Abstract
Multifunctional zinc oxide (ZnO) nanoparticles (NPs) with well-integrated multimodality imaging capacities have generated increasing research interest in the last decade. However, limited progress has been made in developing ZnO NP-based multimodality tumor-imaging agents. Here we developed novel red fluorescent ZnO NPs and described the successful conjugation of 64Cu (t1/2 = 12.7 h) and TRC105, a chimeric monoclonal antibody against CD105, to these ZnO NPs via well-developed surface engineering procedures. The produced dual-modality ZnO NPs were readily applicable for positron emission tomography (PET) imaging and fluorescence imaging of the tumor vasculature. Their pharmacokinetics and tumor-targeting efficacy/specificity in mice bearing murine breast 4T1 tumor were thoroughly investigated. ZnO NPs with dual-modality imaging properties can serve as an attractive candidate for future cancer theranostics.
Keywords: Zinc oxide, positron emission tomography (PET), imaging, drug delivery, CD105, angiogenesis
INTRODUCTION
Significant research efforts have been put into biocompatible nano-sized zinc oxide (ZnO) due to its unique physical properties. Nano ZnO has versatile applications in optics, electronics, sensors, drug delivery, and biomedical imaging.1, 2 Particularly, nano-sized ZnO possesses high surface areas, excellent interaction with electro-magnetic wave, and tunable electronic properties.3 Various nanostructures of ZnO have been developed including nanowires, nanotubes, nanopods, and nanorods.2
The majority of nano ZnO surface is decorated with hydroxyl groups (-OH), which can be used for further conjugation with various functional molecules.4 Upon direct contact with aqueous solution, ZnO can dissolve slowly in both acidic (e.g. tumor microenvironment) and strong basic conditions. Since Zn2+ is an essential nutrient for adults, ZnO nanomaterials are considered to be safe in vivo. In fact, ZnO is graded as a “GRAS” (generally recognized as safe) substance (Code of Federal Regulations Title 21, Section 182.8991) by US Food and Drug Administration (FDA). With these properties, ZnO nanomaterials can be selected as biocompatible and biodegradable nano-platforms for targeted cancer therapy.
Molecular imaging can measure the distribution pattern and characterize the interaction events of a given molecule in vivo.5 Among all molecular imaging techniques, fluorescence imaging has intrinsically high sensitivity/safety, optimal spatial resolution, and relatively low instrumentation cost while low tissue penetration is the major curdle for its application.6 At the same time, positron emission tomography (PET) imaging, which has been widely used for cancer diagnosis and therapy response monitoring, possesses high quantitative capacity and no tissue penetration limitation.7, 8 Since the combination of optical imaging and PET can collect synergistic information of molecular events, our goal here is to produce a ZnO-based nanoparticle (ZnO NP) to serve as a PET and fluorescence image-guided drug delivery vector.9
CD105, also known as endoglin, was used as the target on tumor due to its nearly exclusive expression pattern on proliferating endothelial cells (especially inside a tumor).10,11 In our previous studies, radiolabeled TRC105, a monoclonal antibody against CD105, was repeatedly used for non-invasive CD105 imaging in various tumor models.12–17 A recent phase I trial in patients with advanced cancer further confirmed that TRC105 was well tolerated in these patients and could stabilize the disease progression.18 With the support of current evidence, we can conclude that TRC105 and its derivatives can play important roles in multiple aspects of cancer patient management.
Therefore, we develop a tumor-targeted, PET imageable red fluorescent ZnO NPs in the current study by conjugation with TRC105. Red fluorescent ZnO NPs have been synthesized and used as a potentially theranostic reagent with versatile application potentials. By targeting of CD105 on tumor vasculature, more than 3-fold higher uptake (~6 %ID/g at 4 h post-injection [p.i.]) in tumor was observed for the TRC105-conjugated ZnO NPs compared to ZnO NPs without TRC105 conjugation (~2 %ID/g at 4 h p.i.). The targeting efficacy, specificity, and biodistribution of these ZnO nano-conjugates were investigated systematically. The TRC105-ZnO NPs designed here can be used as a cancer theranostic nano-platform in the future upon further optimization.
EXPERIMENTAL SECTION
Synthesis of red fluorescent ZnO NPs
Red fluorescent ZnO nanoparticles were synthesized by a calcination method that was reported in the literature.19 0.6 mmol zinc acetate dehydrate - pre-dissolved in 12 mL dimethylformamide (DMF) - was added by droplets to 60 mL DMF solution containing 0.3 mmol 2-aminoterephthalic acid and 0.3 mmol acetylenedicarboxylic acid while stirring. After stirring for 10 minutes, zinc 2-aminoterephthalate acetylenedicarboxylate coordination polymer formed as yellow precipitates. These precipitates were filtered, washed by DMF, and dried in a vacuum oven at 60 °C for 5 hours. The final product was obtained by calcination of the dried powders at 550 °C with an air flow for 75 minutes after a heating ramp at a rate of 2 degrees per minute. The scanning electron microscopy (SEM) images were taken by LEO 1530 FE-SEM.
