Abstract
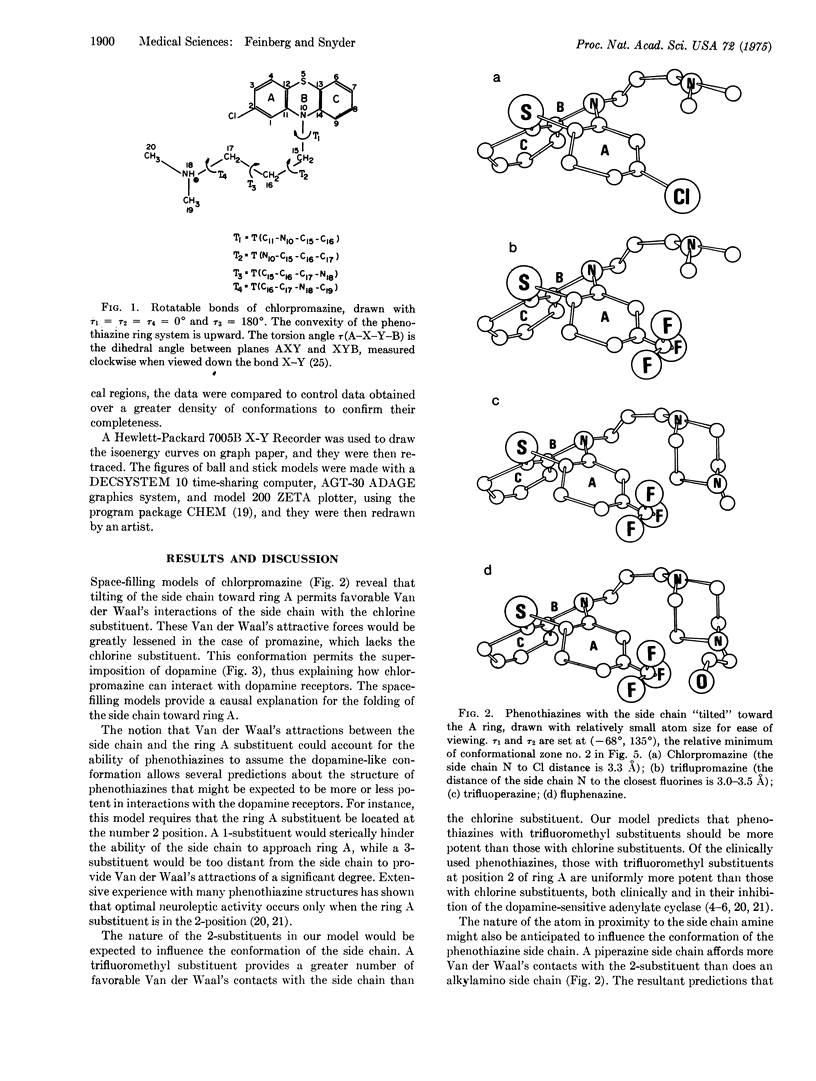
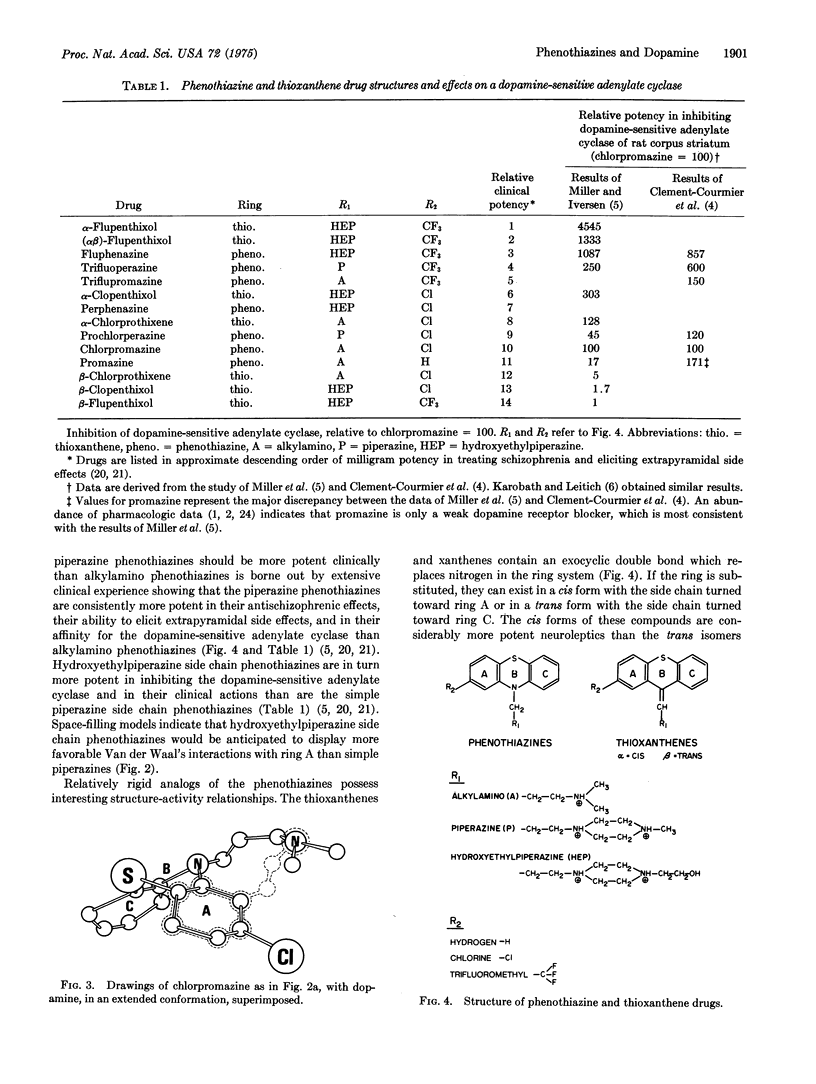
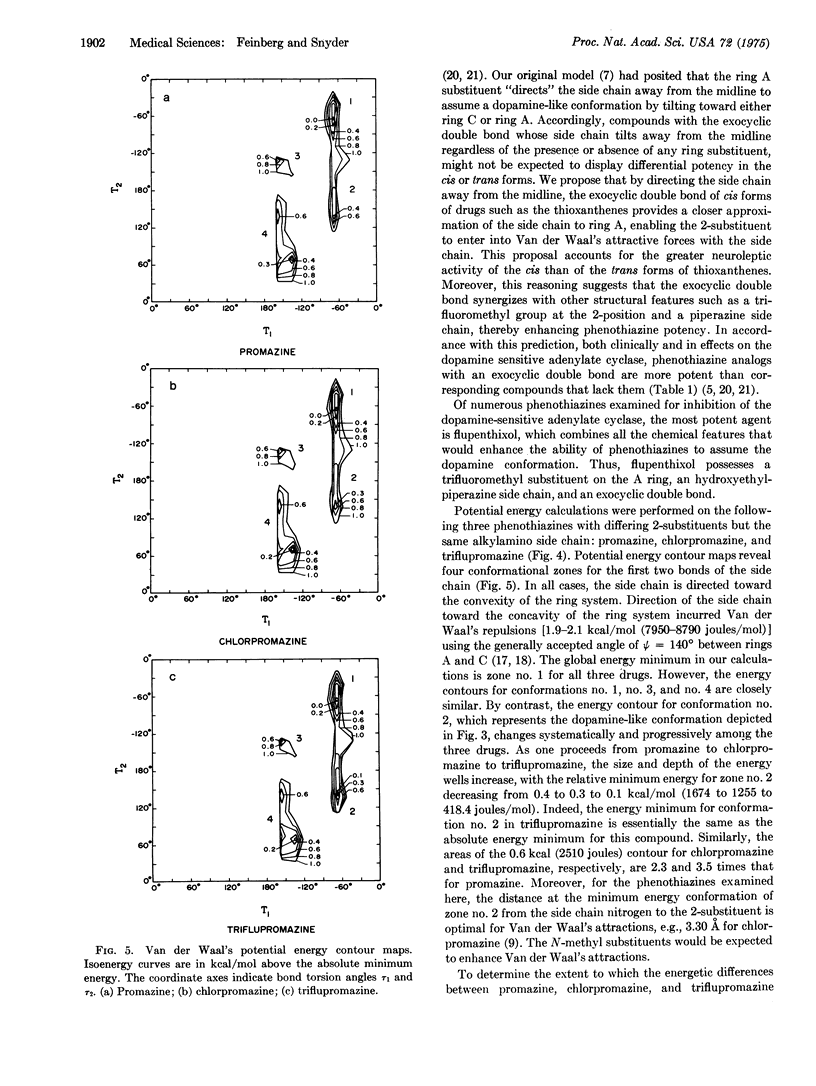
The antischizophrenic activity of phenothiazine drugs and their tendency to elicit extrapyramidal symptoms are thought to involve blockade of synaptic dopamine receptors in the brain. Space filling molecular models show how favorable Van der Waal's interactions between the side chain amino of phenothiazines and the 2-substituent on ring A can promote a conformation mimicking dopamine. These Van der Waal's attractive forces can expain (i) the greater potency of drugs with trifluoromethyl rather than chlorine as a 2-substituent; (ii) the enhanced activity of phenothiazines with piperazine instead of alkylamino side chains; (iii) the increased potency associated with hydroxyethylpiperazines as contrasted to piperazine side chains; (iv) the greater potency of cis rather than trans thioxanthenes; and (v) the crucial location of the ring A substituent at carbon no. 2. Potential energy calculations support the observations with molecular models and suggest an active conformation for the phenothiazines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clement-Cormier Y. C., Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A. S., Snyder S. H. Chlorpromazine and dopamine: conformational similarities that correlate with the antischizophrenic activity of phenothiazine drugs. