Abstract
Rationale
Varenicline (VAR), a smoking cessation aid that is a partial agonist at nicotinic receptors, mimics the reinforcement-enhancing effects of nicotine. Varenicline, when accompanied by non-drug cues, is self-administered by rats, though it is unclear whether this results from varenicline acting as a primary reinforcer or a reinforcement-enhancer of the cues.
Objectives
This study sought to disentangle these two potential actions.
Methods
Rats were allowed to self-administer intravenous nicotine, saline, or varenicline during 1-h sessions in operant chambers equipped with two levers. Five groups had concurrent access to drug infusions and a moderately reinforcing visual stimulus (VS) for responding on separate levers. Meeting the reinforcement schedule on one lever was reinforced with VAR (0.01, 0.06, 0.1 mg/kg/infusion), nicotine (0.06 mg/kg/infusion), or saline, while meeting the same schedule on the other lever delivered the VS. Additional groups were reinforced for pressing a single “active” lever, and received VAR paired with the VS, the VS with response-independent infusions of VAR, or VAR alone (0.1 mg/kg/infusion).
Results
Rats readily responded for VAR paired with VS on a single lever. However, when VAR was the only reinforcer contingent on a response, rats did not respond more than for saline.
Conclusions
These findings show that VAR does not serve as a primary reinforcer in rats at doses that increase responding for non-drug reinforcers. These data are consistent with research showing that the primary reinforcing effects of VAR are weak, at best, and that the primary reinforcing and reinforcement-enhancing actions of nicotinic drugs are pharmacologically distinct.
Keywords: varenicline, nicotine, self-administration, reinforcement, rats
Introduction
Varenicline (Chantix®) is currently the most effective pharmacotherapy for promoting smoking cessation (Aubin et al. 2008; Eisenberg et al. 2008; Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006). Nonetheless, its effectiveness is limited, and only a small percentage of quitters remain abstinent. The mechanism by which varenicline is thought to be effective as a smoking cessation aid relates to its partial agonist activity at α4β2 containing nicotinic receptors (nAChRs) (Foulds 2006; Mihalak et al. 2006; Rollema et al. 2007). Thus, varenicline is thought to substitute for the reinforcing actions of nicotine and prevent symptoms of withdrawal, while at the same time limiting the extent to which the reinforcing properties are realized.
Nicotine has two important, yet distinct, reinforcing actions, as has been clearly demonstrated in studies in animal models (Caggiula et al. 2009; Chaudhri et al. 2006; Chaudhri et al. 2007; Donny et al. 2003). Nicotine acts as a primary reinforcer, but this action is relatively weak (Caggiula et al. 2009). On the other hand, nicotine also acts to enhance responding for other reinforcing stimuli, including those associated with nicotine (Caggiula et al. 2009; Chaudhri et al. 2006; Chaudhri et al. 2007; Donny et al. 2003). Therefore, the two reinforcing properties of nicotine are difficult to dissociate in single-lever protocols. Previous research from our laboratory has shown that the two reinforcing properties of nicotine can be dissociated using a “two-operant procedure,” in which rats respond on one lever for nicotine and on a separate lever for a mildly reinforcing visual stimulus (VS, 1-s white cue light on and 60-s white house light off) (Palmatier et al. 2006; Palmatier et al. 2007; Palmatier et al. 2008). This protocol has revealed that the two actions of nicotine are pharmacologically and behaviorally distinct. Palmatier et al. (2007) demonstrated that acute injections of mecamylamine potently reduced the reinforcement enhancing effects of nicotine (i.e., reduced responding for VS), whereas repeated pretreatment injections of mecamylamine were required to reduce responding for nicotine infusions, suggesting that the primary reinforcing effects of nicotine underwent ‘extinction’ in response to the antagonist. Using a similar operant procedure, antagonists of the metabotropic glutamate receptor 5 (mGluR5) were found to block the primary reinforcing effects of nicotine without altering the reinforcement-enhancing actions of nicotine (Palmatier et al. 2008). A more comprehensive understanding of the behavioral and pharmacological effects of varenicline can be established by investigating whether the partial agonist mimics both the primary reinforcing and reinforcement enhancing effects of nicotine using this approach.