Conjugation of ZnO NPs
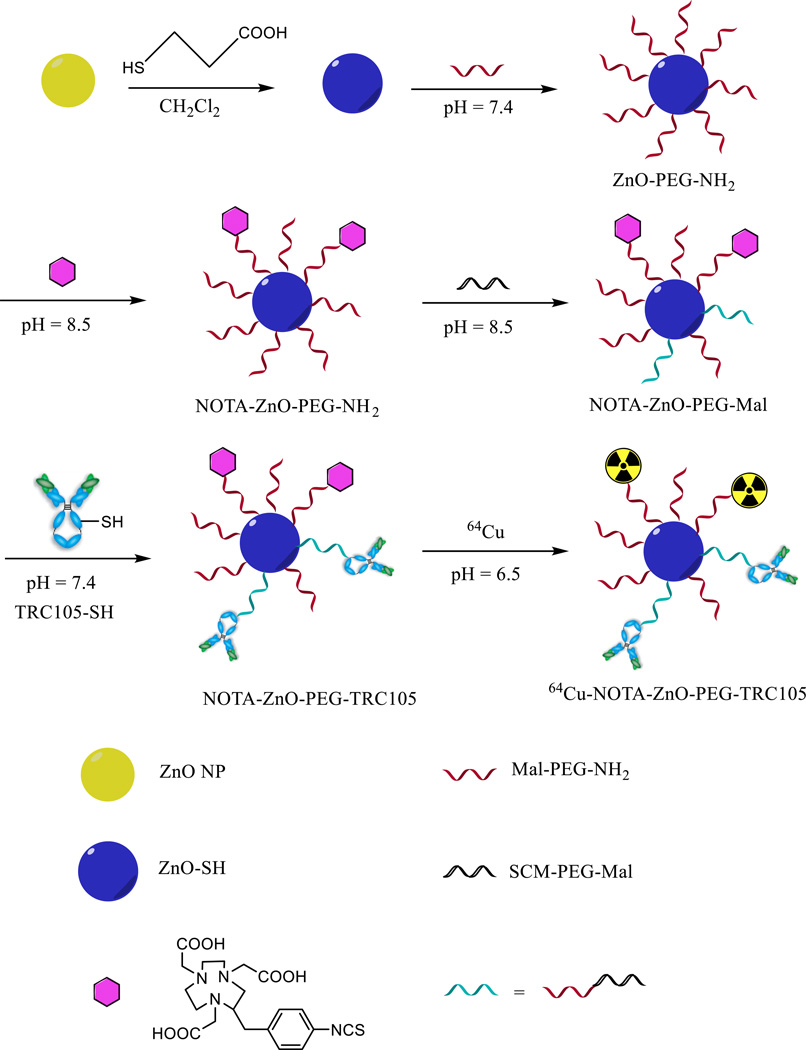
In this study, the surface of ZnO NPs was functionalized by carboxylic acids that can form bidentate coordination bonds with the zinc atoms. The detailed procedure was previously reported by our group.20, 21 Briefly, 3 mg of 3-mercaptopropionic acid (Sigma-Aldrich, St. Louis, MO) were added into 2 ml of dichloromethane (DCM) suspension of ZnO NPs (3 mg/ml) and stirred at 350 rpm for 1 h (room temperature [RT]). After removing DCM by evaporation, the intermediate product (ZnO-SH) was repeatedly washed by ethanol. Two milligrams of ZnO-SH were incubated with 10 mg of maleimide-polyethylene glycol-succinimidyl carboxy methyl ester (Mal-PEG-NH2; Creative PEGWorks; MW: 5 kDa) for 1 h at RT in 2 mL of phosphate-buffered saline (PBS). Tris(2-carboxyethyl)phosphine hydrochloride (TCEP; Sigma-Aldrich) was also added into the mixture for prevention of thiol oxidation and the final pH was maintained at 7 – 7.5. The formed ZnO-PEG-NH2 was purified by 100 kDa cutoff Amicon filter with subsequent wash by ethanol and water. A molar ratio of 1:10 was adopted in the 2 h reaction between ZnO-PEG-NH2 and S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) at the pH of 8.5. 100 kDa cutoff Amicon filters was again used to purify the resulting NOTA-ZnO-PEG-NH2. Another 2 h reaction between NOTA-ZnO-PEG-NH2 and succinimidyl carboxymethyl PEG maleimide (SCM-PEG-Mal; Creactive PEGWorks; MW: 5 kDa) was carried out at pH 8.5 in a molar ratio of 1:30 to acquire the important intermediate NOTA-ZnO-PEG-Mal.
Meanwhile, the thiolation of TRC105 (named TRC105-SH) was conducted using our previously published procedure.13 Subsequently, NOTA-ZnO-PEG-Mal was reacted with TRC105-SH (molar ratio 1:5) at pH 7.5 and RT under TCEP protection. The final product, NOTA-ZnO-PEG-TRC105, was purified by centrifugation filtration. The detailed reaction procedures were shown in Scheme 1.
Scheme 1.
Schematic synthesis route of NOTA-ZnO-PEG-TRC105 and NOTA-ZnO-PEG-NH2.
Cellular uptake assays
The interactions between ZnO NP conjugates and cellular CD105 were examined by a fluorescence microscope. CD105-positive Human umbilical vein endothelia cells (HUVEC22) were cultured onto 24-well plates with a density of 1 × 106 per well for overnight. Before treatment with NOTA-ZnO-PEG-TRC105, NOTA-ZnO-PEG-NH2, or NOTA-ZnO-PEG-TRC105 with TRC105 blocking (0.5 mg/mL TRC105) for 120 min (ZnO concentration: 50 µg/ml), a cell fixing solution (methanol/water/acetic acid, 5:4:1) was used to fix cells. Repeated PBS washing was used after incubation. Fluorescence from ZnO was acquired with a Nikon Eclipse Ti-U inverted microscope (Nikon, Melville, NY) and analyzed using the NIS-Elements BR Software.
Animal model
All animal studies were conducted in compliance with the regulations by the University of Wisconsin Institutional Animal Care and Use Committee. Four- to five-week-old female Balb/c mice from Harlan (Indianapolis, IN) were used for tumor establishment via subcutaneous injection of 2 × 106 cells (formulated in 100 µL of PBS) into the front mouse flank. Caliper measurement (twice per week) was carried out to monitor the tumor sizes. The tumor-bearing animals were used for imaging/biodistribution studies when tumor diameter reached 6 to 8 mm.
64Cu-labeling of ZnO nanoparticles
70–120 MBq of 64CuCl2 was diluted in 300 µl of 0.1 M sodium acetate buffer (pH 6.5) before incubation with NOTA-ZnO-PEG-TRC105 or NOTA-ZnO-PEG-NH2 (concentration: ~2 mg/ml) at a ratio of 30 µg per 37 MBq of 64CuCl2. The reaction condition is stirring (350 rpm) at 37 °C for 30 min. 64Cu-NOTA-ZnO-TRC105 or 64Cu-NOTA-ZnO-PEG-NH2 was purified by Amicon filter (molecular weight cut-off: 50 kDa) and re-suspended in PBS.
PET imaging and biodistribution studies
PET scans were performed by a Siemens Inveon PET/CT hybrid scanner (Siemens Medical Solutions USA, Inc.) as described previously.13, 22, 23 Following the image acquisition, maximum a posteriori (MAP) algorithm was used for image reconstruction (no attenuation or scatter correction was applied). Tumor-bearing mouse was intravenously injected with 5 to 10 MBq of 64Cu-NOTA-ZnO-TRC105 or 64Cu-NOTA-ZnO-PEG-NH2 prior to 3 – 15 min static PET scans at various time points post-injection (p.i.). Percent injected dose per gram of tissue (%ID/g) of injected materials was also calculated by a vendor software (Inveon Research Workplace). Pre-injection of TRC105 (1 mg) one hour before 64Cu-NOTA-ZnO-PEG-TRC105 was also carried out in a separate group of mice (n = 3) to evaluate the CD105 targeting specificity of 64Cu-NOTA-ZnO-PEG-TRC105. Gamma counter (Perkin Elmer) measurements of radioactivity in major tissues/organs (i.e. biodistribution studies) were carried out after the last PET scan in the format of %ID/g (mean ± SD).
