Abstract
Null mutations in the p67phox subunit of NADPH-oxidase confer protection from salt-sensitivity on Dahl salt-sensitive (SS) rats. Here we track the sequential changes in medullary blood flow, glomerular filtration rate, urinary protein and mean arterial pressure in SSp67phox null rats and wild-type littermates during 21-days of 4.0% NaCl (high-salt [HS]) diet. Optical fibers were implanted in the renal medulla and medullary blood flow measured in conscious rats by laser-Doppler flowmetry. Separate groups of rats were prepared with femoral venous catheters and glomerular filtration rate measured by the transcutaneous assessment of fluorescein isothiocyanate-sinistrin disappearance curves. Mean arterial blood pressure was measured by telemetry. In wild-type rats HS caused a rapid reduction in medullary blood flow which was significantly lower than control values by HS day-6. Reduced medullary blood flow was associated with a progressive increase in mean arterial pressure, averaging 170 ± 5 mmHg by HS salt day-21. A significant reduction in glomerular filtration rate was evident at day-14 HS, after the onset of hypertension and reduced medullary blood flow. In contrast, HS had no significant effect on medullary blood flow in SSp67phox null rats and the pressor response to sodium was blunted, averaging 150 ± 3 mmHg at day-21 HS. Glomerular filtration rate was maintained throughout the study and proteinuria was reduced. In summary, when p67phox is not functional in the SS rat HS does not cause reduced medullary blood flow and salt-sensitive hypertension is attenuated, consequently renal injury is reduced and glomerular filtration rate is maintained.
Keywords: Salt-Sensitive, NADPH-Oxidase, Hypertension, Dahl-S Rat, Kidney
Introduction
Essential hypertension is driven by a complex interplay of genetic and environmental factors. Consumption of a high salt diet is a major environmental factor, with blood pressure elevation in response to salt (salt-sensitivity) occurring in ~50% of essential hypertension patients1. In African-American populations this figure increases dramatically, with 75% of hypertensive patients being salt-sensitive1–3 along with a higher incidence of end stage renal disease4–6. Despite this, the mechanisms underlying the progression of salt-sensitivity are poorly understood, which may explain why current treatments are effective in only ~50% of patients7. An area of particular controversy is the relationship between hypertension and chronic kidney disease, as recently reviewed by Pirkle and Freedman8, with clinical trials reporting disappointing results when evaluating the effect of blood pressure control on the rate of decline of glomerular filtration rate and the progression of chronic kidney disease9–12. Therefore, characterizing the interplay between increased blood pressure, renal injury and reduced glomerular filtration rate is of interest.
In the Dahl salt-sensitive SS/JrHsdMcwi (SS) rat, as in the clinical condition, hypertension is greatly accelerated by a high salt (NaCl) diet, a trait that is genetically determined13. Many of the clinical characteristics of the disease are mimicked in these rats which present with progressive, low-renin hypertension, end stage renal disease, hyperinsulinemia and increased reactive oxygen species (ROS) production14–19. Defining the causes and consequences of salt-induced hypertension through the evaluation of its temporal progression in the SS rat will provide valuable insights into the pathology of the clinical condition.
Increased ROS production has been implicated in the development of salt-sensitive hypertension. Increased oxidative stress in the kidneys of SS rats has profound effects resulting in anti-natriuresis, reduced blood flow in the cortex and medulla, reduced glomerular filtration rate, chronic kidney disease and hypertension19–24. Notably, reduction of intramedullary ROS alone greatly attenuates the pressor response to a high salt diet in SS rats19, 21.
ROS production is mediated by NADPH-oxidase, a complex enzyme consisting of six subunits: two membrane-bound (gp91phox and p22phox) and three cytosolic components (p67phox, p47phox and p40phox) plus one G-protein (rac1/2)25. Chromosome substitution studies performed in our laboratory revealed that a 16 Mbp genomic region of chromosome 13 from Brown Norway salt-resistant rats conferred protection from salt-sensitivity onto SS rats26. One of the genes identified in this introgressed region of the congenic strain was p67phox, which plays a crucial role in the activation of NADPH-oxidase27. We have recently demonstrated that SS rats exhibit higher NADPH-oxidase activity in the outer medulla compared to salt-resistant control rats21 and uniquely overexpress the p67phox subunit of the enzyme28. Based on these data, a rodent model was developed in which the p67phox gene was mutated in SS rats (SSp67phox null rat). Study of the SSp67phox null rat provided the first direct evidence of the physiological relevance of p67phox in the etiology of blood pressure salt-sensitivity in the SS rat as the SSp67phox null rats exhibited a substantial attenuation of both the hypertensive response and renal injury28.
In the current study, the SSp67phox null rat was used to determine the physiological role of increased ROS production in the initiation and maintenance of salt-sensitive hypertension in SS rats. Changes of medullary blood flow (MBF), glomerular filtration rate (GFR) and mean arterial blood pressure (MAP) were determined throughout three weeks of the study to track the temporal progression of salt-sensitive hypertension in conscious SSp67phox null rats and their salt-sensitive wild-type (WT) littermates. Importantly, this provided the sequential order of the pathological changes associated with the development of the salt-induced hypertension, with a focus on the role of increased ROS production.
