Abstract
Interleukin-23 (IL-23) responsive group 3 innate lymphoid cells (ILC3s) have been implicated in immune homeostasis and pathogenesis in the adult, but little is known about their roles in the newborn. Here we show that IL-23 promotes conversion of embryonic intestinal Lin−IL-23R+Thy1+ cells into IL-22-producing Thy1+Sca-1hi ILC3s in vitro. Gut-specific expression of IL-23 also activated and expanded Thy1+Sca-1hi ILC3s, which produced IL-22, IL-17, IFN-γ, and GM-CSF and were distinct from canonical CD4+ lymphoid tissue inducer (LTi) cells. These ILC3s accumulated under the epithelium in intercellular adhesion molecule (ICAM)-1 positive cell aggregates together with neutrophils that disrupted the epithelium, leading to the formation of discrete intestinal erosions, bleeding, and neonatal death. Genetic and antibody depletion of ILC3s rescued the mice from neonatal death. Antibiotic treatment of pregnant mothers and offspring prolonged survival of IL-23 transgenic mice, suggesting a role for the commensal flora on ILC3-induced pathogenesis. Our results reveal a novel role for the IL-23-ILC3s axis in the pathogenesis of neonatal intestinal inflammation.
Introduction
Interleukin-23 (IL-23) is a heterodimeric cytokine formed by the IL-23-specific p19 subunit and the IL-12p40 subunit1. IL-23 plays a pivotal role in adult intestinal inflammation2. Recent evidence suggests that IL-23 acts as a molecular switch to promote pathological T cell and innate lymphoid cell (ILC) responses in murine models of colitis and in human inflammatory bowel disease (IBD) 3-9.
ILCs regulate immunity, inflammation, tissue repair and remodeling in multiple anatomical compartments, particularly at mucosal surfaces10. ILCs are currently categorized into three distinct populations based on their developmental requirements for defined transcription factors, expression of cell surface markers, and secretion of specific cytokines 11-13. IL-23 has been implicated in the activation and induction of group 3 ILCs (ILC3s) 13. ILC3s depend on the transcription factor RORγt and are also called RORγt+ ILCs. ILC3s can be further categorized into several subsets with distinct but overlapping phenotypic and functional markers. Lymphoid tissue inducer (LTi) cells represent the subset of ILC3s responsible for the formation of secondary lymphoid organs during embryogenesis14, 15. Intestinal LTi cells do not generally express lineage-specific markers but can express the T cell surface molecule CD416, 17. Two other subsets of group 3 ILCs are defined by their expression of natural cytotoxicity receptors (NCRs, including NKp46 and NKp44) and are thus categorized into NCR+ILC3s and NCR−ILC3s12, 13. NCR+ILC3s also play a role in gut-associated lymphoid tissue generation18, 19, and appear to be essential for host protective immunity in the intestine20. The development/maintenance of NCR+ILC3s, different from LTi cells, appears to be dependent of commensal-derived signals21-23. Another subset within the group 3 ILCs was found to mediate pathology in a mouse model of innate colitis4. These colitogenic NCR−ILC3s lack expression of NKp46 and do express thymocyte differentiation antigen 1 (Thy1), stem cell antigen 1 (Sca-1) and IL-23 receptor (IL-23R) and secrete both IFN-γ and IL-17 in addition to IL-22 under IL-23 stimulation 4. These NCR−ILC3s accumulate in the inflamed colon and are directly responsible for the chronic pathology.
While it is established that IL-23 affects both the maintenance of a Th17 response 3, 8 and activation and function of group 3 ILC3 in adults12, little is know about its role in early development. It is generally accepted that the immune system is immature at birth because the structure of bone marrow, spleen, and lymphoid nodes is not fully defined, there are few B and T cells in circulation and in the periphery, and there are no memory B and T cells 24-26. However, some aspects of the immune response are already in place. For instance, human neonatal dendritic cells (DCs), respond to Toll-like receptor (TLR) stimulation by secreting large amounts of IL-23 27-29. Human neonatal DC-derived IL-23 promotes the differentiation of neonatal CD8+ T cells into IL-17-producing cells 30. In addition, human neonatal DC-derived IL-23 combined with specific TCR signaling drives the generation of neonatal γδT cells equipped with a range of cytotoxic mediators and distinct subpopulations producing IFN-γ and IL-17 25. Isolated DCs from neonatal mice are able to produce relatively higher levels of IL-23p40, but lower levels of IL-12p70 than their DC counterparts in adult mice31, 32.