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2325–2328. doi: 10.1073/pnas.68.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karobath M., Leitich H. Antipsychotic drugs and dopamine-stimulated adenylate cyclase prepared from corpus striatum of rat brain. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2915–2918. doi: 10.1073/pnas.71.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach S. J., Némethy G., Scheraga H. A. Computation of the sterically allowed conformations of peptides. Biopolymers. 1966 Apr-May;4(4):369–407. doi: 10.1002/bip.1966.360040402. [DOI] [PubMed] [Google Scholar]
- Marshall G. R., Bosshard H. E. Angiotensin II. Studies on the biologically active conformation. Circ Res. 1972 Sep;31(9 Suppl):143–150. [PubMed] [Google Scholar]
- McDowell J. J. The crystal and molecular structure of thiethylperazine, a derivative of phenothiazine. Acta Crystallogr B. 1970 Jul 15;26(7):954–964. doi: 10.1107/s0567740870003424. [DOI] [PubMed] [Google Scholar]
- Schussler G. C. Thyroxine-binding globulin: specificity for the hormonally active conformation of triiodothyronine. Science. 1972 Oct 13;178(4057):172–174. doi: 10.1126/science.178.4057.172. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Banerjee S. P., Yamamura H. I., Greenberg D. Drugs, neurotransmitters, and schizophrenia. Science. 1974 Jun 21;184(4143):1243–1253. doi: 10.1126/science.184.4143.1243. [DOI] [PubMed] [Google Scholar]