Materials and Methods
Experimental Animals
Male SSp67phox null rats and WT littermates, generated from heterozygous crosses, were obtained at weaning from colonies developed and maintained at the Medical College of Wisconsin. Breeders and offspring at weaning were fed a purified AIN-76A rodent food diet (Dyets, Bethleham, PA) containing 0.4% NaCl with ad libitium water. The high salt diet (HS) contained 4.0% NaCl. All experimental protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Experimental methods describing the phenotyping protocols and statistics are detailed in the online-only supplemental data.
Results
MBF and MAP
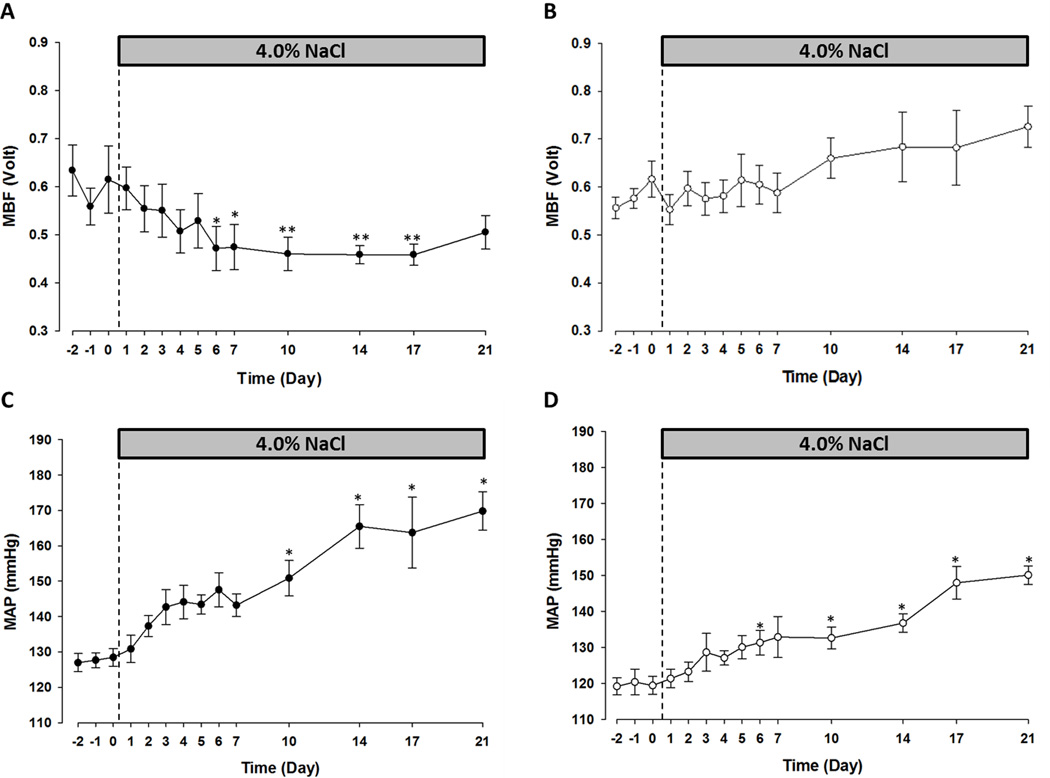
Figure 1 summarizes the MBF and MAP in SSp67phox null rats and their WT littermates over the course of the study. In the salt-sensitive WT rats, HS caused a rapid and sustained reduction in MBF, which fell by 20% over the first week of the salt challenge: from 0.59 ± 0.03 volts to 0.47 ± 0.05 volts. By day-6 HS MBF was significantly lower than the average control value. After this initial reduction, attenuated medullary perfusion persisted over the course of the study, with day-7 HS values being comparable to those recorded on day-14 HS (0.46 ± 0.02 volts) and day-21 HS (0.51 ± 0.03 volts) (Figure 1A). In the WT rats reduced MBF was associated with a progressive rise in MAP which increased from 128 ± 2 mmHg during the control period to 143 ± 3 mmHg at day-7 HS. Blood pressure continued to increase throughout the study, reaching 165 ± 6 mmHg at day-14 and 170 ± 5 mmHg at day-21 HS (Figure 1C). In contrast, null mutation of the p67phox gene protected the kidneys of SS rats from salt-induced reductions of MBF. Notably, MBF was stable during the first week of HS, day-7 values (0.59 ± 0.04 volts) were equivalent to those recorded during the control period (0.58 ± 0.02 volts). Protection from reduced MBF persisted throughout the 3-week salt challenge and day-21 HS values were not significantly different from those recorded when the rats were maintained on 0.4% NaCl. Indeed at day-21 HS MBF had trended upward by ~20% from 0.58 ± 0.02 volts to 0.73 ± 0.04 volts suggesting an increased rather than decreased, medullary perfusion (Figure 1B). In parallel with the sustained MBF, the hypertensive response to HS was blunted in the SSp67phox null rats throughout the study, increasing from 120 ± 3 mmHg during the control period to 150 ± 3 mmHg at day-21 HS (Figure 1D).
Figure 1.