We have recently reported that varenicline acts as a reinforcement enhancer, in a manner consistent with it being a partial agonist at the relevant receptors (Levin et al. 2012). In particular, rats responding for a mildly reinforcing VS exhibited enhanced responding after receiving a pre-session subcutaneous injection of varenicline. Consistent with varenicline acting as a partial nicotinic agonist, the maximal effect of varenicline was less than the maximal effect of nicotine, and the maximal effect of nicotine was blunted by co-treatment with varenicline.
Varenicline has also been reported to support self-administration behavior (Paterson et al. 2010; Rollema et al. 2007) and these data have been interpreted as varenicline, like nicotine, acting as a primary reinforcer. However, these self-administration procedures have utilized a single response to earn the delivery of both the cue and varenicline, and therefore could not distinguish between varenicline acting as a primary reinforcer or a reinforcer enhancer, or as is the case with nicotine, both. Notably, Rollema and colleagues (2007) found that varenicline, at doses that supported peak levels of responding under fixed ratio schedules of reinforcement, was a weaker reinforcer than nicotine under a progressive ratio (PR) reinforcement schedule, which provides an index of the magnitude of drug reinforcement (Richardson and Roberts 1996). However, because both reinforcers were delivered for making a single response, it is impossible to determine which effect of varenicline (primary reinforcing or reinforcement enhancing) was weaker than nicotine.
Therefore, the present study sought to determine whether the potential primary reinforcing effects of varenicline could be dissociated from reinforcement enhancing effects using the two-operant procedure developed previously (Palmatier et al. 2006; Palmatier et al. 2007; Palmatier et al. 2008).
Methods
Subjects
Male Sprague-Dawley rats (Harlan Farms, Indianapolis, IN) weighing 200-225 g on arrival were individually housed in hanging, stainless steel cages. The colony room was temperature- and humidity-controlled, and maintained on a reversed 12:12 h light-dark cycle (lights off at 7 am). Rats had unlimited access to water in their home cage throughout the experimental period. Animals had unrestricted access to food for the first seven days after arrival, and were handled and weighed daily. Following this initial period, food was restricted to 15 g/day for one week, during habituation and training in the operant-conditioning chambers. When training was complete, animals were switched to 20 g chow/day for the remainder of the study (Donny et al. 1995). All procedures conformed with the 2003 National Research Council’s “Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research” and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Apparatus
All habituation, training, and experimental sessions were conducted in 25 × 31 × 28 cm (w × l × h) operant-conditioning chambers (Med Associates, St. Albans, VT). Two retractable levers were located on one wall, approximately 2 cm from the floor, with a food pellet trough centrally located between the levers. A house light was located directly above the pellet trough, approximately 1 cm from the ceiling of the chamber. A white stimulus light was located above each lever, but only the light above the active lever was activated as part of the visual stimulus (VS) (Palmatier et al. 2006). The VS consisted of a 1-s illumination of the stimulus light and the white house light being turned off for 60-s. During self-administration sessions, rats were attached to a swivel system that delivered intravenous infusions via an infusion pump and allowed for relatively unrestricted movement.
Drugs
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) and varenicline tartrate (Pfizer Inc., New York, NY) were dissolved in 0.9% saline. The dose of nicotine used for self-administration was 0.06 mg/kg/infusion (reported as free base). This dose was selected because it reliably supports nicotine self-administration (Donny et al. 2003). The doses of varenicline administered were 0.01, 0.06, and 0.1 mg/kg/infusion (reported as free base, corresponding to doses of 0.017, 0.10, and 0.17 mg/kg/infusion of the tartrate salt); these doses were chosen based on previous varenicline self-administration studies (Paterson et al. 2010; Rollema et al. 2007). All solutions were pH balanced to 7.0 (±0.2) with NaOH and sterilized by being passed through a 0.22 μm filter. All injections were delivered intravenously in approximately 1 s (0.1 mL/kg/infusion).
Procedures
Habituation
Animals were placed into operant chambers for a 20 min habituation period prior to training. During the habituation period levers were retracted and houselights were illuminated red.
Magazine Training
Rats went through two days of magazine training to establish an association between the sound of the food dispenser and food delivery. Animals were placed in operant-conditioning chambers for approximately 1 h, and food pellets were delivered automatically approximately every minute (i.e., 45-75 s) for a total of 60 pellets. Levers remained retracted, and the red house light was illuminated for the duration of the session.