RESULTS
Synthesis and characterization of ZnO conjugates
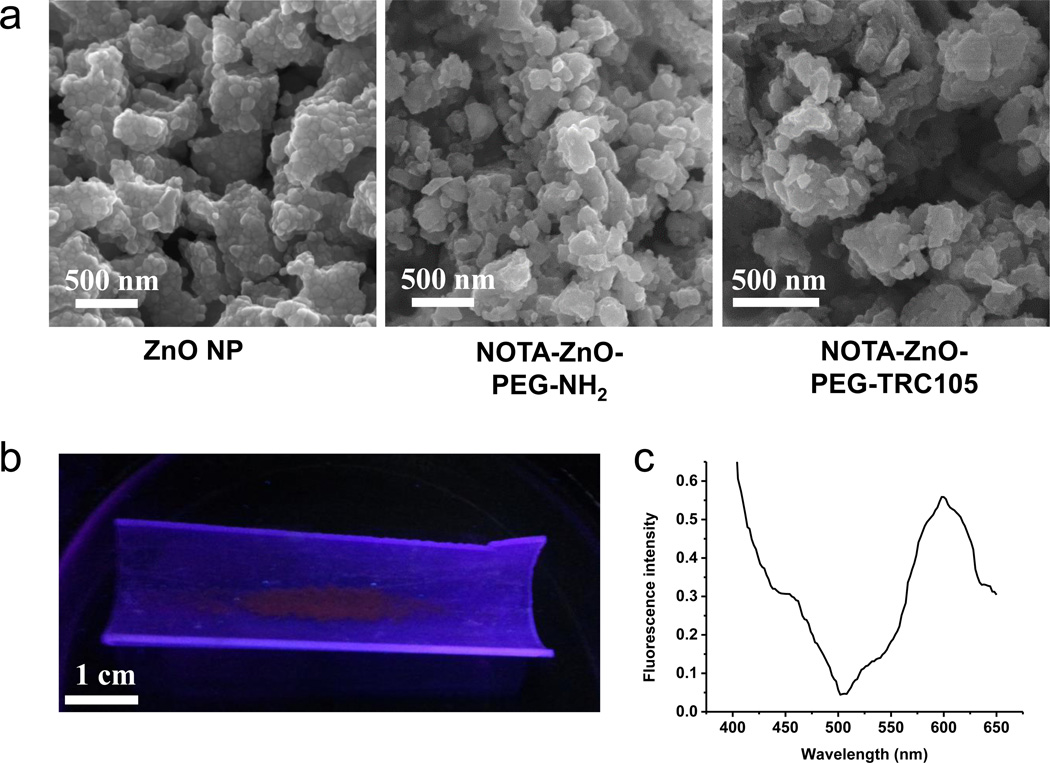
A schematic reaction protocol of NOTA-ZnO-PEG-NH2 and NOTA-ZnO-PEG-TRC105 is shown in Scheme 1. Based on SEM measurements, ZnO NP, NOTA-ZnO-PEG-NH2 and NOTA-ZnO-PEG-TRC105 have the size range of 50 – 100 nm (Figure 1a). The hydrodynamic sizes of ZnO NP, NOTA-ZnO-PEG-NH2, and NOTA-ZnO-PEG-TRC105 (measured by dynamic light scattering (DLS)) were 78.4 ± 8.2 nm, 83.3 ± 7.6 nm, and 101.2 ± 10.7 nm, respectively. Although a certain percentage of aggregates (> 1 µm) could also be observed in DLS measurement, the overall uniformity of all three samples was high and the samples could stay stable in PBS for over one week. Since NOTA is quite small in size, the diameter alteration of the ZnO NP conjugates is primarily from the conjugation of TRC105 (5–10 nm in size) and PEG. The zeta-potential values of ZnO NP, NOTA-ZnO-PEG-NH2, and NOTA-ZnO-PEG-TRC105 were measured to be −33.2 ± 3.1 mV, −27.3 ± 2.7 mV, and −11.2 ± 2.1 mV, respectively. We notice that NOTA conjugation altered the zeta-potential (i.e. surface charge) of ZnO-NP and so did TRC105 conjugation. TEM and zeta-potential measurements served as strong evidence of NOTA/TRC105 attachment onto ZnO NPs. When excited with UV light (~350 nm), red fluorescence could be observed from ZnO NPs (Figure 1b), which was further confirmed by a fluorescence spectrum measurement to possess a peak emission at 598 nm (Figure 1c).
Figure 1.
(a) SEM images of ZnO NP, NOTA-ZnO-PEG-NH2, and NOTA-ZnO-PEG-TRC105. (b) Red fluorescence emitted from ZnO NPs after the excitation from UV light. (c) Fluorescence spectrum of ZnO NPs at the excitation of 350 nm.
CD105-mediated cellular uptake
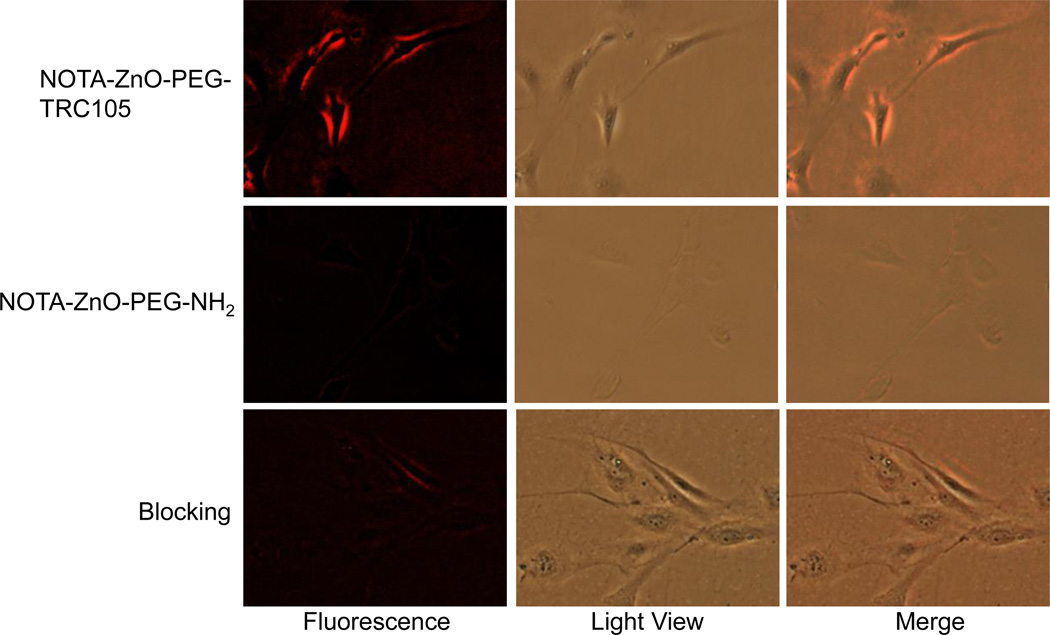
Fluorescent microscopy analysis in HUVEC (CD105 positive17, 22) and MCF-7 (CD105 negative17, 22) was used to examine the interaction of NOTA-ZnO-PEG-NH2 or NOTA-ZnO-PEG-TRC105 with the cells. HUVEC treated with NOTA-ZnO-PEG-TRC105 showed high levels of cellular fluorescence emitted from ZnO NPs. In contrast, cells treated with NOTA-ZnO-PEG-NH2 showed low levels of cellular fluorescence. Competition of TRC105 (0.5 mg/mL) resulted in significantly decreased cellular fluorescence of NOTA-ZnO-PEG-TRC105 to a level comparable to that of NOTA-ZnO-PEG-NH2 (Figure 2).
Figure 2.
Representative fluorescence images of ZnO NP conjugates in HUVEC (CD105-positive) after 2 h incubation and subsequent washing (images were taken by Nikon Eclipse Ti-U at 200 ×).
64Cu-Labeling and PET Imaging
64Cu-labeling, including purification using Amicon filters, took 90 ± 15 min (n = 6). The decay-corrected radiochemical yield was 78.5 ± 10.9 % with a radiochemical purity of > 95%. Assuming complete recovery of ZnO NPs after Amicon filtration, the ratio of 64Cu activity to ZnO NPs was ~0.97 GBq/mg for both 64Cu-NOTA-ZnO-PEG-NH2 and 64Cu-NOTA-ZnO-PEG-TRC105.