SSp67phox null rats are protected from salt-sensitive medullary ischemia and hypertension compared to wild-type (WT) littermates. Medullary blood flow (MBF) in WT (A) and SSp67phox null (B) rats on 0.4% NaCl and 4.0% NaCl diets (n=6/7 rats per strain). Mean arterial pressure (MAP) in WT (C) and SSp67phox null (D). n=5/7 per strain, *p<0.05, **p<0.01 compared to the average 0.4% NaCl measurement. Data are presented as mean values ± SE.
GFR and MAP
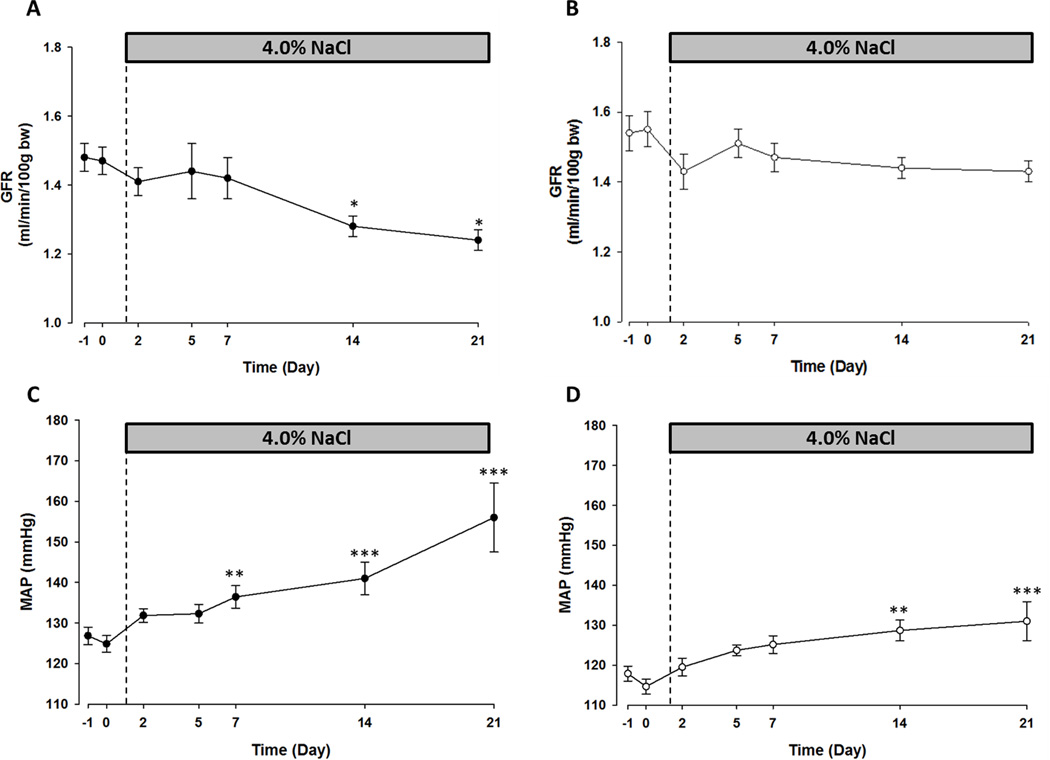
Figure 2 shows the MAP and GFR recorded in unrestrained SSp67phox null and WT rats over 21-days HS diet. As in the MBF study, HS caused a progressive increase in MAP in the WT rats, from 126 ± 2 mmHg in the control period to 156 ± 8 mmHg on day-21 HS (Figure 2C). Although a small initial reduction in GFR was observed on day-2 HS, significant changes from the control period were not observed until day-14 HS in the WT rats, at which point GFR had reduced from an average of 1.48 ± 0.04 ml/min/100g bw to 1.28 ± 0.03 ml/min/100g bw. By day-21 GFR had reduced further, to 1.24 ± 0.03 ml/min/100g bw (Figure 2A). Notably, a significant increase in MAP was observed at day-7 HS in the WT rats, which preceded the reduction in GFR (Figure 2C). In contrast, the pressor response to the HS diet was blunted in the SSp67phox null rats with MAP averaging only 132 ± 5 mmHg at day-21 with a significant increase not observed until day-14 HS (Figure 2D). There was no significant change in GFR over the 21-days HS in the SSp67phox null rats, control values of 1.54 ± 0.05 ml/min/100g bw were comparable to day-21 HS values of 1.43 ± 0.03 ml/min/100g bw (Figure 2B).
Figure 2.
Salt-sensitive hypertension is attenuated and glomerular filtration rate maintained in SSp67phox null rats over 21-days 4.0% NaCl diet compared to wild-type (WT) littermates. Glomerular filtration rate (GFR) in WT (A) and SSp67phox null (B) rats on 0.4% and 4.0% NaCl diets. Mean arterial blood pressure (MAP) in WT (C) and SSp67phox null (D) rats. n=7/8 per strain, *=p<0.05, **p<0.01, ***p<0.001 compared to average 0.4% NaCl measurement. Data are mean values ± SE.