Here we show that IL-23 promotes differentiation of embryonic Thy1+IL-23R+ intestinal cells into Thy1+Sca-1hi ILC3s in vitro. Gut-specific expression of IL-23 in transgenic mice promoted significant expansion of a subset of ILC3s that together with neutrophils and the intestinal flora promoted development of erosive lesions, bleeding and neonatal death. These results indicate that increased IL-23 expression can promote development of pathogenic ILC3s in the newborn intestine.
Results
IL-23 activates embryonic IL-23R+Thy1+ cells to become IL-22-producing RORγ t+Thy1+Sca-1hi cells in vitro
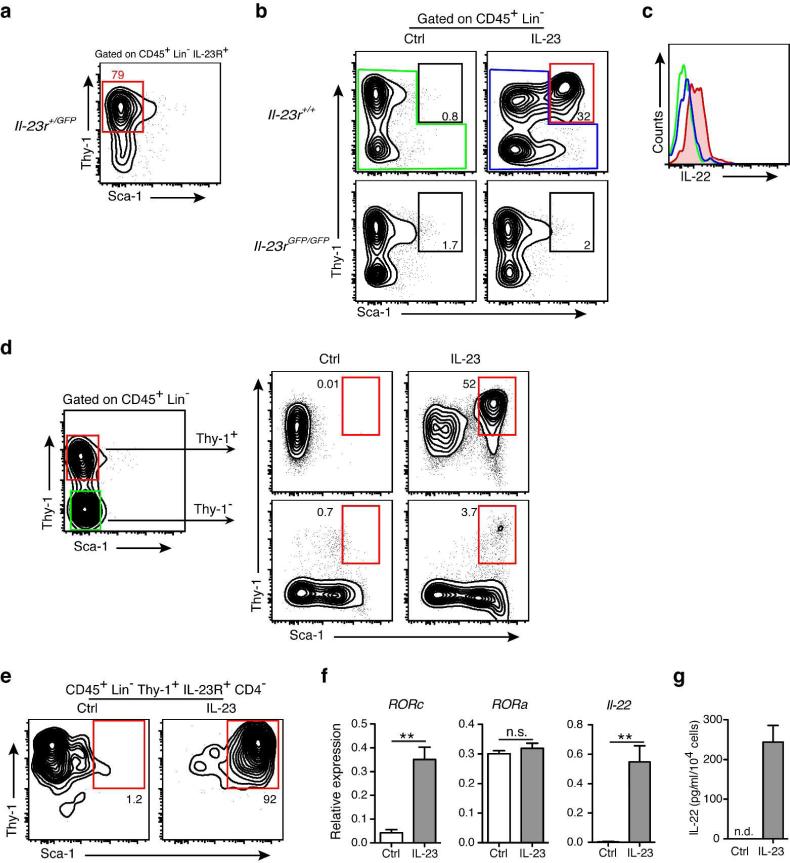
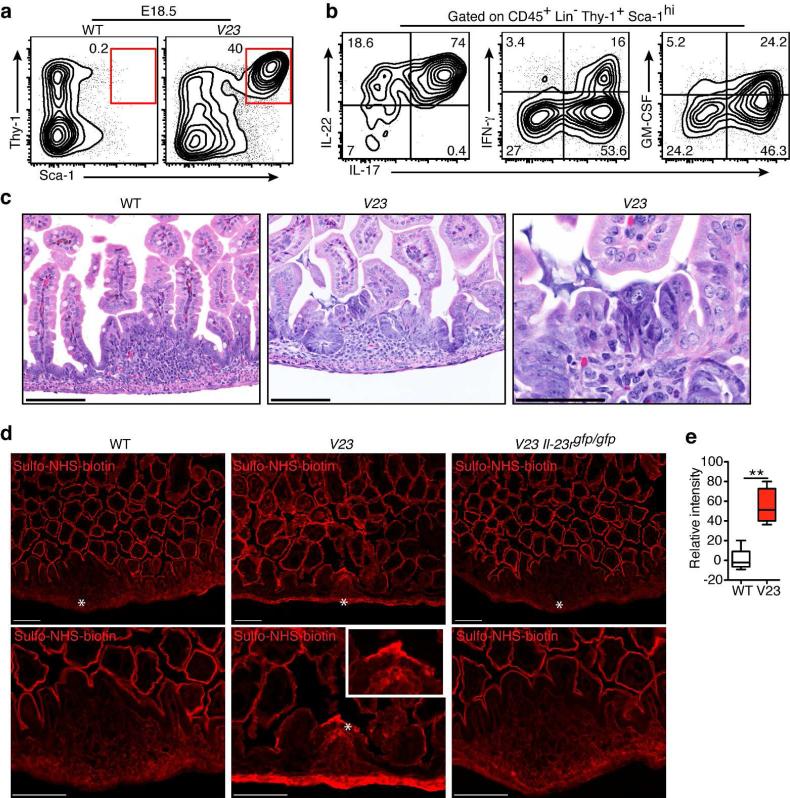
While a role for IL-23 in regulating immune homeostasis and pathogenesis in the adult is firmly established 3, 13, its role in the prenatal/neonatal immune regulation is poorly understood. To start studying the role of IL-23 in this period, we examined the distribution of IL-23 responsive cells in the intestine. IL-23 interacts with cells that co-express the IL-23R subunit and the shared IL-12R β1 chain 33. We took advantage of IL-23R green fluorescent protein (GFP) reporter mice34 to determine which cells expressed IL-23R. Most (~70%) CD45+ IL-23RGFP+ cells in the gut of prenatal mice were lineage-negative (Lin−) cells. The remaining cells were CD11b+ (~18%) and CD11c+ (~12 %) (Supplementary fig. S1). Most Lin− IL-23RGFP+ cells (80%) were Thy1+Sca-1− cells in the intestine of Il-23r+/gfp mice at embryonic day E18.5 (Fig. 1a). Culture of embryonic leukocytes from the Il-23r+/+ intestine with recombinant IL-23 promoted development of Lin−Thy1+Sca-1hi cells (Fig. 1b). Incubation of intestinal cells from IL-23R-deficient (Il-23rgfp/gfp) embryos with IL-23, as expected, did not result in the appearance of Lin−Thy1+Sca-1hi cells (Fig. 1b). Further characterization of this IL-23 stimulated Lin−Thy1+Sca-1hi cell population showed that they were negative for CD4, NKp46, and CCR6 (Supplementary fig. S2). Adult IL-23-responsive cells produce IL-17 and/or IL-22 when challenged with IL-23 13. Lin−Thy1+Sca-1hi cells generated in vitro after stimulation with IL-23 produced IL-22 (Fig. 1c), but not IL-17.
Figure 1. IL-23 promotes development of Thy1+Sca-1hi ILCs in vitro.
(a) Flow cytometric analysis of Thy1 and Sca-1 expression of gated CD45+Lin−IL-23R+ cells from the intestine of Il-23r+/GFP mice at embryonic day E18.5. (b) In vitro stimulated CD45+Lin− leukocyte subpopulations from the intestine of Il-23r+/+ and Il-23rGFP/GFP mice at embryonic day E18.5 with IL-23 (10ng/ml) or vehicle control for 72 hr. Representative flow cytometry plots showing CD45+Lin−Thy1+Sca-1hi population after culture. (c) Intracellular cytokine stain of IL-22 expression by the populations shown in (b), colors correspond to the populations analyzed. (d) Culture of sorted Lin−Thy1+ and Lin−Thy1− cells from the wild-type intestine at embryonic day E18.5 respond to IL-23 (10ng/ml) or vehicle (Ctrl) stimulation after 72 hr. Representative flow cytometry plots showing CD45+Lin−Thy1+Sca-1hi population after culture. (e) Representative flow cytometry plots showing sorted Lin−Thy1+IL-23R+CD4− cells from the intestine of Il-23r+/GFP mice at embryonic day E18.5 respond to IL-23 (10ng/ml) or vehicle (Ctrl) stimulation after 72 hr. (f) Quantitative RT-PCR analysis of Il-22, Rorc and Rora mRNA expression in the Lin-Thy1+IL-23R+CD4− cells stimulated with control media (Ctrl) or IL-23. NS, not significant. ** P < 0.01. (g) ELISA evaluation of IL-22 in the culture supernatant of the Lin−Thy1+IL-23R+CD4− cells stimulated with control media (Ctrl) or IL-23. Data are shown as means ± s.e.m., n = 3–5 per group. ND, not detectable. Results are representative of three independent experiments.
To further confirm that IL-23 acted directly on the Lin−Thy1+ cells, we sorted Lin-Thy1+ and Lin−Thy1− cells from the intestine of embryonic wild-type (WT) mice and cultured them in the presence of IL-23 or vehicle. We found that the Lin−Thy1+ cells converted to Lin−Thy1+Sca-1hi cells after IL-23 stimulation (Fig. 1d). As CD3−CD4+ LTi cells are also Thy1+ 13, we asked next whether Lin−Thy1+IL-23R+CD4− cells could respond to IL-23. We sorted Lin−Thy1+IL-23R+CD4− cells from the intestine of Il-23r+/gfp mice and challenged them with IL-23. We found that more than 90% of the Lin−Thy1+IL-23R+CD4−cells became Lin−Thy1+Sca-1hi cells (Fig. 1e).
To further gain insight into how IL-23 promoted the development of Lin−Thy1+Sca-1hi cells, we examined expression of RORγt and IL-22 . Treatment of the Lin−Thy1+ IL-23R+ CD4− cells with IL-23 increased expression of Rorc (Fig. 1f) and Il-22 (Fig. 1f and g). Incubation of intestinal cells from RORγt-deficient embryos with IL-23, as expected, did not result in the appearance of Lin−Thy1+Sca-1hi cells (Supplementary fig. S3), suggesting that RORγt is critical for Lin−Thy1+Sca-1hi cells development. Together, these results indicate that IL-23 activates embryonic Lin−IL-23R+Thy1+ cells to become IL-22-producing RORγ t+Thy1+Sca-1hi group 3 ILCs in vitro.