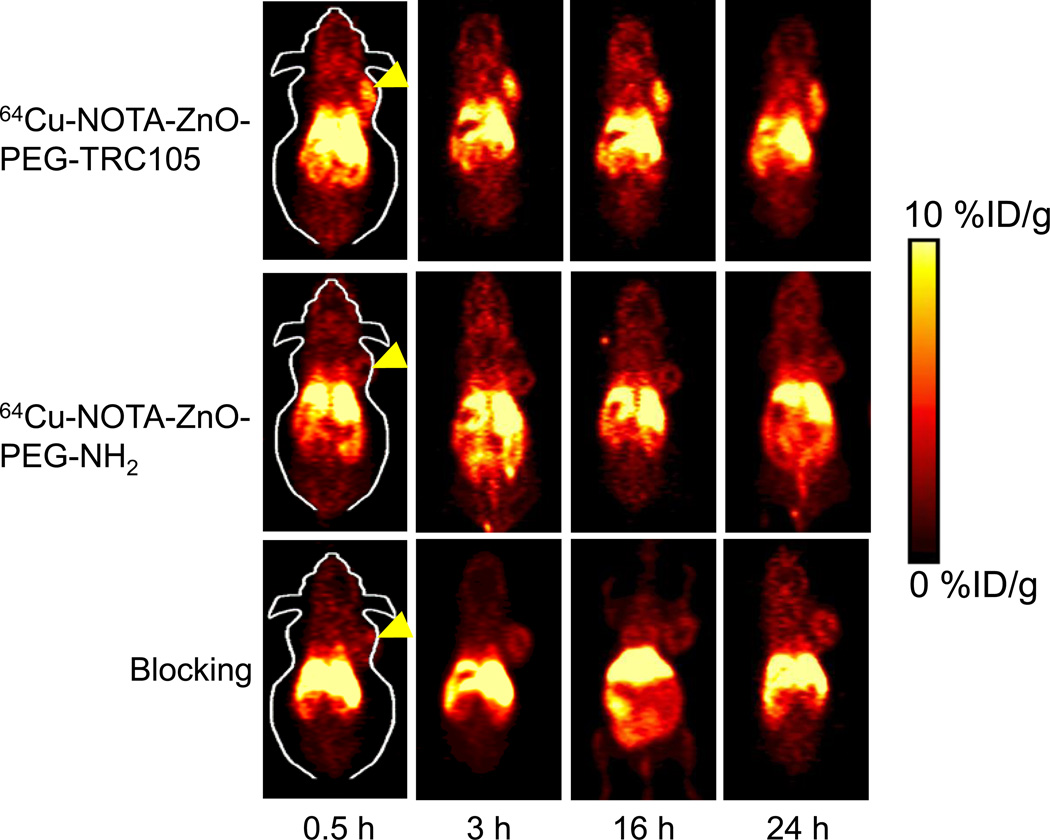
Despite being CD105-negative in 4T1 cells, 4T1 tumors were used due to their rapid growth speed that leads to abundant tumor angiogenesis and hence expression of high levels of CD105.12–17 We utilized our previous experience to choose the time points of 0.5, 3, 16, and 24 h p.i. for PET scans following the injection of 64Cu-NOTA-ZnO-PEG-NH2 or 64Cu-NOTA-ZnO-PEG-TRC105.13, 23–25 The coronal PET slices, containing 4T1 tumors, are presented in Figure 3. The blood pool radioactivity indicated that the few-hour circulation t1/2 of 64Cu-NOTA-ZnO-PEG-TRC105 was similar to that of 64Cu-NOTA-ZnO-PEG-NH2. This should be sufficient for in vivo tumor targeting. Besides blood, high radioactivity accumulation from 64Cu-NOTA-ZnO-PEG-TRC105 was observable in the liver, tumor, and abdominal area. However, there was no accumulation in most other tissues, thus exhibiting good tumor targeting and image contrast. In comparison, tumor deposit of 64Cu-NOTA-ZnO-PEG-NH2, as well as 64Cu-NOTA-ZnO-PEG-TRC105 with TRC105 blocking (~ 50 mg/kg), was very low (Figure 3). The detailed quantitative analysis of the PET data will be discussed below.
Figure 3.
Serial coronal PET images of 4T1 tumor-bearing mice at chosen time points post-injection of 64Cu-NOTA-ZnO-PEG-TRC105, 64Cu-NOTA-ZnO-PEG-NH2, and blocking group. Yellow arrowheads showed tumor locations in mice.
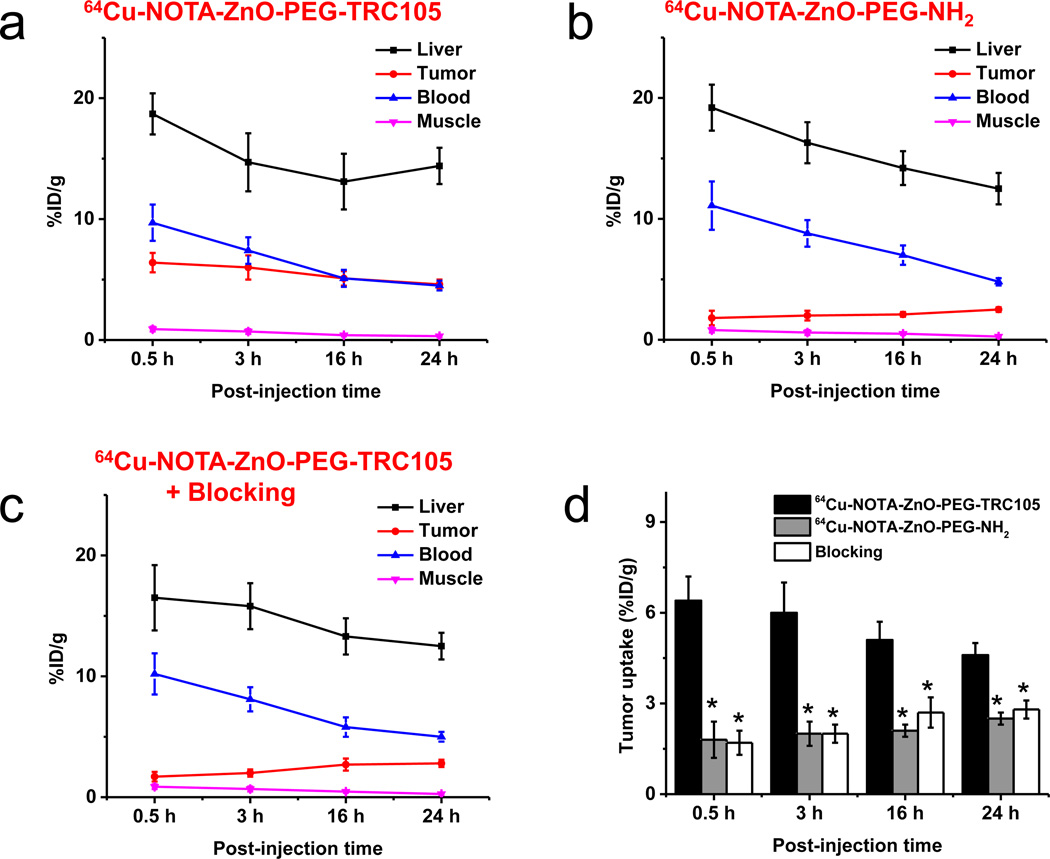
Quantitative ROI analysis is displayed in Figure 4. The uptake of 64Cu-NOTA-ZnO-PEG-TRC105 in the liver was obvious and demonstrated a gradual decay pattern (18.7 ± 1.7, 14.7 ± 2.4, 13.1 ± 2.3, and 14.4 ± 1.5 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively (n = 3, Figure 4a). The blood retention of 64Cu-NOTA-ZnO-PEG-TRC105 was calculated to be 9.7 ± 1.5, 7.4 ± 1.1, 5.1 ± 0.7, and 4.5 ± 0.4 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively (n = 3, Figure 4a). The penetration of 64Cu-NOTA-ZnO-PEG-TRC105 into 4T1 tumor was fast and stayed stable over the time (6.4 ± 0.8, 6.0 ± 1.0, 5.1 ± 0.6, and 4.6 ± 0.4 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively; n = 3, Figure 4a). Good contrast in tumor was witnessed by a tumor-to-muscle ratio of 12.1 ± 2.6 for 64Cu-NOTA-ZnO-PEG-TRC105 at as early as 3 h p.i. (n = 3).