Creatinine, Urinary Protein and Nitrate and Histological Assessment of Glomerular Damage
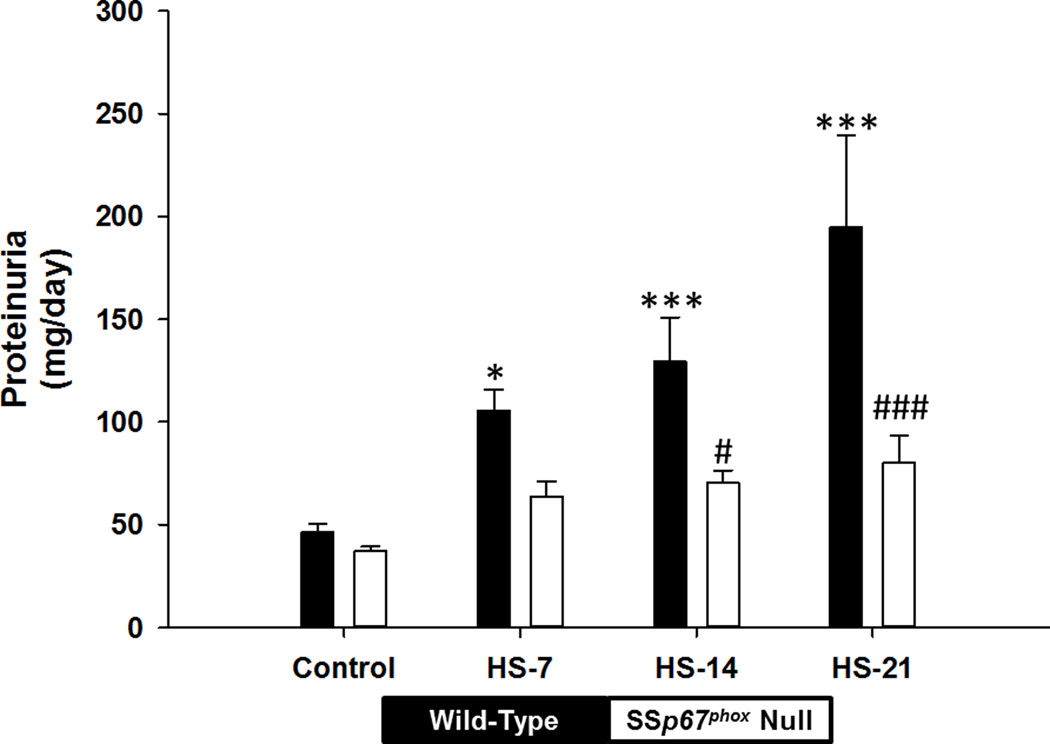
Figure 3 summarizes the urinary protein data over the course of the study. HS caused a significant increase in urinary protein excretion in the WT rats. In contrast to an absence of change of GFR, significant increases in urinary protein were evident at HS day-7 in the WT rats; levels of urinary protein were 2-fold higher than control values in all WT rats at this point. Urinary protein increased progressively throughout the study and was 4-fold higher than control values at HS-21 (Figure 3). In contrast, the protein measured in the urine of SSp67phox null rats was substantially lower than WT rats throughout the study, and by day-14 HS this had reached significance (Figure 3). In correlation with the increased MBF, urinary nitrate excretion was significantly higher in the SSp67phox null rats than the WT rats at day-21 HS (Figure S1).
Figure 3.
Urinary protein is attenuated in SSp67phox null rats compared to wild-type littermates. Proteinuria in wild-type (black bars) and SSp67phox null (white bars) rats on a 0.4% NaCl control day, HS-7, HS-14, HS-21. *p<0.05, ***p<0.001 compared to 0.4% NaCl control measurement. #p<0.05 compared to wild-type rats at the same time point. Data are presented as mean values ± SE.