Transgenic expression of IL-23 in the neonatal intestine causes erosive lesions, bleeding and neonatal death
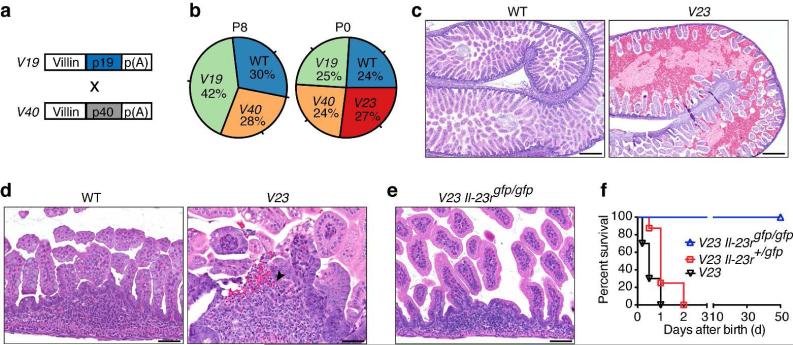
To study if IL-23 had a role in the activation of ILC3s in the intestine, we generated IL-23 transgenic mice. To do so, we first developed sets of mice expressing IL-23p19 (V19 mice) and IL-23p40 (V40 mice) from the villin promoter, which targets expression of transgenes to the intestinal epithelium35. V19 and V40 mice were then intercrossed to generate V23 mice (Fig. 2a). Surprisingly, no V23 transgenic mice were found alive at postnatal day 8 (P8) (Fig. 2b), suggesting early mortality. Further genotypic analysis showed that V23 mice survived gestation but died at P0-P1 (Fig. 2b). To confirm transgene expression, we performed enzyme linked immunosorbent assay (ELISA) in gut extracts and found that IL-23 levels were ~ 7 fold higher in the intestine of transgenic mice than controls (Supplementary fig. S4). These levels are comparable to those induced by administration of CD40-specific antibodies to activate IL-23 expression in Rag−/− mice 36.
Figure 2. Transgenic expression of IL-23 in the intestine causes formation of erosive lesions, bleeding, and neonatal death.
(a) Scheme for generation of V23 mice. Independent sets of murine villin promoter (9kb)-driven transgenes encoding IL-23p19 or p40 were used to generate V19 and V40 mice, respectively. (b) Genotypic ratios of WT, V19, V40 and V23 mice at different ages P0 (n = 97) and P8 (n = 69). (c and d) Representative H&E stained sections of the small intestine of WT and V23 mice at P0. Scale bars, 250 μm in (c) and 50 μm in (d). Arrow indicates an erosive lesion. (e) Representative H&E stained section of the small intestine of V23 Il-23rGFP/GFP mice at P0. Scale bars, 50 μm. (f) The survival curves of V23 (n=16), V23 Il-23r+/GFP (n=15), and V23 Il-23rGFP/GFP (n=18) mice. P < 0.001 between V23 Il-23rGFP/GFP and V23 mice by Log-rank test. Results are representative of three independent experiments.
Further examination of abdominal organs revealed that the small intestine was prominently affected in the transgenic mice (Fig. 2c). On gross examination, the V23 mice had congested and dilated small bowels compared with littermate WT control mice (Fig. 2c). Histologically, the general architecture of the intestine was preserved, but the lumen appeared distended and showed hemorrhage (Fig. 2c). The most distinguished finding was the presence of discrete epithelial lesions overlying lamina propria lymphoid aggregates (Fig. 2d). The lesions consisted of disrupted epithelium in association with intraepithelial and superficial subepithelial neutrophilic infiltrates (Fig. 2d). Neutrophils were also seen in the intestinal lumen, at the sites of epithelial disruption (Fig. 2d). Scattered epithelial apoptotic bodies and reactive/regenerative epithelial changes were also seen at the site of the lesions (Fig. 2, c and d).
To further ascertain that the biology observed was dependent on IL-23, and to rule out the possibility that the early lethality in the V23 mice could be a consequence of a non-specific effect caused by expression of two independent transgenes (Fig. 2, a and b), we intercrossed the V19 and V40 to mice deficient in the IL-23R (Il-23rGFP/GFP) to generate V23 Il-23rGFP/GFP mice. Similar to V23 mice, V23 Il-23r+/GFP mice succumbed immediately after birth. In great contrast, V23 Il-23rGFP/GFP mice survived to adulthood and did not show any signs of disease (Fig. 2, e and f). These results indicate that the phenotype observed in the V23 mice is directly elicited by the interaction of IL-23 with its receptor, and not the consequence of a spurious artifact induced by expression of the transgenes.
IL-23 expression drives expansion of intestinal Lin−Thy1+Sca-1hi cells in mice
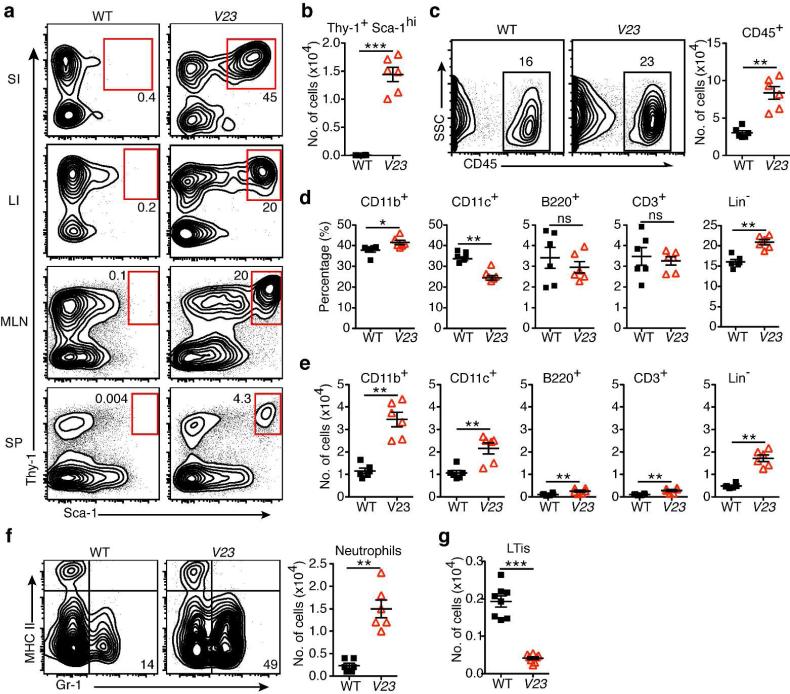
Next we asked whether transgenic expression of IL-23 in vivo affected development of Lin−Thy1+Sca-1hi ILCs in the neonatal intestine. First we examined the frequency of Lin-Thy1+Sca-1hi population in the different organs of V23 transgenic mice at P0. IL-23-responsive Lin−Thy1+Sca-1hi cells were most abundant in the small intestine (SI), representing 40-50% of all Lin− population, but were also present in the large intestine (LI), mesenteric lymph nodes (MLN), and at lower frequency in the spleen (Fig. 3a). In contrast, no Lin−Thy1+Sca-1hi cells were detected in the intestine, MLN or spleen of WT mice (Fig. 3a). The total number of Lin−Thy1+Sca-1hi cells in the SI of transgenic mice was dramatically increased compared to that of WT mice (Fig. 3b).
Figure 3. Gut-specific expression of IL-23 activated and expanded Thy1+Sca-1hi ILC3s in the neonatal intestine.