Figure 4.
PET region-of-interest (ROI) analysis. Time-activity curves (TAC) of the liver, 4T1 tumor, blood and muscle were shown p.i. of (a) 64Cu-NOTA-ZnO-PEG-TRC105, (b) 64Cu-NOTA-ZnO-PEG-NH2, and (c) 64Cu-NOTA-ZnO-PEG-TRC105 with a blocking dose of TRC105 (50 mg/kg) are shown (n = 3). The tumor uptakes for these ZnO nanoconjugates were highlighted in (d).
64Cu-NOTA-ZnO-PEG-NH2 served as a control to study the accumulation behavior of ZnO NPs without TRC105 conjugation in 4T1 tumors. From Figure 4b, the tumor uptake of 64Cu-NOTA-ZnO-PEG-NH2 (1.8 ± 0.6, 2.0 ± 0.4, 2.1 ± 0.2, and 2.5 ± 0.2 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively; n = 3) was significantly lower than that of 64Cu-NOTA-ZnO-PEG-TRC105 within the whole time frame of PET imaging (p < 0.05; n = 3, Figure 4d). This further confirmed that TRC105 conjugation is the leading factor for the elevated uptake of 64Cu-NOTA-ZnO-PEG-TRC105 in 4T1 tumor. The radioactivity of 64Cu-NOTA-ZnO-PEG-NH2 in the blood was similar to that of 64Cu-NOTA-ZnO-PEG-TRC105 (11.1 ± 2.0, 8.8 ± 1.1, 7.0 ± 0.8, and 4.8 ± 0.3 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively; n = 3).
Administering high amount of TRC105 (50 mg/kg) reduced the 4T1 tumor uptake of 64Cu-NOTA-ZnO-PEG-TRC105 significantly (1.7 ± 0.4, 2.0 ± 0.3, 2.7± 0.5, and 2.8 ± 0.3 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively; p < 0.05, n = 3; Figure 4c&d). This again confirmed the CD105 specificity of 64Cu-NOTA-ZnO-PEG-TRC105 in vivo. TRC105 administration did not alter the liver uptake of 64Cu-NOTA-ZnO-PEG-TRC105 (16.5 ± 2.7, 15.8 ± 1.9, 13.3 ± 1.5, and 12.5 ± 1.1 %ID/g at 0.5, 3, 16, and 24 h p.i., respectively; n = 3; Figure 4c).
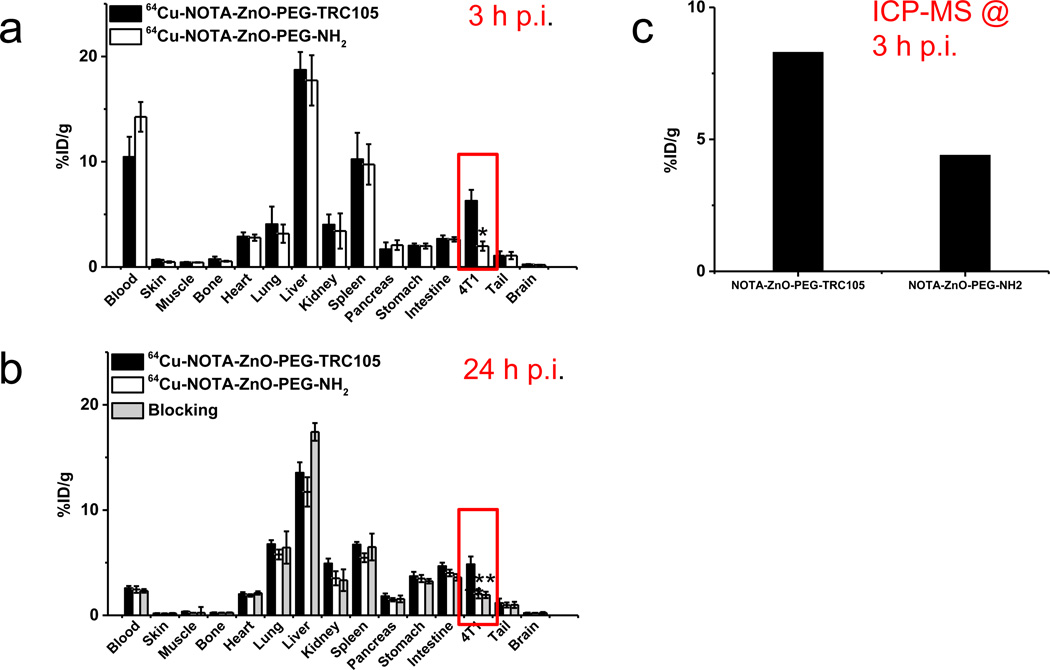
Biodistribution studies was carried out post the last PET scans (Figure 5a&b). Two more separate groups of mice (n = 4 per group) were injected with 64Cu-NOTA-ZnO-PEG-TRC105 or 64Cu-NOTA-ZnO-PEG-NH2 and used for determination of their distribution profiles at 3 h p.i. (early time point with a peak 4T1 tumor uptake based on PET). Consistent with PET studies, the uptake of 64Cu-NOTA-ZnO-PEG-TRC105 in the 4T1 tumor was higher than all the major organs/tissues except liver and spleen (mostly responsible for clearance). The radioactivity observed in kidneys probably came from two pathways – the degradation of ZnO NPs and/or small amount of 64Cu detachment from NOTA chelators on the surface of ZnO NPs. In summary, the quantitative data acquired from the biodistribution studies demonstrated good agreement with PET ROI quantification.
Figure 5.
(a) Biodistribution of 64Cu-NOTA-ZnO-PEG-TRC105 and 64Cu-NOTA-ZnO-PEG-NH2, at 3 h post-injection. (b) Biodistribution of 64Cu-NOTA-ZnO-PEG-TRC105, 64Cu-NOTA-ZnO-PEG-NH2, and blocking group (50 mg/kg TRC105 injected 1 h prior to 64Cu-NOTA-ZnO-PEG-TRC105) at 24 h post-injection. (c) ICP-MS measurement of tumor homogenates 3 h post-injection of NOTA-ZnO-PEG-TRC105 or NOTA-ZnO-PEG-NH2.