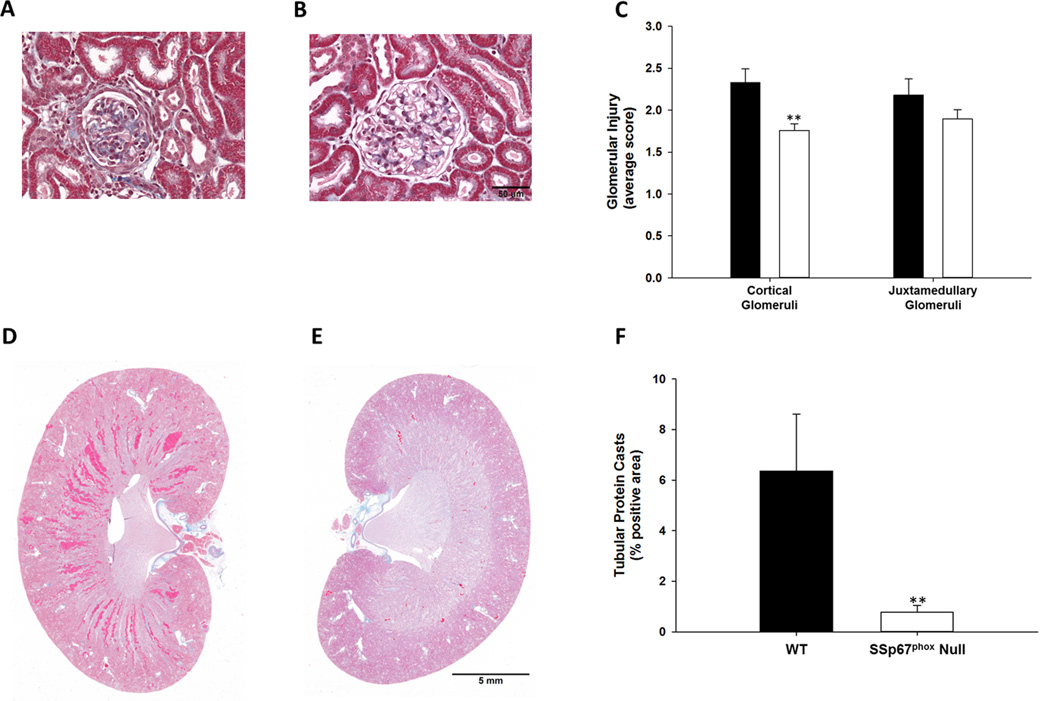
Glomeruli injury was quantified in both the cortex and juxtamedullary boundary of WT and SSp67phox null rats after 21-days HS. The data presented in Figure 4 show that glomeruli injury was significantly higher in the cortex of WT rats compared to the SSp67phox null rats. In contrast there was no difference in the extent of injury observed in the juxtamedullay border region (Figure 4C). Trichrome staining was used for the assessment of tubular protein casts in the outer medulla in both groups of rats. At day-21 HS tubular injury was significantly attenuated in the SSp67phox null rats (Figure 4F).
Figure 4.
Histological assessment indicates reduced renal injury in SSp67phox null rats compared to wild-type (WT) littermates. Cortical glomerular injury in WT (A) and SSp67phox null (B) rats, quantification of glomeruli injury in cortical and juxtamedullary glomeruli (C) in WT (black bars) and SSp67phox null (white bars) rats. Trichrome staining of protein casts in WT (D) and SSp67phox null (E) rats, quantification of tubular casts (F).
Finally, Table 1 summarizes the urinary creatinine clearance over the 21-day HS challenge. Plasma and urinary collections were made on a control day and days 7, 14 and 21 HS. Creatinine concentrations were measured by mass spectrometry with the aim of comparing creatinine clearance and the transcutaneous method as assessments of GFR. No significant reduction in creatinine clearance was observed over the 21-days HS in the WT rats, with day-21 HS values (1.00 ± 0.06 ml/min/100g bw) being comparable to control values (0.84 ± 0.04 ml/min/100g bw). Despite the significant reductions in GFR detected by the FITC-sinistrin excretion kinetics at HS-14, creatinine clearance was significantly higher than control values at this time point in the WT rats. Throughout the study we found no correlation between creatinine clearance and GFR as determined by FITC-sinistrin clearance, further highlighting the limitation of this technique (Figure S2).
Table 1.
| Day | 0.4% NaCl Control | HS-7 | HS-14 | HS-21 | ||||
|---|---|---|---|---|---|---|---|---|
| Group | WT | SSp67phoxNull | WT | SSp67phoxNull | WT | SSp67phox Null | WT | SSp67phox Null |
| Creatinine Clearance (ml/min/100g bw) | 0.84±0.04 | 0.89±0.06 | 1.13±0.05‡ | 1.07±0.05* | 1.08±0.02† | 1.08±0.04 | 1.00±0.06 | 0.96±0.08 |
Creatinine Clearance is not reflective of GFR in either wild-type (WT) or SSp67phox null rats. Creatinine clearance was compared on a 0.4% NaCl control day and 4.0% NaCl day-7 (HS-7), day-14 (HS-14) and day-21 (HS-21). bw indicates body weight.
p<0.05,
p<0.01,
p<0.001 compared to control measurements.
Discussion
The present study characterized the temporal changes in MBF, MAP and GFR in the development of hypertension in Dahl salt-sensitive (WT) and salt-resistant (SSp67phox null) rats to examine the role of increased oxidative stress in the initiation and maintenance of salt-sensitive hypertension.
The data presented show that during the initiation of salt-sensitivity the hypertensive response to a HS diet is directly paralleled by a reduction in MBF in WT salt-sensitive rats. Sustained elevations in blood pressure were associated with the development of glomeruli injury, increased urinary protein, and subsequently reduced GFR. Null mutations in the p67phox gene, which significantly reduces the production of ROS in the renal interstitium28, protected the renal medulla from the ischemic effects of a HS diet. During the first week of salt-loading MBF was stable in the null rats, with HS day-7 values being comparable to those recorded during the control period. Moreover, protection from reductions of MBF persisted over the three weeks, and by HS-21 there was a tendency towards increased rather than decreased perfusion. Sustained MBF was associated with an attenuated pressor response to HS, reduced pressure-induced renal injury and the maintenance of GFR. Glomeruli and tubular injury were exacerbated in the kidneys of WT rats compared to SSp67phox null rats at HS day-21. These data support the concept that reduced MBF, driven by increased ROS production in the renal medulla, plays a central role in the initiation of salt-sensitive hypertension and its resultant pathologies.