(a) Relative number of Lin−Thy1+Sca-1hi cells in small intestine (SI), large intestine (LI), mesenteric lymph nodes (MLN) and spleen (SP) of WT and V23 mice at P0. Representative flow cytometry plots gated on CD45+Lin−. (b) Absolute number of Lin−Thy1+Sca-1hi cells in the small intestine of WT and V23 mice at P0. Means ± s.e.m., n = 5–6 per group, ***P < 0.001. (c) Relative (left) and absolute (right) number of CD45+ cells in the small intestine of WT and V23 mice at P0. Dot plots show cells gated on live cells. Means ± s.e.m., n = 5–6 per group, ** P < 0.01. (d and e) Relative (d) and absolute (e) number of CD11b+, CD11c+, CD3+, B220+ and Lin− cells in the small intestine of WT and V23 mice at P0. Means ± s.e.m., n = 5–6 per group. NS, not significant, * P < 0.05, ** P < 0.01, ***P < 0.001. (f) Relative (left) and absolute (right) number of CD45+CD11b+MHCII−Gr-1+ cells in the small intestine of WT and V23 mice at P0. Dot plots show cells gated on CD45+CD11b+. Means ± s.e.m., n = 5–6 per group, ** P < 0.01. (g) Absolute number of LTi cells (Lin−CD4+) in the small intestine of WT and V23 mice at P0. Means ± s.e.m., n = 7–8 per group, ***P < 0.001. Data are representative of three independent experiments.
At birth the relative and absolute number of leukocytes (CD45+ cells) in the small intestine V23 mice was increased (Fig. 3c). The most increased leukocytes in Lin+ population were CD11b+ cells (Fig. 3, d and e). The dominant population among the CD11b+ cells were neutrophils (CD11b+MHCII−Gr-1+ cells), whose numbers were markedly increased in the intestine of V23 mice compared with WT mice (Fig. 3f). Further analyses showed that there were no changes in the relative number of most leukocytes (Fig. 3d), with only modest increases in the absolute number of CD3+ cells, B220+ cells, and CD11c+ cells in V23 mice compared to WT mice (Fig. 3e). As expected, Thy1+Sca-1hi cells were the most abundant Lin− cells (Fig. 3, b, d and e). Other CD45+ Lin− cells such as the canonical intestinal LTi cells (Lin−CD4+) were decreased in V23 mice compared with WT mice (Fig. 3g). In addition, no NKp46+ILC3s were detected in transgenic nor WT mice at this stage, consistent with the fact that NKp46+ILC3s develop several days after birth and are normally induced by the intestinal commensal flora 21, 23.
Together these results indicate that IL-23 expression promoted the expansion of a distinct set of Thy1+Sca-1hi ILCs and increased in the numbers of neutrophils in the small intestine of V23 neonates.
Lin−Thy1+Sca-1hi cells present in the intestine of the V23 mice are NCR−ILC3s
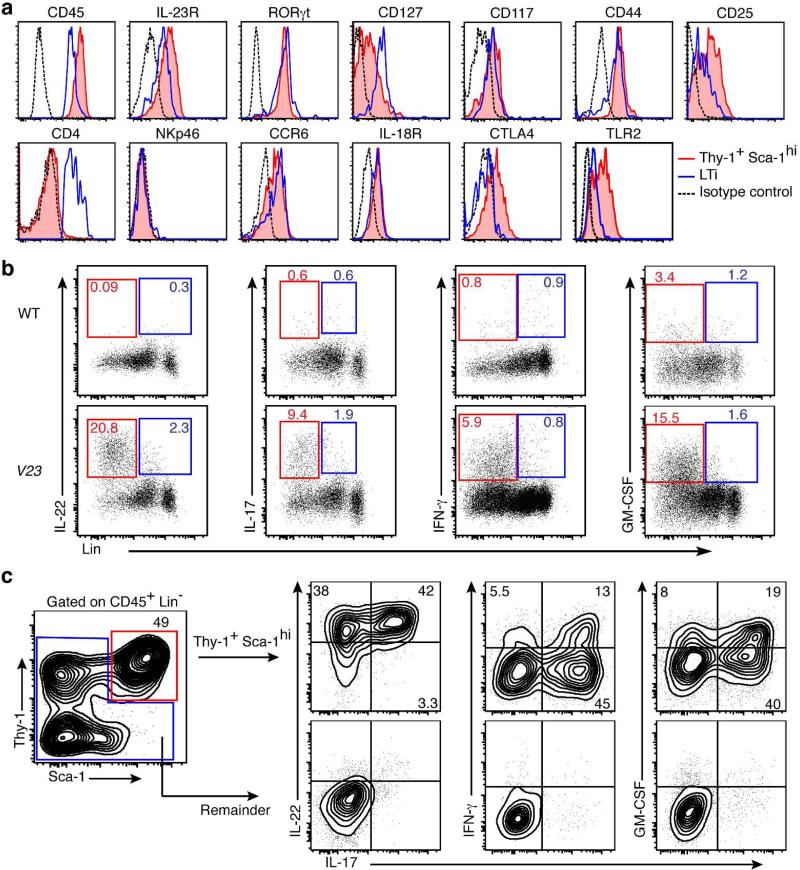
To characterize the Lin−Thy1+Sca-1hi cells further, we examined expression of surface markers, transcription factors, and cytokines by flow cytometry. The Lin−Thy1+Sca-1hi cells present in the neonatal small intestine of V23 mice coexpressed markers normally associated with ILC3s, such as IL-23R, RORγt, IL-7R (CD127), CD44, CD25, c-Kit (CD117) and CCR6 (Fig. 4a). Lin−Thy1+Sca-1hi cells did not express CD4 or NKp46 (Fig. 4a) but did express high levels of Sca-1, which suggests that they were phenotypically distinct from LTi cells and NKp46+ILC3s 12, 13. Further phenotypic analysis showed that IL-18R and cytotoxic T-lymphocyte antigen 4 (CTLA-4) were expressed in Lin−Thy1+Sca-1hi cells (Fig. 4a). Interestingly, CTLA-4 was expressed by Lin−Thy1+Sca-1hi cells but not by classical LTi cells (Fig. 4a). Similar to human LTi-like ILCs 37, Lin−Thy1+Sca-1hi cells expressed TLR2 (Fig. 4a) .
Figure 4. Thy1+Sca-1hi cells in the intestine of the V23 mice are NCR−ILC3s.
(a) Phenotype of lineage-negative (Lin−, CD3−B220−CD11b−Gr-1−Ter119−) Thy1+Sca-1hi cells. Histograms are electronically gated on Lin− cells. Red lines indicate Lin−Thy1+Sca-1hi ILC3, blue lines denote LTi cells (Lin−CD4+), and dotted black lines indicate isotype controls. (b) Expression of indicated cytokines analyzed by flow cytometry on the CD45+Lin+ and CD45+Lin− populations after stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. Dot plots show cells gated on CD45+. Outlines areas in red indicate Lin− and outlines areas in blue indicate Lin+. (c) Intracellular cytokine expression by Lin−Thy1+Sca-1hi cells (red) in comparison with remaining cells (blue) from the small intestine of V23 mice at P0 following stimulation with PMA and ionomycin. Data are representative of at least three independent experiments.
IL-22, IL-17 and IFN-γ are produced not only by Lin+ cells, such as Th17 cells and γδT cells, but also by mucosal ILCs. Intracellular flow cytometry revealed that neonatal CD45+Lin− cells, but not Lin+ cells, produced most of IL-22, IL-17, IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the intestine of V23 mice (Fig. 4b), which is consistent with the fact that very few CD3+ cells were present at this stage in the intestine (Fig. 3, d and e). Further analysis showed that most of these cytokines (IL-22, IL-17, IFN-γ and GM-CSF) were produced by Lin−Thy1+Sca-1hi ILCs (Fig. 4c). The vast majority (80-95%) of Thy1+Sca-1hi ILCs produced IL-22 (Supplementary fig. S5a). Approximately 30% of the Thy1+Sca-1hi ILCs produced only IL-22 and the remaining cells co-expressed it with IL-17, IFN- γ or GM-CSF (Supplementary fig. S5a). Of note, we observed that ~6% of the cytokine expressing cells, expressed IL-22, IL-17, IFN-γ and GM-CSF (Supplementary fig. S5b). Together these results indicate that the distinct Thy1+Sca-1hi ILC population present in the neonatal intestine of V23 transgenic mice most closely resembles the NCR-negative group 3 ILCs reported in adult host 4, 5.
