: 10.1016/j.visres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA, Luck SJ. Electrocortical Substrates of Visual Selective Attention. In: Meyers DE, Kornblum S, editors. Attention and Performance XIV: synergies in Experimental Psychology, Artificial Intelligence, and Cognitive Neuroscience. 1990. pp. 219–243. [Google Scholar]
- Martin T, Das A, Huxlin KR. Visual cortical activity reflects faster accumulation of information from cortically blind fields. Brain. 2012;135:3440–3452. doi: 10.1093/brain/aws272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Huxlin KR, Kavcic V. Motion-onset visual evoked potentials predict performance during a global direction discrimination task. Neuropsychologia. 2010;48:3563–3572. doi: 10.1016/j.neuropsychologia.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi CA, Bisiacchi P, Nicoletti R. Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia. 1991;34(2):1163–1177. doi: 10.1016/0028-3932(91)90031-3. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Topographic organization of the middle temporal visual area in the macaque monkey: representational biases and the relationship to callosal connections and myeloarchitectonic boundaries. Journal of Comparative Neurology. 1987;266(4):535–555. doi: 10.1002/cne.902660407. [DOI] [PubMed] [Google Scholar]
- McDonald SA, Spitsyna G, Shillcock RC, Wise RJ, Leff AP. Patients with hemianopic alexia adopt an inefficient eye movement strategy when reading text. Brain. 2006;129:158–167. doi: 10.1093/brain/awh678. [DOI] [PubMed] [Google Scholar]
- Miller R. Axonal Conduction Time and Human Cerebral Laterality: A Psychobiological Theory. Amsterdam: Harwood Academic; 1996. [Google Scholar]
- Muller NG, Knight RT. Age-related changes in fronto-parietal networks during spatial memory: an ERP study. Cognitive Brain Research. 2002;13(2):221–234. doi: 10.1016/s0926-6410(01)00119-7. [DOI] [PubMed] [Google Scholar]
- Natale E, Marzi CA, Girelli M, Pavone EF, Pollmann S. ERP and fMRI correlates of endogenous and exogenous focusing of visual spatial attention. European Journal of Neuroscience. 2006;23:2511–2521. doi: 10.1111/j.1460-9568.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Paré EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrj M, Bodis-Wollner I, Mylin L. Visual evoked potential diagnosis of field defects in patients with chiasmatic and retrochiasmatic lesions. Journal of Neurology, Neurosurgery & Psychiatry. 1982;45:294–302. doi: 10.1136/jnnp.45.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambakian ALM, Kennard C. Can visual function be restored in patients with homonymous hemianopia. British Journal of Ophthalmology. 1997;81:324–328. doi: 10.1136/bjo.81.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Merigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cerebral Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- Patston LLM, Kirk IJ, Rolfe MHS, Corballis MC, Tippett LJ. The unusual symmetry of musicians: Musicians have equilateral interhemispheric transfer for visual information. Neuropsychologia. 2007;45:2059–2065. doi: 10.1016/j.neuropsychologia.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Perenin MT, Jeannerod M. Residual vision in cortically blind hemifields. Neuropsychologia. 1975;13:1–7. doi: 10.1016/0028-3932(75)90041-x. [DOI] [PubMed] [Google Scholar]
- Pöppel E, Held R, Frost D. Residual visual function after brain wounds involving the central visual pathways in man. Nature. 1973;243:295–296. doi: 10.1038/243295a0. [DOI] [PubMed] [Google Scholar]
- Ptito M, Johannsen P, Faubert J, Gjedde A. Activation of human extrageniculostriate pathways after damage to area V1. NeuroImage. 1999;9:97–107. doi: 10.1006/nimg.1998.0390. [DOI] [PubMed] [Google Scholar]
- Raninen A, Vanni S, Hyvärinen L, Näsänen R. Temporal sensitivity in a hemianopic visual field can be improved by long-term training using flicker stimulation. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;78:66–73. doi: 10.1136/jnnp.2006.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, S A, Schiefer U, Sabel BA, Kenkel S, Vontheim R, Trauzettel-Klosinski S. Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. British Journal of Ophthalmology. 2005;89:30–35. doi: 10.1136/bjo.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch G. Dissociations of visual perceptions due to occipital injuries, with especial reference to appreciation of movement. Brain. 1917;40:15–57. [Google Scholar]
- Rossion B, de Gelder B, Pourtois G, Guerit JM, Weiskrantz L. Early extrastriate activity without primary visual cortex in humans. Neuroscience Letters. 2000;279:25–28. doi: 10.1016/s0304-3940(99)00926-x. [DOI] [PubMed] [Google Scholar]