Previous studies from our laboratory have highlighted the key role that increased oxidative stress in the renal medulla plays in the development of salt-sensitive hypertension. We have demonstrated that DETC (superoxide dismutase inhibitor) infusion into the renal medulla of anaesthetised rats resulted in an immediate reduction in MBF and sodium excretion; MAP however, did not change during the 2-hour acute study29. In contrast, in comparable chronic studies 24-hours of DETC-infusion into the renal medulla did cause a significant increase in MAP which was paralleled by reduced MBF30. These data suggest that increased oxidative stress in the renal medulla induces reduced MBF and sodium retention which leads to the development of hypertension29. Accordingly, direct infusion of H2O2 into the renal medulla of normotensive rats caused a rapid and sustained increase in blood pressure31. Together these data indicate that endogenous ROS in the renal medulla is vasoconstrictive and the resultant reductions in MBF play a pathological role in the development of salt-sensitive hypertension. The observation that SSp67phox null rats are protected from reduced MBF is therefore consistent with the demonstration that H2O2 and superoxide (O2−) production are significantly reduced in the renal medulla of SSp67phox null rats compared to their WT littermates28.
The balance between ROS and nitric oxide (NO) in the renal medulla is finely regulated, with NO acting as a buffer to the vasoconstrictive actions of ROS32. The medullary thick ascending limb (mTAL) is a major site for both ROS and NO production, mediated by NADPH oxidase and NOS respectively33–35. Studies in isolated mTAL have demonstrated the importance of the balance between ROS and NO and coined the concept of tubulovascular crosstalk. We have demonstrated that NO and O2− produced by the mTAL can interact with surrounding outer medullary descending vasa recta promoting vasodilation and vasoconstriction respectively34, 36. Under normal physiological conditions NO is capable of buffering the vasoconstrictive effects of O2−36. In situations of increased ROS production, as during the consumption of a HS diet in SS rats, the balance is skewed towards excessive ROS production and MBF is compromised30, 37. In contrast, the results in this study show a tendency towards increased medullary perfusion from day-10 HS onwards in the SSp67phox null rats. This may reflect a relative increase in NO in the renal medulla, occurring as a secondary consequence of the attenuated ROS production in the kidneys of SSp67phox null rats. In support of this hypothesis we found that urinary nitrate levels were augmented in the SSp67phox null rats relative to the WT rats at day-21 HS.
In the current study MBF was lower than the average control value from HS day-2 onwards in the WT rats and was significantly reduced by HS day-6. It may be of note that despite comparable pressor responses to 4.0% NaCl during week one and week two of salt loading in the WT rats, maximal reductions in MBF were observed in the first week of HS, after which medullary perfusion appeared to plateau. This observation is consistent with the concept that reductions in MBF are involved in the initiation of the hypertensive response to a HS diet and are not merely the consequence of increased blood pressure.
Pressure-induced renal injury is reduced in SSp67phox null rats
In the current study we have used the transcutaneous assessment of FITC-sinistrin clearance to determine GFR in conscious, unrestrained rats. Sinistrin is a biologically inert fructose polymer which is freely filtered at the glomerulus and neither secreted nor reabsorbed by the renal tubules, making its clearance an ideal assessment of GFR38. The power of this novel technique is that the results are not confounded by the effects of repeated blood sampling or anaesthesia as is required in more traditional renal clearance studies. Here we have demonstrated that reductions in GFR occur after significant elevations in blood pressure in the WT rats. Blood pressure was significantly higher than control values at day-7 HS, at which point urinary protein excretion had also increased significantly suggestive of glomeruli injury. However, significant reductions in GFR were not observed until day-14 HS. Given these results we hypothesize that reduced GFR is the consequence of pressure-induced glomerular injury rather than a cause of hypertension. Similar observations were recently made in SS rats, in which reductions in GFR were observed 9-days after significant increases in blood pressure39.
Increased salt consumption has been shown to increase p67phox abundance and augment NADPH-oxidase activity in the renal cortex of SS rats24. In addition to increased cortical O2−, maintaining SS rats on 8.0% NaCl for 5-weeks was associated with increased MAP, reduced GFR and cortical glomerular sclerosis. Treatment with the anti-oxidant apocynin reduced NADPH-oxidase activity and consequently O2− production in the cortex. In correlation with the reduced cortical ROS, GFR was improved and MAP reduced24. The current study shows that in SSp67phox null rats, in which NADPH-oxidase activity is reduced28, GFR is maintained throughout the salt challenge and glomeruli injury is reduced. These results implicate a role for increased ROS production in cortical injury and reduced GFR. Whether the improved glomeruli function is the direct result of reduced ROS or secondary to the reduced MAP cannot be conclusively determined. However, given that reductions in GFR occurred after the onset in hypertension in WT rats it is likely that attenuated pressure, as a result of reduced ROS production, conferred protection from cortical injury to the SSp67phox null rats.
The concept of pressure-induced glomerular injury is consistent with results from studies in which one kidney of SS rats was protected from the hypertensive effects of a 14-day HS diet. Specifically, when renal perfusion pressure to the left kidney was servo-controlled with an aortic balloon implanted between the renal arteries, glomeruli injury was significantly reduced compared to the non-controlled, hypertensive right kidney. These results highlight the contribution of elevated renal perfusion pressure to glomeruli injury in salt-sensitive rats40. Whether extending the HS protocol would lead to the surpassing of a “pressure threshold” and a reduction in GFR in the SSp67phox null rats is an interesting area of future research.