Lever Training/Autoshaping
Following habituation and magazine training, rats were food deprived for 24 h and trained to lever press on both levers over four successive sessions. During the two initial sessions, levers (one at a time) were randomly extended for 15-s (with a random interval of 45-105-s at 5-s intervals), followed immediately by delivery of one food pellet. Pressing the lever during the 15-s extension resulted in the delivery of two pellets. During the latter two sessions, each lever was randomly extended for up to 15-s at a random interval of 30, 45 or 60-s and food delivery was contingent on lever pressing with the lever retracted upon food delivery. Rats receiving <30 pellets during the first session had lever pressing shaped by reinforcing successive approximation to the terminal response. All rats completed a second session to ensure that all would respond to receive ≥30 pellets within the 1 h session. The house-light was illuminated red for all four sessions. Rats received equal training on both levers and at the end of training there was no lever bias in any of the groups.
Surgery
Rats were anesthetized with isoflurane and implanted with chronic indwelling catheters in the right jugular vein, similar to those detailed by Corrigall and Coen (1989). Bupivacaine, an analgesic agent, was applied to the incision site before suturing. Rats were allowed to recover for a minimum of five days in their home cages. To prevent infection and maintain catheter patency, catheters were flushed once daily with 0.1 mL sterile, heparinized (30 U/mL) saline solution containing Timentin (66.67 mg/mL) and streptokinase (8333 U/mL) for three days following surgery. Thereafter, for the remainder of the study, catheters were flushed daily with 0.1 mL sterile, heparinized saline (30 U/mL) prior to and following each session.
Experimental Drug Phase
Rats were randomly assigned to one of eight groups (n=11/group; see table 1), as follows: 2-lever saline or VS (2L SAL), 2-lever nicotine or VS (2L NIC, 0.06 mg/kg), 2-lever 0.01 mg/kg varenicline or VS (2L 0.01 VAR), 2-lever 0.06 mg/kg varenicline or VS (2L 0.06 VAR), 2-lever 0.1 mg/kg varenicline or VS (2L 0.1 VAR), 1-lever 0.1 mg/kg varenicline + VS (1L VAR+VS), l-lever yoked 0.1 mg/kg varenicline + VS (1L YOKED VAR+VS), and 1-lever 0.1 mg/kg varenicline only (1L VAR). Self-administration sessions were 1-h in duration and occurred 5 days a week. For all 2-lever groups, the left lever was the infusion lever and the right lever was the VS lever. For the remaining three groups (labeled as 1L groups), the left lever was the active lever, and the right lever was the inactive lever (responses were recorded but had no consequence). Responses on the active lever in the VAR+VS group resulted in animals receiving both an infusion and a VS presentation. Responses on the active lever in the YOKED VAR+VS and VS only groups resulted in a VS presentation only. Infusions to animals in the YOKED VAR+VS group occurred when a paired animal in the VAR+VS group responded on the active lever to deliver the combined infusion + VS presentation. After each infusion, nicotine or varenicline was unavailable for a 1-min time-out period. The VS consists of the 1-s illumination of a stimulus light above the assigned VS lever, and the termination of the white house light for 60-s. Further responses on the VS lever during the 61-s VS period were recorded but not reinforced. Initially lever pressing was maintained under a fixed ratio (FR) 1 schedule of reinforcement for session 1-5, then sessions 6-11 were under an FR2 schedule, and finally sessions 12-26 were under an FR5. Schedule requirements were equal for both levers in 2-lever groups, and were identical to groups with one active lever.
Table 1.