ILC3s and neutrophils co-localize in cellular aggregates beneath the intestinal erosive lesions
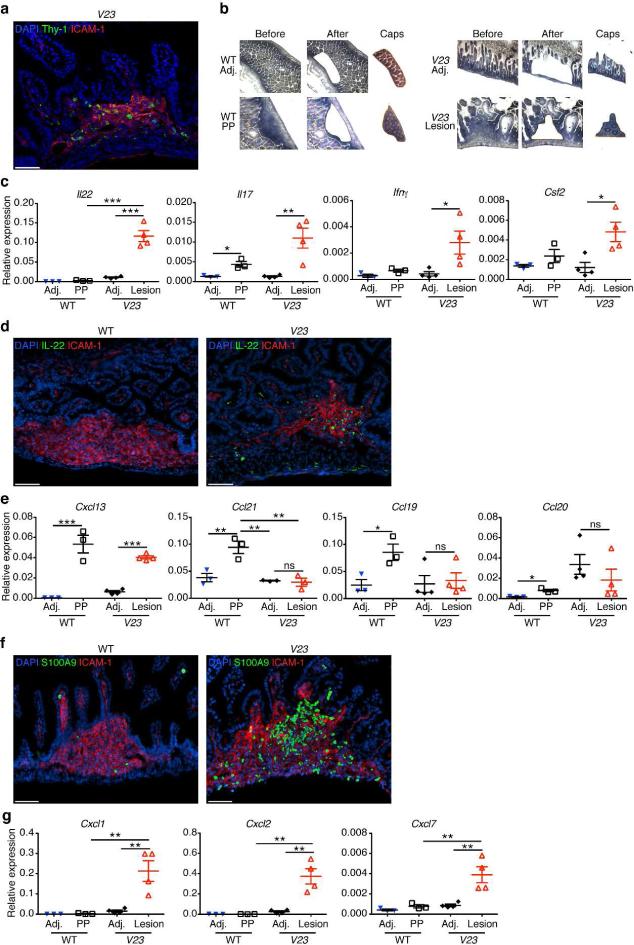
Having detected the presence of Thy1+Sca-1hi ILC3s in the small intestine of V23 mice, we investigated next their distribution. To do so, we first performed anti-Thy1 immunostaining. We found Thy1+ cells in the erosive lesions, but not in adjacent areas (Fig. 5a). These cells were interspersed among cells expressing the intercellular adhesion molecule (ICAM)-1, which is commonly expressed within lymph nodes and Peyer's patches (PP) anlagen (Fig. 5a). To further confirm the presence of cytokine-producing cells in these areas, we performed laser-capture microdissection of the erosive lesions and the adjacent areas of the intestine of V23 mice, extracted mRNA and performed quantitative RT-PCR analysis for cytokines (Fig. 5b). As control, we investigated cytokine mRNA expression in samples collected from PP's anlagen and adjacent areas of WT mice (Fig. 5b). Quantitative RT-PCR results showed that with the exception of IL-17, no cytokines were expressed in PP of WT mice at this point (Fig. 5c). In contrast, IL-22, IL-17, IFN-γ and GM-CSF mRNA were all expressed in the areas corresponding to the erosive lesions of V23 mice compared to all other microdissected samples (Fig. 5c), suggesting that cytokine-producing ILC3s clustered in the erosive lesions. IL-22 immunofluorescence staining subsequently confirmed that cells expressing IL-22 localized to cell clusters under the erosive lesions in the small intestine of V23 mice (Fig. 5d).
Figure 5. Thy1+Sca-1hi ILC3s are located within erosive lesions in the intestine.
(a) Immunofluorescence staining of the small intestine at P0 showing the presence of Thy1+ cells in ICAM+ cells aggregates in V23 mice (Thy1, green; ICAM-1, red; cell nuclei, DAPI). Scale bars, 50μm. (b) Histology of snap-frozen small intestine from WT and V23 mice. Slides show tissue before (left), after (middle) laser microdissection, as well as excised tissue in caps (right). (c) Cytokine expression measured by quantitative RT-PCR in microdissected Peyer's patches anlagen (PP)/erosive lesions areas (Lesion) and adjacent control areas (Adj) isolated from P0 WT and V23 mice. Means ± s.e.m., n = 4 per group. * P < 0.05, ** P < 0.01, ***P < 0.001. (d) Immunofluorescence staining of the small intestine of V23 mice at P0 showing the presence of IL-22+ cells within ICAM+ aggregates (IL-22, green; ICAM-1, red; cell nuclei, DAPI). Scale bars, 50μm. (e) Chemokine expression in microdissected Peyer's patches anlagen (PP)/erosive lesions areas (Lesion) and adjacent control areas (Adj) isolated from P0 WT and V23 mice. Means ± s.e.m., n = 4 per group. NS, not significant, * P < 0.05, ** P < 0.01, ***P < 0.001. (f) Immunofluorescence staining the small intestine at P0 showing the presence of neutrophils (S100A9+) in ICAM+ cells aggregates (S100A9, green; ICAM-1, red; cell nuclei, DAPI). Scale bars, 50μm. (g) Expression of neutrophil chemoattracting chemokines in microdissected Peyer's patches anlagen (PP)/erosive lesions areas (Lesion) and adjacent control areas (Adj) isolated from P0 WT and V23 mice. Means ± s.e.m., n = 4 per group, * *P < 0.01.
Besides expressing cytokines, ILC3s express chemokine receptors such as CXCR5 (Supplementary fig. S6) and CCR6 (Fig. 4a). We tested next if the presence of ILC3s in the erosive lesions was associated with increased expression of CXCL13 and CCL20, ligands for CXCR5 and CCR6 respectively. In the WT intestine CXCL13, along with CCL19 and CCL21, is expressed constitutively in PP38-40. Consistent with these previous findings, we detected increased expression of Ccl19, Ccl21 and Cxcl13 within ICAM-1+ PP anlagen of P0 WT mice (Fig. 5e). In V23 mice, expression of ICAM-1 localized to the erosive lesions, and increased levels of Cxcl13 mRNA were detected compared to neighboring areas (Fig. 5e). Ccl19 and Ccl21 mRNA levels did not differ between erosive and adjacent areas (Fig. 5e). Ccl20 mRNA was only modestly increased in PP of controls and did not differ between erosive and adjacent areas of V23 intestine (Fig. 5e). These results suggest that CXCL13 may be important for recruitment of ILC3s into the ICAM-1+ erosive areas of the V23 intestine.
Because neutrophil numbers were also increased in the small intestine of V23 mice (Fig. 3f), we examined next their distribution. Neutrophils were abundant in the same areas where ILC3s were found (Fig. 5f). Ultrastructural analysis showed that neutrophils migrated from the lamina propria to the lumen and disrupted the epithelium (Supplementary fig. S7), leading to formation of the erosive lesions. Of note, we found that cells isolated from the erosive lesions in V23 mice expressed higher levels of neutrophil-recruiting chemokines Cxcl1, Cxcl2 and Cxcl7 than those from adjacent control areas or from PP anlagen of WT mice (Fig. 5g).