In the current study we have shown that reductions in GFR, which are secondary to increased blood pressure, are preceded by two fold increases in urinary protein. These data suggest that clinically there could be a period in the initial stages of hypertension during which the identification of proteinuria and the initiation of blood pressure treatments may reduce the progression of renal injury and the decline of GFR. The concept that anti-hypertensive therapies have a more beneficial effect on GFR in individuals with increased proteinuria may be reflective of this41, 42.
In addition to the assessment of FITC-sinistrin clearance, creatinine clearance was measured in the rats to compare the sensitivity of this novel technique to that of one of the most commonly used experimental and clinical estimates of GFR. The results of the current study are in agreement with others which have shown the limitations of using creatinine clearance as a surrogate marker of GFR as we have previously discussed39. Specifically, at day-14 HS creatinine clearance increased in the WT rats relative to the control values and was comparable to those recorded in the SSp67phox null rats at the same time point; this is despite the demonstration that GFR had significantly reduced in the WT rats at day-14 HS when using the FITC-sinistrin clearance method. Moreover, we found no correlation between creatinine clearance and GFR as determined by the elimination of FITC-sinistrin. Given that creatinine clearance is considered an overestimation of GFR it is surprising that these values were consistently lower than the GFR values obtained using FITC-sinistrin disappearance, an observation also made in our previous characterization of GFR in SS rats over a 21-d HS challenge39. The cause of this discrepancy is unclear, however it possible that factors such as skin perfusion and thickness affect light penetration and lead to a further over-estimation of GFR when using the transcutaneous method; the investigation of these factors is an important area of future research.
It is a technical limitation of these studies that GFR, MBF and MAP cannot be simultaneously measured in the same rat. The requirement of restraint in the MBF studies prevents concurrent assessment of GFR, and may account for the consistently higher blood pressures recorded over the 21-days of HS in the MBF study compared to the GFR study. Nevertheless, in both studies null mutation of the p67phox gene conferred protection from salt-sensitivity to the SS rat, with day-21 HS MAP being at least 20 mmHg lower in the SSp67phox null rats than the WT rats in both experiments.
Perspectives
In these studies we have made repeated measurements of MBF, GFR, MAP and urinary protein in conscious SSp67phox null and WT rats over 21-days consumption of a HS diet. This is the first study to simultaneously track these parameters over a three week chronic salt challenge. The results presented have allowed us to determine both the sequential order of events involved in the initiation and maintenance of salt-sensitive hypertension, and the role of increased ROS production in its development. We have shown that reduced MBF, occurring as a result of increased NADPH oxidase-mediated ROS production, is involved in the initiation of salt-sensitive hypertension in the SS rat. In the SSp67phox null rat, medullary perfusion was sustained throughout the study and the hypertensive response to HS was blunted. In the later stages of the condition we hypothesize that pressure-induced renal injury results in a reduction in GFR; indeed renal injury was reduced in the SSp67phox null and GFR was maintained. Two fold increases in proteinuria occurred prior to significant reductions in GFR in WT rats. Clinically, increased proteinuria can be used to predict the rate of decline of GFR41, 42. Our data suggest that in hypertensive renal injury, early initiation of therapies to reduce the progression of salt-sensitive hypertension may improve clinical outcomes by preventing/delaying reductions in GFR. Screening for elevated proteinuria may help identify patients during the initiation of salt-sensitive hypertension, who could be at an increased risk of developing hypertensive renal injury.
Supplementary Material
Novelty and Significance.
1) What is new
These studies are the first to measure MBF in conscious rats over 3 weeks. The combination of these studies with the chronic assessment of GFR has allowed the delineation of events involved in the initiation of salt-sensitive hypertension in the SS rat, with a focus on the role of oxidative stress generated by NADPH-oxidase
2) What is relevant
The SS rat is frequently used as a model of clinical salt-sensitive hypertension. The mechanisms involved in the initiation and progression of salt-sensitivity have not been fully defined. The data presented suggest that increased oxidative stress in the renal medulla results in medullary ischemia which is involved in the initiation of hypertension. Increased blood pressure leads to proteinuria and subsequently reduced GFR. Identification of these time points clinically may improve therapeutic management.
3) Summary
Reducing oxidative stress in the renal medulla, through null mutations in p67phox, improves medullary blood flow during the initial stages of salt-sensitive hypertension, which promotes a blunted pressor response to salt and improved renal function.
Acknowledgments
We thank Glenn Slocum for assistance with microscopy and image analysis and Camille Taylor and Jenifer Philips for the measurement of protein and albumin.
Sources of Funding
This study was supported by National Institutes of Health grants HL-116264 (A.C.) and HL-082798 (A.C.). L.C. Evans is funded by an AHA postdoctoral fellowship grant AHA13POST15230000.