At the same time and in order to confirm that the uptake in tumor is relevant to ZnO NP delivery, we did inductively coupled plasma mass spectrometry (ICP-MS) on excised 4T1 tumors from two individual mice at 3 h p.i. of NOTA-ZnO-PEG-TRC105 or NOTA-ZnO-PEG-NH2 (5 mg/kg each). Almost two-fold higher zinc concentration was confirmed in the tumor injected with NOTA-ZnO-PEG-TRC105, when compared to NOTA-ZnO-PEG-NH2 (8.3 %ID/g and 4.4 %ID/g, respectively (Figure 5c). This matched very well the results from the biodistribution studies and PET scans.
Histology
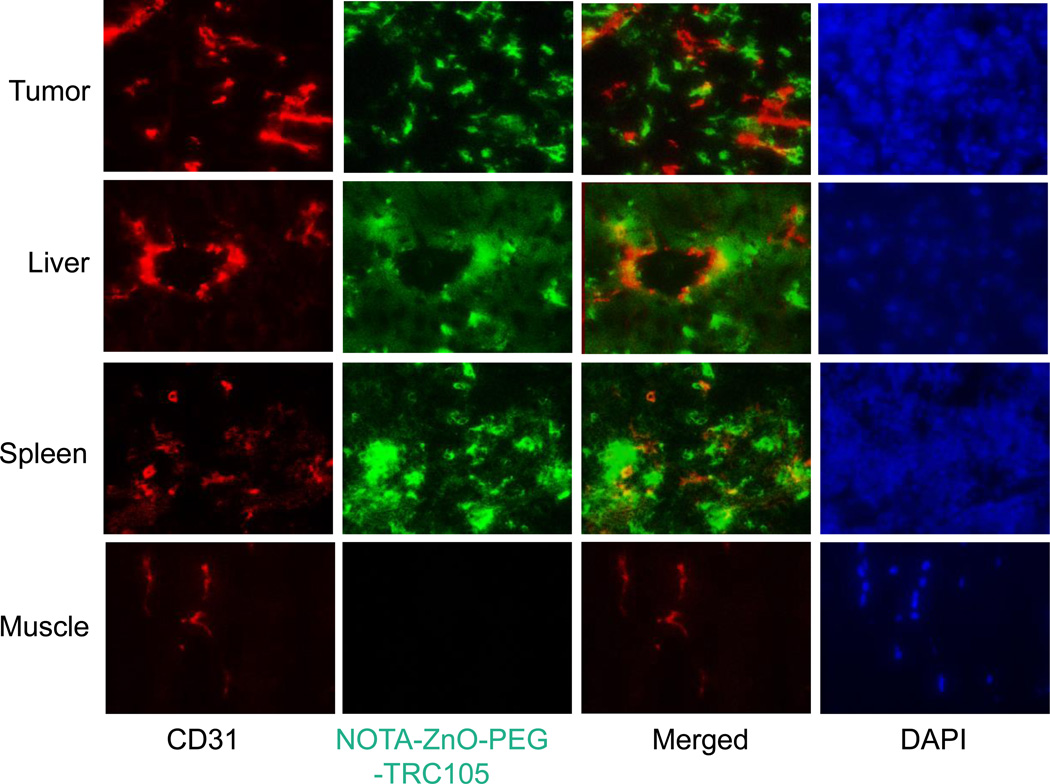
To confirm that the enhanced ZnO NPs delivery to 4T1 tumor was primarily via the introduction of TRC105, each of three 4T1 tumor-bearing mice was injected with 5 mg/kg of NOTA-ZnO-PEG-TRC105 and euthanized at 3 h p.i. Tissues showing high accumulation of 64Cu-NOTA-ZnO-PEG-TRC105, i.e. the tumor, liver, and spleen, were used for histological analysis. With minimal 64Cu-NOTA-ZnO-PEG-TRC105 absorption, the muscle tissue was used a standard control for normal tissues.
TRC105 attached onto ZnO NPs was used as the primary antibody label, thus only an Alexa Fluor488-labeled antihuman IgG was required for visualization purpose. Based on that, the green fluorescence in Figure 6 was a location indicator of NOTA-ZnO-PEG-TRC105. From the clear separation of the red (stands for CD31, an endothelial cell marker on vessels) and green fluorescence at 3 h p.i., we could confirm significant extravasation of NOTA-ZnO-PEG-TRC105 in 4T1 tumor at that time. Significant uptake of NOTA-ZnO-PEG-TRC105 in liver and spleen slices at 3 h p.i. (Figure 6) was also observed. We have to emphasize here that the green fluorescence from NOTA-ZnO-PEG-TRC105 displayed weak overlay with CD31 staining, which indicates that liver/spleen uptake of NOTA-ZnO-PEG-TRC105 was not mediated by CD105. At the same time, green fluorescence was undetectable in muscle.
Figure 6.
Histological analysis of 4T1 tumor, liver, spleen, and muscle at 3 h post-injection of NOTA-ZnO-PEG-TRC105. Red fluorescence stands for CD31 staining and the location of NOTA-ZnO-PEG-TRC105 is indicated by green fluorescence. Blue fluorescence from DAPI clarified the nuclei locations.
DISCUSSIONS
Various nanomaterials have been used in targeted drug delivery to significantly enhance the therapeutic outcome and minimize undesirable side effects. Compared with other nanomaterials, ZnO-based nanomaterials are attractive due to their low toxicity and biodegradable characteristics.26 Based on these desirable properties, ZnO nanomaterials have gained enormous interest in biomedical applications. Different types of chemo/gene drugs (e.g. doxorubicin, daunorubicin, or DNA fragments) have been loaded on ZnO nanomaterials for effective delivery into cancer cells.2 The data from the current study confirmed the enhanced uptake of ZnO nanoparticles in tumor sites as a result of a targeting ligand (TRC105) conjugation, which could enable ZnO nanomaterials as a tumor-selective drug delivery nanoplatform in the future.
The abundance of -OH groups on the surface of ZnO can be readily functionalized by molecules with various activities.4, 27 Successful surface modification of ZnO-based nanomaterials is critical for both their stability and drug delivery efficacy. Surface PEGylation of ZnO NPs was performed twice in this study to facilitate the attachment of NOTA and TRC105, improve the stability of ZnO NPs in a biological scenario, and reduce the opsonization, thus improving the blood circulation lifetime of 64Cu-NOTA-ZnO-PEG-TRC105. SEM, DLS and zeta potential characterizations confirmed the successful surface functionalization. Well dispersion in PBS could be achieved for NOTA-ZnO-PEG-TRC105 for weeks.