Together the data indicate that ILC3s and neutrophils co-localize in cellular aggregates beneath the intestinal erosive lesions, and suggest that specific chemokines may be involved in their recruitment to those sites.
Thy1+Sca-1hi ILCs contribute to neonatal intestinal pathology
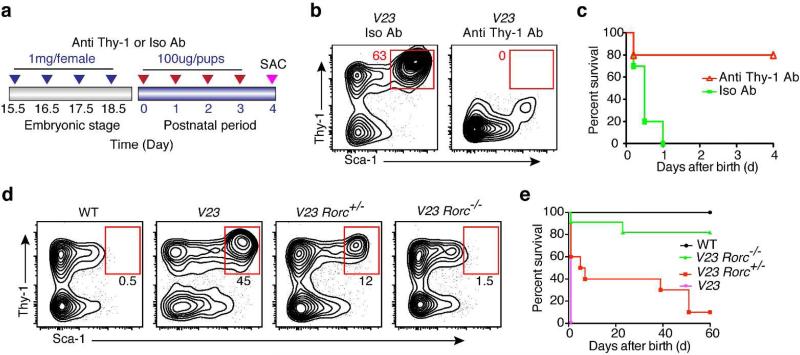
IL-23-responsive NCR−ILC3s mediate intestinal immune pathology in adult murine models of colitis 4, 6. To test if the Thy1+Sca-1hi ILC3s could contribute to the neonatal pathology observed in the V23 mice, we first injected an anti-Thy1 mAb into pregnant mice and newborn pups and estimated the frequency of Thy1+Sca-1hi ILCs in their offspring at P4 (Fig. 6a). Anti-Thy1 mAb injection reduced the total number of Thy1+Sca-1hi cells in V23 mice (Fig. 6b). These animals had fewer erosive lesions in the intestine (Supplementary fig. S8a) and survived longer than the V23 mice treated with isotype control antibodies (Fig. 6c).
Figure 6. Thy1+Sca-1hi ILC3s are the main drivers of the intestinal pathogenesis.
(a) Protocol used for in vivo depletion of Thy1+ cells in newborn V23 mice. The pregnant mothers were administered intravenously with 1 mg rat anti-Thy1 mAb or 1 mg isotype control mAb at gestational days 15.5, 16.5, 17.5 and 18.5. After birth pups were injected i.p. daily with 100 μg anti-Thy1 mAb or 100 μg isotype control per mouse for 4 days. (b) Flow cytometric analysis of the Lin−Thy1+Sca-1hi population in the small intestine after antibody injection. Dot plots show cells gated on CD45+Lin−. (c) Survival curves of V23 pups treated with anti-Thy1 (n = 12) or isotype (n = 12). ** P < 0.01 between groups by Log-rank test. (d) Flow cytometry plot showing the proportion of Lin−Thy1+Sca-1hi cells in the small intestine of V23, V23Rorc(γt)+/−, and V23Rorc(γt)−/− mice at P0. Dot plots show cells gated on CD45+Lin−. (e) Survival curves of V23 (n = 15), V23Rorc(γt)+/− (n = 18), and V23Rorc(γt)−/− (n = 22) mice. *** P < 0.001 between V23Rorc(γt)+/− /V23Rorc( γt)−/− mice and V23 mice by Log-rank test. Data are representative of three independent experiments.
To genetically deplete the ILC3 population we intercrossed the V19 and V40 mice to mice deficient in the Rorc to generate V23Rorc −/− mice. RORγt deficient mice lack all ILC3s 12. At birth, V23 Rorc +/− mice had fewer Thy1+Sca-1hi ILC3s than V23 mice (Fig. 6d) and survived longer than V23 mice (Fig. 6e). As expected, V23 Rorc −/− mice did not have Thy1+Sca-1hi ILCs (Fig. 6d) and survived to adulthood (Fig. 6e). Accordingly, there were no erosive lesions present in the intestine of V23 Rorc −/− mice (Supplementary fig. S8b) consistent with the fact that RORγt deficient mice lack Peyer's patches41. Similar to V23 Rorc −/− mice, no Thy1+Sca-1hi ILCs were present in V23 Il-23r gfp/gfp mice (Supplementary fig. S9) that survived to adulthood (Fig. 2f).
At birth, few T cells are present in the intestine (Fig 3, d and e). To rule out a contribution of T cells to the phenotype observed in V23 mice, we intercrossed the V19 and V40 mice to RAG1−/− mice and to mice deficient in the gamma delta T-cell receptor (Tcrd−/−) to generate V23 RAG1−/− and V23 Tcrd−/− mice respectively. V23 RAG1−/− and V23 Tcrd−/− mice succumbed immediately after birth and presented erosive lesions (Supplementary fig. S10) similar to those noted in V23 mice (Fig 2d and Supplementary fig. S10). Together these results indicate that Thy1+Sca-1hi ILC3s, but not T and B cells, have a critical role in neonatal intestinal pathology and perinatal death of V23 mice.
Intestinal embryonic Thy1+Sca-1hi ILC3s dysregulate epithelial permeability
We examined next whether the NCR−ILC3s were present during the prenatal stage. We found that Thy1+Sca-1hi ILC3s were present in the V23, but not WT fetuses, prior to birth (Fig. 7a) and that the relative number of these cells was not different between late fetal stages and birth (Figs. 3a and 7a). As some studies indicate that commensal-derived signals may also contribute to ILCs function22, 42, we analyzed next the cytokine production by Thy1+Sca-1hi ILC3s in prenatal period. We found that fetal Thy1+Sca-1hi ILC3s (Fig. 7b) expressed IL-22, IL-17, IFN-γ and GM-CSF similar to the postnatal Thy1+Sca-1hi ILC3s (Fig. 4c).
Figure 7. Intestinal Thy1+Sca-1hi ILCs dysregulate epithelial permeability at prenatal stage.
(a) Flow cytometric analysis of the Lin−Thy1+Sca-1hi cells in the small intestine of mice at embryonic day E18.5. Dot plots show cells gated on CD45+Lin−. (b) Representative cytokine expression profile of Lin−Thy1+Sca-1hi ILC3s present in the small intestine of embryonic V23 mice (E18.5). (c) Representative H&E stained sections of the small intestine of WT and V23 embryo mice (E18.5). Scale bars, 100 μm. (d) The fluorescence staining of proteins labeled with sulfo-NHS-biotin in the small intestine after injection of the probe into the lumen of the intestine of embryos at E18.5. Stars indicate cellular aggregates in the gut. Scale bars, 100μm. Zoomed-in boxed area shows fluorescence staining of cellular aggregates in V23 mice. Data are representative of three independent experiments. (e) Quantification of relative fluorescent intensity in the PP anlagen of WT mice (2-5 sections for each mouse) and in erosive lesions of V23 mice (4-10 sections for each mouse). Data are shown as means ± s.e.m. of n = 5 mice per group. **P < 0.01, nonparametric Mann-Whitney test.