- Sahraie A, MacLeod MJ, Trevathan CT, Robson S, Olson JA, Callaghan P, Yip B. Improved detection following Neuro-Eye Therapy in patients with post-geniculate damage. Experimental Brain Research. 2010;206:25–34. doi: 10.1007/s00221-010-2395-z. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ, Murray AD, Olson JA, Weiskrantz L. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(40):14971–14976. doi: 10.1073/pnas.0607073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saron CD, Davidson RJ. Visual evoked potential measures of interhemispheric transfer time in humans. Behavioral Neuroscience. 1989;103:1115–1138. doi: 10.1037//0735-7044.103.5.1115. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, et al. Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clinical Neurophysiology. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Kutas M. Neurophysiological evidence for the time course of activation of global shape, part, and local contour representations during visual object categorization and memory. Journal of Cognitive Neuroscience. 2007;19(5):734–749. doi: 10.1162/jocn.2007.19.5.734. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Walsh V, Cowey A. Abnormal functional connectivity between ipsilesional V5/MT+ and contralesional striate cortex (v1) in blindsight. Experimental Brain Research. 2009;193:645–650. doi: 10.1007/s00221-009-1712-x. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct genicular input fo area MT. Nature Neuroscience. 2004;7(10):1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Spitzyna GA, Wise RJS, McDonald SA, Plant GT, Kidd D, Crewes H, et al. Optokinetic therapy improves test reading in patients with hemianopic alexia. Neurology. 2007;68:1922–1930. doi: 10.1212/01.wnl.0000264002.30134.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerig P. Functional rehabilitation of partial cortical blindness? Restorative Neurology and Neuroscience. 2008;26:291–303. [PubMed] [Google Scholar]
- Talsma D, Kok A. Intermodal spatial attention differs between vision and audition: an event-related potential analysis. Psychophysiology. 2002;39(6):689–706. [PubMed] [Google Scholar]
- Teuber HL, Battersby WS, Bender MB. Visual Field Defects After Penetrating Missile Wounds of the Brain. Cambridge, Massachusetts: Harvard University Press; 1960. [Google Scholar]
- Thompson B, Liu Z. Learning motion discrimination with suppressed and un-suppressed MT. Vision Research. 2006;46:2110–2121. doi: 10.1016/j.visres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, et al. Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. Journal of Neuroscience. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobe JD, Lorber ML, Schlezinger NS. Isolated homonymous hemianopia: a review of 104 cases. Archives of Ophthalmology. 1973;89:377–381. doi: 10.1001/archopht.1973.01000040379005. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JR. Hierarchical organization and functional streams in the visual cortex. Trends in Neurosciences. 1983;6(September):370–375. [Google Scholar]
- Vargas-Martin F, Peli E. Eye movement patterns in walking hemianopic patient. Investigative Ophthalmology and Visual Science. 2002;43(Suppl. 2):3809. [Google Scholar]
- Warren M. Pilot Study on Activities of Daily Living Limitations in Adults With Hemianopsia. American Journal of Occupational Therapy. 2009;63:626–633. doi: 10.5014/ajot.63.5.626. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Shinoda K, Kimura I, Mashima Y, Oguchi Y, Ohde H. Discordance between subjective perimetric visual fields and objective multifocal visual evoked potential-determined visual fields in patients with hemianopsia. American Journal of Ophthalmology. 2007;143(2):295–304. doi: 10.1016/j.ajo.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Blindsight revisited. Current Opinion in Neurobiology. 1996;6(2):215–220. doi: 10.1016/s0959-4388(96)80075-4. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Barbur JL, Sahraie A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (v1) Proceedings of the National Academy of Science USA. 1995;92:6122–6126. doi: 10.1073/pnas.92.13.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Wolach I, Pratt H. The mode of short-term memory encoding as indicated by event-related potentials in a memory scanning task with distractions. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2001;112(1):186–197. doi: 10.1016/s1388-2457(00)00501-0. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, et al. Jerabek P. Retinotopic Organization of Early Visual Spatial Attention Effects as Revealed by PET and ERP's. Human Brain Mapping. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zeki S, ffytche DH. The Riddoch Syndrome: insights into the neurobiology of conscious vision. Brain. 1998;121:25–45. doi: 10.1093/brain/121.1.25. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Homonymous hemianopias: Clinical-anatomic correlations in 904 cases. Neurology. 2006a;66:906–910. doi: 10.1212/01.wnl.0000203913.12088.93. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology. 2006b;66:901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]