In vivo distribution profiles of ZnO NPs are very critical for their drug delivery applications. The ZnO NPs showed strong red fluorescence at room temperature (Figure 1b&c). We believe that the fluorescence came from the non-stoichiometric defects, e.g. Zn interstitials or oxygen vacancies associated with the Zn deposition.28 Such defects usually result in a green fluorescence, sometimes with slightly blue or red shift depending on the synthesis conditions.29, 30 Hence it is very rare and intriguing that the ZnO nanoparticles we synthesized exhibited red fluorescence. In our synthesis, we adopted a polymer condensation method that is largely different from the hydrolysis/alcoholysis used for most other ZnO nanoparticles. In our polymer condensation synthesis, ZnO comes from the condensation of Zn-O-(C=O)- bond in the form of their coordination polymers, while in the prevalent hydrolysis/alcoholysis synthesis, ZnO comes from zinc hydroxide/zinc alcoholoxide. Therefore, the formation mechanism of their intrinsic defects, such as zinc vacancies, oxygen vacancies, as well as their complex defects, may have been radically different. The difference in the species and concentration of such defects may have resulted in a strong defects-related emission at a shifted wavelength. Electron paramagnetic resonance studies suggested that single-ionized oxygen vacancies concentrated on the surface of ZnO nanoparticles may be responsible for the red fluorescence.19 The fluorescent ZnO NPs coupled with PET imaging capacity can enable the in vivo monitoring of ZnO nanomaterials in biomedical applications. Compared with our previously developed green fluorescent ZnO nanowires,20, 21 these red-fluorescing ZnO NPs possess better tissue penetration of the optical signal, making them more preferable for fluorescence-guided drug delivery purposes. However, the requirement of ultraviolet light excitation (excitation wavelength: ~350 nm) for their fluorescence limited their applications in vivo, which serves as the primary reason that no fluorescence imaging was performed in vivo in the current study. Further optimization is under way to make red fluorescent ZnO NPs excitable by visible lights. The conjugation of 64Cu onto the surface of ZnO NPs in this study provided an alternative method and enabled the accurate quantification of these ZnO NPs in vivo by PET.
CD105 is critical for tumor angiogenesis, with a tremendous potential to serve as a theranostic marker for various tumors based on literature reports.10, 11, 31 CD105 exhibits several advantages compared to other markers, which includes the independence from tumor histotype or tumor cell expression, easy circulation accessibility, and universal tumor vasculature expression. The conjugation of TRC105 onto ZnO NPs significantly boosted their interactions with CD105-positive cells (Figure 2) and their tumor vasculature targeting efficacy (Figures 3–5). PET imaging capacity of 64Cu-NOTA-ZnO-PEG-TRC105 makes it more clinically translatable,32–34 and applicable for quantitative and sensitive tumor detection.35–37
Different studies have been carried out to assess the cytotoxicity of ZnO nanomaterials and demonstrate that their toxicity is dependent on the sizes, morphologies, surface chemistry, and cell types.38 Although ZnO-base nanomaterials have been primarily considered to possess no adverse side effects and high biodegradability, the materials’ dosage should be kept as low as possible. In the current study, no cell morphology changes were observed by us despite the fact that no toxicity evaluation was carried out. Another potential research direction is to select better ligands on ZnO NPs. Despite all the advantages discussed in the previous text, TRC105 can still interact nonspecifically with Fc receptor from different types of immune cells.39 To minimize these interactions and the potential immune responses, antibody fragments or peptides can be used for future generation of targeted ZnO NP platform.
The versatile surface chemistry of ZnO nanomaterials enables them to be attached with various molecules (e.g. drugs or imaging labels). In this study, radiolabeled ZnO nanomaterials were used for PET imaging and in vivo tumor targeting. This biocompatible/biodegradable ZnO nanomaterial platform can be used for future tumor-targeted cargo delivery. Other future research directions of ZnO nanomaterials that are of great importance include the following: 1) Combination therapy of cancer (e.g. photo dynamic therapy (PDT) with drug/gene-loaded ZnO nanomaterials); 2) Dual-modality imaging (e.g. PET/optical or PET/MRI), which takes advantage of the quantitation capability of PET and the intrinsic fluorescence signal of ZnO (and/or high resolution of MRI) to track ZnO nanomaterials in vivo; 3) Thorough investigation of the pharmacokinetics and long term toxicity of ZnO nanomaterials with different surface modifications; among others.
CONCLUSIONS
64Cu-NOTA-ZnO-PEG-TRC105 nanoconjugates were reported in this study for tumor vasculature targeting, imageable by PET. Pharmacokinetics and tumor-targeting efficacy/specificity in 4T1 tumor-bearing mice were thoroughly investigated. Around 3-fold enhancement of tumor accumulation over passive targeting alone was confirmed due to CD105 targeting. Given the safety and high accommodation of therapeutic agents, as-designed 64Cu-ZnO-PEG-TRC105 could be employed as image-guided drug delivery vectors for theranostic applications. Other targeting ligands could also be conjugated to ZnO NPs for further improvement of tumor accumulation in vivo.
ACKNOWLEDGEMENTS
We acknowledge the financial support from the University of Wisconsin - Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365 and P30CA014520), the Department of Defense (W81XWH-11-1-0644 and W81XWH-11-1-0648), the Department of Energy (DE-SC0008384), and the American Cancer Society (125246-RSG-13-099-01-CCE).
REFERENCES
- 1.Xiong HM. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013;25:5329–5335. doi: 10.1002/adma.201301732. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Nayak TR, Hong H, Cai W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013;13:1633–1645. doi: 10.2174/1566524013666131111130058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko W, Jung N, Lee M, Yun M, Jeon S. Electronic Nose Based on Multipatterns of ZnO Nanorods on a Quartz Resonator with Remote Electrodes. ACS Nano. 2013;7:6685–6690. doi: 10.1021/nn4027245. [DOI] [PubMed] [Google Scholar]
- 4.Liu D, Wu W, Qiu Y, Yang S, Xiao S, Wang QQ, Ding L, Wang J. Surface Functionalization of ZnO Nanotetrapods with Photoactive and Electroactive Organic Monolayers. Langmuir. 2008;24:5052–5059. doi: 10.1021/la800074f. [DOI] [PubMed] [Google Scholar]
- 5.Mankoff DA. A Definition of Molecular Imaging. J. Nucl. Med. 2007;48:18N–21N. [PubMed] [Google Scholar]
- 6.Moriyama EH, Zheng G, Wilson BC. Optical Molecular Imaging: from Single Cell to Patient. Clin. Pharmacol. Ther. 2008;84:267–271. doi: 10.1038/clpt.2008.58. [DOI] [PubMed] [Google Scholar]