Given that cytokine-producing Thy1+Sca-1hi ILC3s could be found in the embryonic gut, we asked next if they could promote prenatal pathology. Histopathological analysis of the intestine of V23 fetuses at E18.5 showed no epithelial disruption or intestinal bleeding, but revealed the presence of cellular aggregates with few scattered subepithelial neutrophils (Fig. 7c). Quantitative RT-PCR analysis of sorted epithelial cells of V23 mice revealed that epcam, cldn1, 2, 3, 7 and cdh1 expression was downregulated in V23 mice compared to controls (Supplementary fig. S11). These results suggested a possible dysfunction in epithelial permeability. To test this hypothesis we injected the intestinal lumen of E18.5 V23 and WT embryos with sulfo-NHS-biotin, a probe that labels cell membrane proteins 43. As control, we examined the biotin signal at the intestinal epithelium of WT mice. The intestine of the WT mouse was uniformly labeled, with no dye being detected in the lamina propria (Fig. 7d). The intensity of the biotin signal in areas of the lamina propria, especially in the area corresponding to the cellular aggregates, was higher in the V23 mice than in WT mice (Fig. 7d and e), suggesting increased permeability of the epithelium at those sites. We also examined, as an additional control, the intestine of V23 Il-23rgfp/gfp mice injected with sulfo-NHS-biotin. The results were similar to those obtained with WT mice (Fig. 7d), which ruled out the possibility that the dysregulated intestinal epithelial permeability in the V23 mice resulted from a transgenic artifact.
Together the findings indicate that V23 mice had downregulation in the expression of genes involved in maintaining the epithelial barrier, and increased permeability of the epithelium in areas rich in cytokine-producing NCR−Thy1+Sca-1hi ILC3s.
Commensal bacteria aggravate intestinal pathology induced by Thy1+Sca-1hi ILCs after birth
As demonstrated above, cytokine-producing ILC3s were present in the embryonic V23 intestine and their presence was associated with increased epithelial permeability. Yet, no significant damage or mortality was observed in embryos, suggesting that ILC3s were necessary, but not sufficient to induce the full phenotype. We next tested the hypothesis that the microbiota could contribute to the neonatal phenotype. Bacterial colonization of the newborn intestinal tract starts as the fetuses pass the birth canal and continues with the initial feeding and exposure to the environment. We thus tested if there were differences in the initial colonization of the intestine between WT and V23 mice. We subjected DNA collected from the small intestine of P0 V23 and WT littermate controls to 16S rDNA sequencing. Weighted UniFrac distances (PC1, P = 0.82; Supplementary fig. S12a) and alpha diversity analysis (P > 0.9, Supplementary fig. S12b) indicated that the microbiota of V23 mice was similar to that of WT littermate controls. Analysis of the most abundant phyla did not indicate any major changes in the relative abundance between V23 and WT littermate controls (Supplementary fig. S12c). In total, 156 individual OTUs were retained after quality filtering, none of which showed a significant difference between V23 and WT mice (q > 0.05, Supplementary fig. S12d).
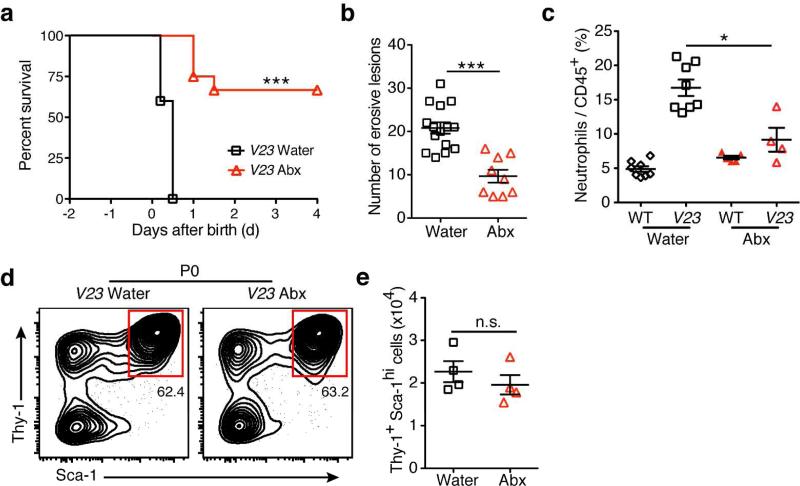
Having demonstrated that the expression of IL-23 and increased numbers of ILC3s did not affect the initial bacterial colonization of the gut, we tested next if a reduction of the bacterial load would affect the phenotype. To reduce the exposure of newborn V23 mice to bacteria, we treated pregnant mothers with antibiotics. Antibiotic treatment prolonged the survival and decreased the number of intestinal erosive lesions in the newborn V23 mice (Fig. 8, a and b), implicating the microbiota in ILC3s-induced pathogenesis. Of note, we found a reduced neutrophil infiltrate in the intestine of antibiotic-treated V23 mice (Fig. 8c), suggesting that neutrophil accumulation in the ulcers could be due in part to the intestinal bacterial colonization.
Figure 8. Commensal bacteria aggravate intestinal pathology induced by Thy1+Sca-1hi ILCs.
(a) Survival curves of V23 mice derived from mothers treated with (n=22) or without (n=15) an antibiotic cocktail in drinking water during pregnancy. ***, P < 0.001 (Log-rank test). (b) Number of erosive lesions in the intestine of V23 mice born from mothers receiving water or antibiotic. Data are shown as means ± s.e.m., n = 10-15 for each group, ***, P < 0.001. (c) Relative number of neutrophils (CD11b+Gr-1+) in the small intestine of V23 mice born from mothers receiving water or antibiotic. Data are shown as means ± s.e.m., n = 4–8 per group, * P < 0.05. (d and e) Relative (d) and absolute (e) number of the Lin−Thy1+Sca-1hi cells in the small intestine of V23 mice born from mothers receiving water or antibiotic. Data are shown as means ± s.e.m., n=4 for each group. Dot plots show cells gated on CD45+Lin−. NS, not significant.
We next examined if bacteria affected the phenotype by affecting the number or activity of ILC3s. There were no significant differences in the number of Thy1+Sca-1hi ILC3s between antibiotic treated and untreated V23 mice (Fig. 8, d and e). We then examined gene expression in highly purified intestinal Thy1+Sca-1hi ILC3s from non-treated, and antibiotic-treated neonatal V23 mice. No significant differences were observed in the expression of surface markers (Il23r, Kit, Cd44, Cxcr5, Ccr6, Il18r1, Ctla4, et al.) or cytokines (Il22, Il17a, Ifng and Csf2) (Supplementary fig. S13) between these two groups. The expression of ILC3s signature cytokine genes was also similar between non-antibiotic treated fetal and postnatal Thy1+Sca-1hi ILC3s (Supplementary fig. S13), corroborating the results reported above (Fig. 4c and 7 a, b).
These results indicated that Thy1+Sca-1hi ILC3s were necessary but not sufficient for the neonatal intestinal pathology observed in V23 mice. Bacterial colonization at birth exacerbates pathology induced by these cells and promotes neonatal death.
Discussion
Most studies to date have examined IL-23 biology during the course of bacterial infections and/or gastrointestinal tract injury in the adult host, but a direct role for IL-23 in the activation and function of neonatal ILC3s has not yet been investigated. Results shown in the present study indicate that dysregulated expression of IL-23 during the immediate neonatal period promotes development of a population of innate lymphoid cells with pathogenic properties.