2-Lever and 1-Lever Group names, drug and/or cue conditions available for self-administration, and final group sample sizes.
| Group | Left Lever | Right Lever | n | |
|---|---|---|---|---|
|
|
||||
| 2-Lever Groups | 2L SAL | Saline | VS | 8 |
| 2L NIC | 0.06 mg/kg Nicotine | VS | 9 | |
| 2L 0.01 VAR | 0.01 mg/kg Varenicline | VS | 11 | |
| 2L 0.06 VAR | 0.06 mg/kg Varenicline | VS | 9 | |
| 2L 0.1 VAR | 0.1 mg/kg Varenicline | VS | 10 | |
|
|
||||
| 1-Lever Groups | 1L VAR+VS | 0.1 mg/kg Varenicline + VS | Inactive | 9 |
| 1L Yoked VAR+VS | VS | Inactive | 10 | |
| 1L VAR | 0.1 mg/kg Varenicline | Inactive | 10 | |
|
|
||||
Test of Catheter Patency
A test of catheter patency was conducted after completion of the experiment. This was initially tested by determining whether blood could be drawn back into the catheter, which was taken as confirmation of patency. Catheters that failed this initial assessment were infused with a solution of chloral hydrate (200 mg/1ml, up to 0.3 mL/rat). Animals not displaying physical signs of ataxia within 5-s of chloral hydrate injection were recorded as failed patency. A total of 12 of 88 animals failed the patency test and were excluded from data analyses, so final group sizes ranged from 8 to 11 (see table 1).
Data Analyses
The primary goal of this experiment was to compare responding for individual reinforcers when two reinforcers were simultaneously present, relative to a single operant response for a combination infusion/VS presentation. Furthermore, this experiment was designed to determine the degree of primary reinforcement for varenicline and to what degree varenicline serves to enhance responding for a nonpharmacological reinforcer. Number of infusions and/or VS presentations earned across the last three sessions of FR5 was the primary dependent variable. Analyses of the effect of drug treatment on infusions earned only use data from groups that had control over drug infusions (excludes 1L YOKED VAR+VS group). Similarly, analyses of the effects of drug treatment on VS presentations earned use only data from groups that were exposed to VS presentations (excludes 1L VAR group). Data were analyzed with both mixed analyses of variance (ANOVA) with group and session as factors and ANOVA based on the averages during the last 3 days. Post hoc tests compared all groups to each other using Least Squares Difference (LSD) test to control for family-wise Type I error rate. Both the repeated measures ANOVA and the ANOVA using the average of the last 3 days provided the same between group differences, though for simplicity only the analysis of the average of the last 3 days is presented in Results. Statistical analyses were performed using SPSS (version 21).
Results
Infusions Earned
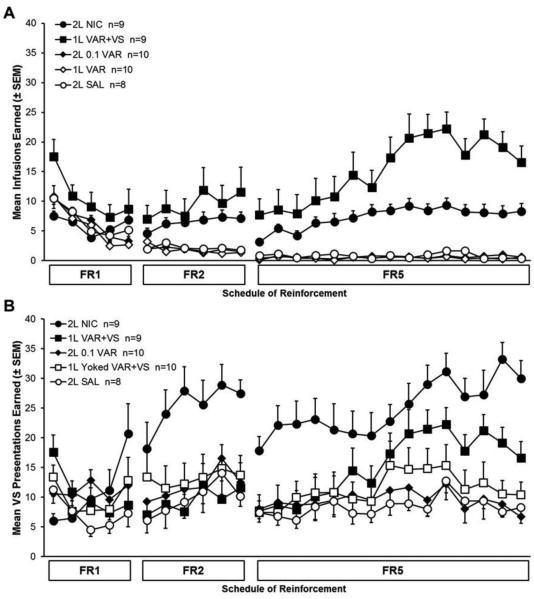
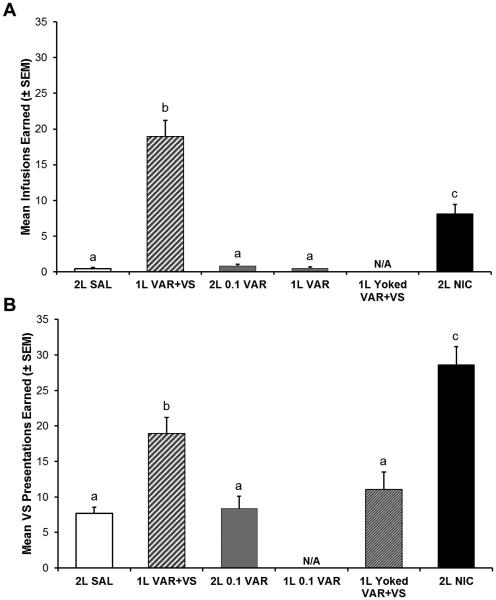
The mean number of infusions earned by each group across each experimental drug phase is displayed in figure 1A, whereas figure 2A presents the average number of infusions earned by groups across the last three sessions of the FR5 phase of the experiment. The two lower doses of varenicline included in the study (0.01 and 0.06 mg/kg) did not produce responding different from saline; they are not included in these figures but are discussed below. There was a main effect of group (F6, 59 = 53.151, p < 0.001) and post-hoc tests revealed that the 1L VAR+VS group earned significantly more infusions than all other groups, including 2L NIC (p < 0.05). The 2L NIC group earned significantly more infusions than all other remaining groups (p < 0.05).