- 7.Cai W, Chen X. Multimodality Molecular Imaging of Tumor Angiogenesis. J. Nucl. Med. 2008;49(Suppl 2):113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 8.Lopci E, Grassi I, Chiti A, Nanni C, Cicoria G, Toschi L, Fonti C, Lodi F, Mattioli S, Fanti S. PET Radiopharmaceuticals for Imaging of Tumor Hypoxia: a Review of the Evidence. Am. J. Nucl. Med. Mol. Imaging. 2014;4:365–384. [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty R, Hong H, Cai W. Positron Emission Tomography Image-Guided Drug Delivery: Current Status and Future Perspectives. Mol. Pharm. 2014 doi: 10.1021/mp500173s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a Marker of Tumor Vasculature and Potential Target for Therapy. Clin. Cancer Res. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 11.Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She X, Harada N, Uneda S, Tsujie T, Toi H, Tsai H, Haruta Y. Endoglin-Targeted Cancer Therapy. Curr. Drug Delivery. 2011;8:135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engle JW, Hong H, Zhang Y, Valdovinos HF, Myklejord DV, Barnhart TE, Theuer CP, Nickles RJ, Cai W. Positron Emission Tomography Imaging of Tumor Angiogenesis with a 66Ga-Labeled Monoclonal Antibody. Mol. Pharm. 2012;9:1441–1448. doi: 10.1021/mp300019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, Nayak TR, Goel S, Bean J, Theuer CP, Barnhart TE, Liu Z, Cai W. In Vivo Targeting and Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. ACS Nano. 2012;6:2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong H, Zhang Y, Engle JW, Nayak TR, Theuer CP, Nickles RJ, Barnhart TE, Cai W. In Vivo Targeting and Positron Emission Tomography Imaging of Tumor Vasculature with 66Ga-Labeled Nano-graphene. Biomaterials. 2012;33:4147–4156. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Hong H, Engle JW, Bean J, Yang Y, Leigh BR, Barnhart TE, Cai W. Positron Emission Tomography Imaging of CD105 Expression with a 64Cu-Labeled Monoclonal Antibody: NOTA is Superior to DOTA. PLoS One. 2011;6:e28005. doi: 10.1371/journal.pone.0028005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y, Theuer CP, Barnhart TE, Cai W, Gong S. Image-Guided and Tumor-Targeted Drug Delivery with Radiolabeled Unimolecular Micelles. Biomaterials. 2013;34:8323–8332. doi: 10.1016/j.biomaterials.2013.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H, Zhang Y, Severin GW, Yang Y, Engle JW, Niu G, Nickles RJ, Chen X, Leigh BR, Barnhart TE, Cai W. Multimodality Imaging of Breast Cancer Experimental Lung Metastasis with Bioluminescence and a Monoclonal Antibody Dual-Labeled with 89Zr and IRDye 800CW. Mol. Pharm. 2012;9:2339–2349. doi: 10.1021/mp300277f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen LS, Hurwitz HI, Wong MK, Goldman J, Mendelson DS, Figg WD, Spencer S, Adams BJ, Alvarez D, Seon BK, Theuer CP, Leigh BR, Gordon MS. A Phase I First-in-human Study of TRC105 (Anti-Endoglin Antibody) in Patients with Advanced Cancer. Clin. Cancer Res. 2012;18:4820–4829. doi: 10.1158/1078-0432.CCR-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi HY, Deng B, Zhong SL, Wang L, Xu AW. Synthesis of Zinc Oxide Nanoparticles with Strong, Tunable and Stable Visible Light Emission by Solid-State Transformation of Zn(II)-Organic Coordination Polymers. J. Mater. Chem. 2011;21:12309–12315. [Google Scholar]
- 20.Hong H, Shi J, Yang Y, Zhang Y, Engle JW, Nickles RJ, Wang X, Cai W. Cancer-Targeted Optical Imaging with Fluorescent Zinc Oxide Nanowires. Nano Lett. 2011;11:3744–3750. doi: 10.1021/nl201782m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, Hong H, Ding Y, Yang Y, Cai W, Wang X. Evolution of Zinc Oxide Nanostructures through Kinetics Control. J. Mater. Chem. 2011;21:9000–9008. doi: 10.1039/C1JM10918A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong H, Yang Y, Zhang Y, Engle JW, Barnhart TE, Nickles RJ, Leigh BR, Cai W. Positron Emission Tomography Imaging of CD105 Expression During Tumor Angiogenesis. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:1335–1343. doi: 10.1007/s00259-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y, Hong H, Javadi A, Engle JW, Xu W, Yang Y, Zhang Y, Barnhart TE, Cai W, Gong S. Multifunctional Unimolecular Micelles for Cancer-Targeted Drug Delivery and Positron Emission Tomography Imaging. Biomaterials. 2012;33:3071–3082. doi: 10.1016/j.biomaterials.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi S, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, Theuer CP, Barnhart TE, Liu Z, Cai W. Tumor Vasculature Targeting and Imaging in Living Mice with Reduced Graphene Oxide. Biomaterials. 2013;34:3002–3009. doi: 10.1016/j.biomaterials.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Xiao Y, Yang Y, Zhang Y, Nickles RJ, Cai W, Steeber DA, Gong S. cRGD-Functionalized, DOX-Conjugated, and 64Cu-Labeled Superparamagnetic Iron Oxide Nanoparticles for Targeted Anticancer Drug Delivery and PET/MR Imaging. Biomaterials. 2011;32:4151–4160. doi: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Xu NS, Wang ZL. Dissolving Behavior and Stability of ZnO Wires in Biofluids: A Study on Biodegradability and Biocompatibility of ZnO Nanostructures. Adv. Mater. 2006;18:2432–2435. [Google Scholar]
- 27.Taratula O, Galoppini E, Wang D, Chu D, Zhang Z, Chen H, Saraf G, Lu Y. Binding Studies of Molecular Linkers to ZnO and MgZnO Nanotip Films. J. Phys. Chem. B. 2006;110:6506–6515. doi: 10.1021/jp0570317. [DOI] [PubMed] [Google Scholar]
- 28.Lin HY, Cheng CL, Chou YY, Huang LL, Chen YF, Tsen KT. Enhancement of Band Gap Emission Stimulated by Defect Loss. Opt. Express. 2006;14:2372–2379. doi: 10.1364/oe.14.002372. [DOI] [PubMed] [Google Scholar]
- 29.Gong Y, Andelman T, Neumark G, O’Brien S, Kuskovsky I. Origin of Defect-Related Green Emission from ZnO Nanoparticles: Effect of Surface Modification. Nanoscale Res. Lett. 2007;2:297–302. [Google Scholar]
- 30.Tang X, Choo ESG, Li L, Ding J, Xue J. Synthesis of ZnO Nanoparticles with Tunable Emission Colors and Their Cell Labeling Applications. Chem. Mater. 2010;22:3383–3388. [Google Scholar]
- 31.Zhang Y, Yang Y, Hong H, Cai W. Multimodality Molecular Imaging of CD105 (Endoglin) Expression. Int. J. Clin. Exp. Med. 2011;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 32.Gambhir SS. Molecular Imaging of Cancer with Positron Emission Tomography. Nat. Rev. Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 33.Alauddin MM. Positron Emission Tomography (PET) Imaging with 18F-Based Radiotracers. Am. J. Nucl. Med. Mol. Imaging. 2012;2:55–76. [PMC free article] [PubMed] [Google Scholar]
- 34.Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The Clinical Use of PET with 11C-acetate. Am. J. Nucl. Med. Mol. Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- 35.Fakhri GE. Ready for Prime Time? Dual Tracer PET and SPECT Imaging. Am. J. Nucl. Med. Mol. Imaging. 2012;2:415–417. [PMC free article] [PubMed] [Google Scholar]
- 36.Nolting DD, Nickels ML, Guo N, Pham W. Molecular Imaging Probe Development: a Chemistry Perspective. Am. J. Nucl. Med. Mol. Imaging. 2012;2:273–306. [PMC free article] [PubMed] [Google Scholar]
- 37.van Dongen GA, Ussi AE, de Man FH, Migliaccio G. EATRIS, a European Initiative to Boost Translational Biomedical Research. Am. J. Nucl. Med. Mol. Imaging. 2013;3:166–174. [PMC free article] [PubMed] [Google Scholar]
- 38.Roy R, Das M, Dwivedi PD. Toxicological Mode of Action of ZnO Nanoparticles: Impact on Immune Cells. Mol. Immunol. 2015;63:184–192. doi: 10.1016/j.molimm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Ravetch JV, Bolland S. IgG Fc Receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]