Footnotes
Disclosures
None
References
- 1.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 2.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713. doi: 10.1161/01.HYP.0000120155.48024.6f. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JM, Prewitt RL, Ratts TE. Sodium sensitivity in normotensive and borderline hypertensive humans. Am J Med Sci. 1988;295:370–377. doi: 10.1097/00000441-198804000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 5.Whittle JC, Whelton PK, Seidler AJ, Klag MJ. Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med. 1991;151:1359–1364. [PubMed] [Google Scholar]
- 6.Lipworth L, Mumma MT, Cavanaugh KL, Edwards TL, Ikizler TA, Tarone RE, McLaughlin JK, Blot WJ. Incidence and predictors of end stage renal disease among low-income blacks and whites. Plos One. 2012;7:e48407. doi: 10.1371/journal.pone.0048407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan BM, Zhao Y. Different definitions of prevalent hypertension impact: the clinical epidemiology of hypertension and attainment of Healthy People goals. J Clin Hypertens (Greenwich) 2013;15:154–161. doi: 10.1111/jch.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirkle JL, Freedman BI. Hypertension and chronic kidney disease: controversies in pathogenesis and treatment. Minerva Urologica E Nefrologica. 2013;65:37–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154:541–548. doi: 10.7326/0003-4819-154-8-201104190-00335. [DOI] [PubMed] [Google Scholar]
- 12.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 13.Kato N. Genetic analysis in Dahl salt-sensitive rats. Clin Exp Pharmacol Physiol. 1999;26:539–540. doi: 10.1046/j.1440-1681.1999.03084.x. [DOI] [PubMed] [Google Scholar]
- 14.Baba K, Mulrow PJ, Franco-Saenz R, Rapp JP. Suppression of adrenal renin in Dahl salt-sensitive rats. Hypertension. 1986;8:1149–1153. doi: 10.1161/01.hyp.8.12.1149. [DOI] [PubMed] [Google Scholar]
- 15.Chen PY, St John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary L-arginine supplementation. Lab Invest. 1993;68:174–184. [PubMed] [Google Scholar]
- 16.Reaven GM, Twersky J, Chang H. Abnormalities of carbohydrate and lipid metabolism in Dahl rats. Hypertension. 1991;18:630–635. doi: 10.1161/01.hyp.18.5.630. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation. 1997;96:2407–2413. doi: 10.1161/01.cir.96.7.2407. [DOI] [PubMed] [Google Scholar]
- 18.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 19.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1573–R1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 20.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 21.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 22.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor NE, Maier KG, Roman RJ, Cowley AW., Jr NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension. 2006;48:1066–1071. doi: 10.1161/01.HYP.0000248751.11383.7c. [DOI] [PubMed] [Google Scholar]
- 24.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Li P, Yang C, Kurth T, Misale M, Skelton M, Moreno C, Roman RJ, Greene AS, Jacob HJ, Lazar J, Liang M, Cowley AW., Jr Dynamic convergence and divergence of renal genomic and biological pathways in protection from Dahl salt-sensitive hypertension. Physiol Genomics. 2010;41:63–70. doi: 10.1152/physiolgenomics.00170.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maehara Y, Miyano K, Sumimoto H. Role for the first SH3 domain of p67phox in activation of superoxide-producing NADPH oxidases. Biochem Biophys Res Commun. 2009;379:589–593. doi: 10.1016/j.bbrc.2008.12.112. [DOI] [PubMed] [Google Scholar]
- 28.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW., Jr Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012;15:201–208. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 30.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 31.Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- 32.Evans RG, Fitzgerald SM. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens. 2005;14:9–15. doi: 10.1097/00041552-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol. 2007;292:F993–F998. doi: 10.1152/ajprenal.00383.2006. [DOI] [PubMed] [Google Scholar]
- 34.Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res. 2002;91:487–493. doi: 10.1161/01.res.0000035243.66189.92. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol. 2004;287:F274–F280. doi: 10.1152/ajprenal.00382.2003. [DOI] [PubMed] [Google Scholar]
- 36.Mori T, O'Connor PM, Abe M, Cowley AW., Jr Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension. 2007;49:1336–1341. doi: 10.1161/HYPERTENSIONAHA.106.085811. [DOI] [PubMed] [Google Scholar]
- 37.Miyata N, Cowley AW., Jr Renal intramedullary infusion of L-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension. 1999;33:446–450. doi: 10.1161/01.hyp.33.1.446. [DOI] [PubMed] [Google Scholar]
- 38.Tett SE, Kirkpatrick CM, Gross AS, McLachlan AJ. Principles and clinical application of assessing alterations in renal elimination pathways. Clin Pharmacokinet. 2003;42:1193–1211. doi: 10.2165/00003088-200342140-00002. [DOI] [PubMed] [Google Scholar]
- 39.Cowley AW, Jr, Ryan RP, Kurth T, Skelton MM, Schock-Kusch D, Gretz N. Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension. 2013;62:85–90. doi: 10.1161/HYPERTENSIONAHA.113.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1482. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toto RD. Lessons from the African-American study of kidney disease and hypertension: an update. Curr Hypertens Rep. 2006;8:409–412. doi: 10.1007/s11906-006-0087-7. [DOI] [PubMed] [Google Scholar]
- 42.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.