Fig. 1.
Mean infusions (A) and VS presentations (B) earned across experimental sessions. Animals were on an FR1 schedule of reinforcement for 5 sessions, FR2 for 6 sessions, and FR5 for the final 15 sessions. Error bars represent standard error. Data are stable across the final 3 sessions of FR5, as revealed by 2-way repeated measures ANOVA (Infusions F2, 118 = 1.050, p > 0.05; VS presentations F2,118 = 1.210, p > 0.05).
Fig. 2.
Mean number of infusions (A) and VS presentations (B) earned over the last three sessions on an FR5 schedule of reinforcement. Bars with different letters are significantly different from each other (p < 0.05). Error bars represent standard errors. N/A denotes groups that did not have control over drug infusions or were not exposed to VS presentations.
VS Presentations Earned
The mean number of VS presentations earned by each group across sessions and experimental drug phases is displayed in figure 1B, and figure 2B presents the average VS presentations earned by groups across the final three sessions of FR5. There was a main effect of group (F6,59 = 19.330, p < 0.001); follow-up tests revealed that the 2L NIC group earned significantly more VS presentations than all other groups (p < 0.05), and 1L VAR+VS group earned more VS presentations than all remaining groups (p < 0.05). There were no other significant differences.
The groups with access to the two lowest doses of varenicline (0.01 and 0.06 mg/kg), 2L 0.01 VAR and 2L 0.06 VAR, were not different from the 2L SAL group for infusions or VS presentations earned and are therefore not shown in figures 1 and 2. The three day average (mean ± standard error of the mean) number of infusions earned by the 2L 0.01 VAR group was 0.4 ± 0.2, and for the 2L 0.06 VAR group it was 0.5 ± 0.2. The three day average number of VS presentations earned by the 2L 0.01 VAR group was 7.9 ± 1.2, and for the 2L 0.06 VAR group was 5.6 ± 1.0.
Active Versus Inactive lever Responding
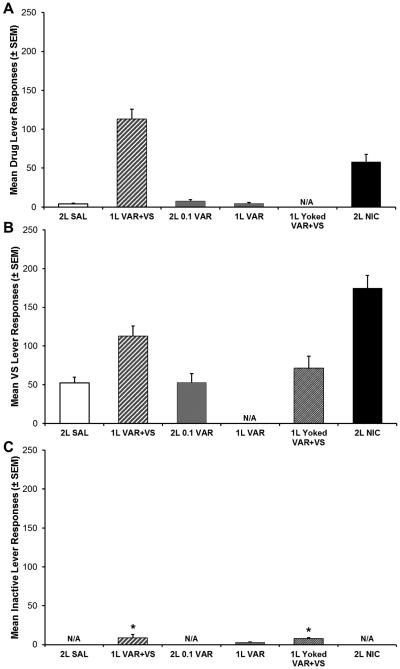
The mean number of drug lever responses, VS lever responses, and inactive lever responses across the last three session of FR5 are shown in figure 3A-C, respectively. Three of the eight experimental groups had one active lever and one inactive lever; the inactive responses made by these groups are shown in panel C. The 1LVAR+VS group earned varenicline infusions and VS presentations from responding on a single “active” lever, so these responses are shown in both panels A and B. A one-way ANOVA of the average of the last three sessions on FR5 revealed that there was no significant difference in the inactive responses made by the 1L VAR, 1L VAR+VS, and 1L YOKED VAR+VS groups (p > 0.05). A paired samples t-test comparing the active responses and inactive responses made by each group revealed that the 1L VAR group did not discriminate between the active and inactive levers (p > 0.05). In contrast, the 1L VAR+VS and 1L YOKED VAR+VS groups made significantly more active responses than inactive responses (1L VAR+VS t8 = 9.004, p < 0.001; 1L YOKED VAR+VS t9 = 4.208, p < 0.01).
Fig. 3.
Mean number of responses on the drug lever (A), VS lever (B), and inactive lever (C) earned over the last three sessions on an FR5 schedule of reinforcement. Three groups had one active lever and one inactive lever (1L groups). The 1L VAR+VS group earned combination varenicline infusions and VS presentations from responses on a single lever; responses on that lever are shown in both panels (A) and (B). Responses include timeout responding. Asterisks denote groups with significantly greater active responses than inactive responses (p < 0.01). Error bars represent standard errors. N/A denotes groups that either did not have control over drug infusions, were not exposed to VS presentations, or did not have an inactive lever.
Discussion
Key Findings
Varenicline, a smoking cessation aid that acts as a partial agonist at certain subtypes of nAChRs, has reinforcement-enhancing actions in rats, similar to those of nicotine (Levin et al. 2012). Consistent with varenicline acting as a partial agonist, the maximal response to varenicline was less than that for nicotine, and high doses of varenicline antagonized the maximal effect of nicotine (Levin et al. 2012). In the present study we sought to determine whether varenicline also acts as a primary reinforcer, using a two-lever self-administration paradigm that dissociates these two actions. A range of doses of varenicline was tested to assess potential effects resulting from partial agonist activity. When lever responding resulted in the delivery of both an intravenous infusion of varenicline (0.1 mg/kg/infusion) and a complex visual stimulus, rats readily responded. However, when responding on the lever resulted only in the delivery of varenicline, rats did not respond at a higher rate than responding for saline vehicle, suggesting that at the doses tested (0.01, 0.06, 0.1 mg/kg/infusion) varenicline failed to act as a primary reinforcer. Thus, varenicline, in doses that rats will administer intravenously when it is coupled to a mildly reinforcing stimulus, does not appear to act as a primary reinforcer.
Lack of Evidence for a Primary Reinforcing Effect of Varenicline
Examining the actions of varenicline in this two-lever procedure, it appears that varenicline lacks the primary reinforcing effect of nicotine. Responding on the lever for varenicline was no different than responding for the saline vehicle and, in the case of rats in the 1L VAR group, no different than responding on the inactive lever. In contrast, rats readily administered nicotine in this procedure, consistent with results that we have previously reported (Palmatier et al. 2006; Palmatier et al. 2007; Palmatier et al. 2008). However, rats will readily respond for varenicline when its delivery is paired with a mildly-reinforcing environmental cue. These results fail to provide evidence that varenicline acts as a primary reinforcer, unlike nicotine.
In rats that had access to varenicline but were not pressing the lever enough to earn a significant number of varenicline infusions (2-lever varenicline groups), responding for the VS was no greater than rats that were responding for the VS while responding for saline on the other lever. However, when both varenicline and the VS were contingent on the same lever, responding was greater than for just the VS alone, consistent with the previously reported reinforcement-enhancing effect of varenicline (Levin et al. 2012). Curiously, rats responding for the VS while receiving injections of varenicline yoked to the VS+VAR group, responded less for the VS despite receiving the same number of varenicline infusions. Indeed, in this group of rats receiving yoked injections of varenicline, responding for the VS was only marginally greater than in rats with access to saline responding for the VS alone, and these groups were not statistically different. In a previous study with nicotine, we observed that the reinforcement enhancement effect of nicotine did not differ between rats receiving the nicotine either by responding for it directly or having it delivered in a yoked design (Chaudhri et al. 2006; Donny et al. 2003). This apparent lack of an enhancement effect of varenicline is not simply related to the dose of varenicline, since subcutaneous injections of varenicline, at a dose of 0.1 mg/kg, the dose used in the present study, elicited reinforcement enhancement (Levin et al. 2012). Why discrete intravenous infusions of varenicline that are temporally dissociated from VS delivery do not support the same magnitude of reinforcement enhancement elicited by either temporally-locked delivery of varenicline and VS or pre-session subcutaneous injection of varenicline is not clear, but may relate to the temporal dynamics of rapid intravenous injections of varenicline. For example, rapid intravenous injections of varenicline may produce spikes in varenicline levels that are cleared or metabolized quickly, such that brain levels of varenicline when administered non-contingently are sufficiently low at the time of VS presentation that enhancement is not observed. Alternatively, the higher responding when both the VS and varenicline are delivered together may not simply be the result of varenicline enhancement of responding for the VS. For example, based on the data from the present experiment, it could be that VS enhances responding for varenicline or they interact in some other manner when the two are presented together. Indeed, Sorge et al. (2009) reported a similar observation when rats responded for slow (30-s) intravenous infusions of nicotine (0.015 mg/kg/infusion) along with an environmental cue (either a cue light or white noise) that was not itself reinforcing; rats did not respond for either nicotine or the cue but readily responded when they were delivered together. Sorge et al. (2009) suggested that the cue could have made the response more salient, or the cue could have served to bridge the temporal gap between the operant response and the pharmacological actions of the drug. Whatever the mechanism underlying the interaction between varenicline and environmental cues, reinforcement-enhancement as shown by Levin et al. (2012), or some other mechanism, the present study does not provide support for a primary reinforcing action of varenicline in the absence of other cues.
Two previous studies have reported varenicline self-administration in rats (Paterson et al. 2010; Rollema et al. 2007), concluding that varenicline, like nicotine, had primary reinforcing actions. However, in both of those studies, the self-administration procedure was cued and the present data demonstrate that the results can be explained completely by an interaction between environmental cues and varenicline. Still, it is important to note that these previous studies, like the present studies, find varenicline to support operant behavior in a similar dose range as nicotine, though with a somewhat reduced maximal response.
Pharmacology of Varenicline
Varenicline has a complex pharmacological profile, acting on nAChRs of different subunit compositions with different affinities and agonist activities (Coe et al. 2005; Foulds 2006; Grady et al. 2010; Mihalak et al. 2006; Rollema et al. 2007). The ability of varenicline to act as an effective smoking cessation aid is often considered to derive from its actions as a partial agonist of α4β2 containing receptors (Brose et al. 2013; Cahill et al. 2012; Gonzales et al. 2006; Jorenby et al. 2006). However, this is far from clear, as varenicline has varying efficacy at different nAChRs and this varies depending upon the specific assay and duration of drug exposure (Coe et al. 2005; Foulds 2006; Mihalak et al. 2006; Rollema et al. 2007). Still, the observation in the present study that varenicline does not appear to act as a primary reinforcer, while being readily self-administered when combined with the mildly-reinforcing VS suggests that these two actions of nicotine result from actions on nAChRs of different subunit compositions. The primary reinforcing action of nicotine would appear to be mediated by some type of nAChR at which varenicline has limited agonistic activity, since this action is not shared by these two drugs. The α4β2 subunit containing nAChRs are potential candidates, as varenicline binds to these receptors but has minimal agonist activity in several in vitro tests (Grady et al. 2010). Consistent with that, in vivo studies in mice suggest that varenicline acts as a potent antagonist at β2-containing nAChRs (Ortiz et al. 2012). Furthermore, multiple lines of evidence suggest that β2-containing nAChRs are necessary for the primary reinforcing actions nicotine (Picciotto et al. 1998; Picciotto and Mineur 2014). In contrast, the reinforcement enhancing action of varenicline demonstrated by Levin et al. (2012), which is shared with nicotine, must be mediated by nAChRs at which varenicline has agonist activity. In both in vitro and in vivo tests varenicline has a high degree of efficacy as an agonist on α3β4* (where * denotes additional unspecified subunits) and α7 nAChRs (Grady et al. 2010; Ortiz et al. 2012). There is little evidence that α7 nAChRs might mediate the reinforcement enhancing effects of nicotine. Notably, even a relatively large dose of the α7-selective antagonist methyllycaconitine did not interfere with the reinforcement enhancing action of nicotine (Liu et al. 2007). On the other hand, there is evidence to support the case for α3β4* nAChRs (Jackson et al. 2013; Toll et al. 2012). In particular, Toll et al. (2012) reported that a novel high affinity and selective α3β4* nAChR antagonist, AT-1001, markedly reduced nicotine self-administration in rats tested using a cued protocol in which the reinforcement enhancing actions of nicotine would be expected to provide a major contribution to the lever-pressing behavior. In a study specifically examining the role of nAChRs in mediating the reinforcement enhancing actions of nicotine, it was noted dihydro-β-erythroidine effectively blocked this action of nicotine. While dihydro-β-erythroidine is often viewed as an α4β2-selective antagonist, it has antagonist activity on β4-containing nAChRs as well (Harvey and Luetje 1996; Harvey et al. 1996). The present data show that varenicline acts as a reinforcer when coupled with a mildly-reinforcing environmental cue while lacking primary reinforcing activity, and it is plausible that this results from the different actions of varenicline on β2-containing nAChRs versus β4-containing nAChRs.
Clinical Efficacy of Varenicline
The results of this experiment may provide some insight regarding the clinical efficacy of varenicline as a smoking cessation aid. Varenicline, by nature of its action as a partial agonist at nAChRs, is thought to act as a smoking cessation aid by decreasing the motivation to obtain nicotine. As varenicline does not appear to act as a primary reinforcer (and might instead block this action of nicotine), the clinical efficacy of varenicline is presumably not related to varenicline partially substituting for nicotine as a primary reinforcer. In contrast, the efficacy of varenicline may relate to its activity as a reinforcement enhancer. As an interaction between nicotine and nicotine-related cues seems to be important in maintaining smoking behavior (Caggiula et al. 2009) it is possible that by substituting in this interaction, varenicline helps smokers manage without their cigarettes. Another possibility that a combination of these actions of varenicline, including its activity as a reinforcement enhancer, is important for its clinical efficacy.
Acknowledgements
This work was supported by the National Institutes of Health grants DA-10464 and DA-24801. Varenicline was generously donated by Pfizer, Inc.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr., Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose LS, West R, Stapleton JA. Comparison of the effectiveness of varenicline and combination nicotine replacement therapy for smoking cessation in clinical practice. Mayo Clin Proc. 2013;88:226–233. doi: 10.1016/j.mayocp.2012.11.013. doi: 10.1016/j.mayocp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. doi: 10.1002/14651858.CD006103.pub6. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chamber LK, Rovetti CC, Schulz DW, Tingley FD, III, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, Gervais A, O'Loughlin J, Paradis G, Rinfret S, Pilote L. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–144. doi: 10.1503/cmaj.070256. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J Clin Pract. 2006;60:571–576. doi: 10.1111/j.1368-5031.2006.00955.x. doi: 10.1111/j.1368-5031.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study G Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, Marks MJ. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. J Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, Damaj MI. The alpha3beta4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the alpha5 subunit in the mouse. Neuropharmacology. 2013;70:228–235. doi: 10.1016/j.neuropharm.2013.01.017. doi: 10.1016/j.neuropharm.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study G Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res. 2012;14:299–305. doi: 10.1093/ntr/ntr213. doi: 10.1093/ntr/ntr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology. 2007;194:463–473. doi: 10.1007/s00213-007-0863-3. doi: 10.1007/s00213-007-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Ortiz NC, O'Neill HC, Marks MJ, Grady SR. Varenicline blocks beta2*-nAChR-mediated response and activates beta4*-nAChR-mediated responses in mice in vivo. Nicotine Tob Res. 2012;14:711–719. doi: 10.1093/ntr/ntr284. doi: 10.1093/ntr/ntr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology. 2007;32:1098–1108. doi: 10.1038/sj.npp.1301228. doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology. 2008;33:2139–2147. doi: 10.1038/sj.npp.1301623. doi: 10.1038/sj.npp.1301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Min W, Hackett A, Lowe D, Hanania T, Caldarone B, Ghavami A. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1455–1464. doi: 10.1016/j.pnpbp.2010.07.037. doi: 10.1016/j.pnpbp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Mineur YS. Molecules and circuits involved in nicotine addiction: The many faces of smoking. Neuropharmacology. 2014;76:545–553. doi: 10.1016/j.neuropharm.2013.04.028. Pt B. doi: 10.1016/j.neuropharm.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, III, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM. AT-1001: a high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology. 2012;37:1367–1376. doi: 10.1038/npp.2011.322. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]