At birth the mouse intestine is populated by few T cells (Fig. 3, d and e), but is home to myeloid cells and innate lymphoid cells that express the IL-23 receptor2, 12, 13. Here, we have used in vitro approaches (Fig. 1) and reductionist genetic models (Fig. 2) to examine the contribution of IL-23 to the activation and function of these IL-23R+ ILC3s. Using an in vitro culture system, we demonstrated that IL-23 directly promoted development of IL-22-producing Thy1+Sca-1hi ILC3s. We also show that gut-specific expression of IL-23 expanded this population in the neonatal intestine. Together these results indicate that expression of IL-23 promotes development and function of Thy1+Sca-1hi ILC3s in the neonatal intestine. These neonatal Thy1+Sca-1hi ILC3s (Fig. 4) shared similarities with a NCR−ILC3 population found in the adult colon by other groups 4, 6. Neonatal Thy1+Sca-1hi NCR−ILC3s concentrated in the small intestine and were found in clusters with ICAM+ cells and neutrophils in areas that resembled Peyer's patches anlagen (Fig. 5). Thy1+Sca-1hi NCR−ILC3s expressed LTi-related genes such as LT-α, LT-β, and TRANCE 4, but were not canonical LTis, because they did not express CD4 (Fig. 4a). However, similar to LTi, Thy1+Sca-1hi NCR−ILC3s expressed IL23R and the chemokine receptor CXCR5, which is responsive to CXCL13, a chemokine expressed in lymphoid anlagen. The fact that these two cell populations express many similar markers and are located in the same intestinal area suggests that they may share a common developmental origin. It remains to be tested if IL-23 stimulates further differentiation of LTi into pathogenic Thy1+Sca-1hi NCR−ILC3s or whether it acts on a common precursor that is recruited into the anlagen.
There appears to be significant heterogeneity within group 3 ILCs in the adult intestine. Some ILC3s are predominantly IL-22 producers, some primarily produce IL-17, while others can produce IFN-γ 4, 6, 44. It is unclear if these represent different subsets of cells, or if this reflects different cytokine expression profiles in response to environmental signals. In a murine model of Helicobacter hepaticus-mediated colitis, RORγt+ ILCs in the colon produce IL-17A and IFN-γ and depletion of these cells prevents colitis4. ILC3s secreting IL-17A, but little IFN-γ, have also been implicated in the pathogenesis of the Tbx21−/−Rag2−/− ulcerative colitis (TRUC) disease model. Treatment with a Thy1-specific antibody also ameliorates disease in this setting6. Here we show that IL-23 induced Thy1+Sca-1hi ILCs express only IL-22 in vitro (Fig. 1g). In vivo, the Thy1+Sca-1hi ILCs induced by IL-23 produce several cytokines, including IL-17, IL-22, IFN-γ, and GM-CSF (Fig. 4c). Most cells produced IL-22 and IL-17, but cells expressing different combinations of cytokines, or even all cytokines simultaneously, were also identified. To our knowledge, the existence of a population expressing all these cytokines simultaneously has not been previously demonstrated.
Our results indicate that increased IL-23 signaling in the immature, early intestine, led to an exaggerated generation of NCR−ILC3s, and to uncontrolled production of cytokines. These ILC3s are likely to be the main drivers of the pathogenesis because they were the main cytokine producers. The few T cells present at this stage did not produce cytokines, nor do the γ δ T cells or other Lin+/Lin− populations. Furthermore, reduction or ablation of NCR−ILC3, but not T and B cells (Supplementary fig. S10), reduced or eliminated disease, suggesting that this population is critical for pathogenesis in the neonatal gut.
Group 3 ILCs include LTi cells, NCR+ILC3s and NCR−ILC3s13. LTi cells develop in the microbiota-free environment of the fetus before birth14, 15, whereas NKp46+ RORγ t+ ILC3s only appear after birth and are not found in embryonic mice 21, 23. Thy1+Sca-1hi ILC3s were present in the V23 fetal intestine (Fig. 7a), but did not appear to cause pathology, suggesting that they were necessary, but not sufficient to cause disease. Results shown here indicate that antibiotic-treatment of pregnant mothers prolonged survival of V23 offspring (Fig. 8a), suggesting that commensal bacterial-derived signals affected the pathology induced by Thy1+Sca-1hi ILC3s. Despite being localized near barrier surfaces populated by commensal flora45, ILC3s did not affect the initial bacterial colonization of the intestine (Supplementary fig. S12). Bacterial colonization did not affect the numbers of ILC3s or the expression of signature cytokines by these cells (Fig. 8e and Supplementary fig. S13). Our results suggest that ILC3s induced pathology by altering barrier integrity (Fig. 7d and Supplementary fig. S11). Penetration of bacteria or bacterial products through areas of ILC3-induced increased permeability located in the anlagen, could lead to uncontrolled bacterial proliferation, cytokine storm and death.
Our findings may have relevance for understanding the development of intestinal inflammatory diseases of the neonate. The fetus and young infant have a high susceptibility to infections with pathogens, suggesting that immune responses are different in early life 46. It is generally accepted that this increased susceptibility is related to the immaturity/deficiencies of the neonatal immune system. However, there is evidence that some responses rather than inhibited, are exaggerated. For instance, human neonatal dendritic cells, respond to Toll-like receptor stimulation by secreting large amounts of IL-23 27-29. Robust expression of IL-23 by DC after bacterial exposure could trigger development and activation of ILC3 in the neonatal gut and lead to disease. In this context, it is important to note that IL-23 expression has been detected in the neonatal ileum of experimental models of necrotizing enterocolitis (NEC) 47. NEC is the most common inflammatory disease of the gastrointestinal tract of preterm infants 48. The etiology of NEC is obscure, but the disease is characterized by intestinal bleeding, mucosal intestinal necrosis and bacterial translocation. Increased bacterial translocation could trigger IL-23 production and expansion of pathogenic NCR−ILC3s. It will be thus important to investigate if IL-23 and IL-23 responsive-ILC3s play a role in NEC pathogenesis.
In conclusion, the data presented in this study demonstrate that increased levels of IL-23 in the neonatal intestine can trigger development of Lin− RORγt+Thy1+Sca-1hi ILC3s that act in concert with commensal bacteria to promote severe pathology. Thus, our results reveal a novel role for the IL-23-ILC3s axis in the pathogenesis of neonatal intestinal inflammation.
Methods
Mice
The cDNA of IL-23p19 and IL-23p40 were cloned into a pBS-Villin vector that contained a 9kb segment of the mouse villin promoter35. Transgenic mice were produced in the C57BL/6J background using conventional methods49. Identification of the transgenic V19 mice was done by PCR amplification using the following primers: 5'-GCC AGT TTC CCT TCT TCC TC-3' and 5'- GGC TAG CAT GCA GAG ATT CC-3'. Identification of the transgenic V40 mice was done by PCR amplification using the following primers: 5'- AAT CCA GCG CAA GAA AGA AA-3' and 5'- CAA ATG TGG TAT GGC TGA TTA TG-3'. V19 mice crossed to V40 mice to obtain V23 mice. C57BL/6 , Rorc(γt)−/−, Tcrd−/−, and RAG1−/− mice were purchased from The Jackson laboratory (Bar Harbor, ME). Il-23r−/− mice were described before 34. Mice were maintained under specific pathogen-free conditions. All experiments involving animals were performed following guidelines of the Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai.
Flow cytometry and sorting
The small intestine, large intestine, and mesenteric lymph nodes of embryonic and P0 mice were micro-dissected using a stereo microscope and further digested with 2 mg/ml collagenase D (Roche). Cell suspension was passed through a 70-μm cell strainer and mononuclear cells were isolated. Further details and list of antibodies used have been included in Supplementary Procedures.
For the intracellular cytokine staining was measured after cells were stimulated for 6 hr with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml), and ionomycin (1 μg/ml, both from Sigma-Aldrich) in the presence of monensin (2μM) (eBioscience) for the final 4 hr at 37°C culture (for details, see Supplementary Procedures).
Histology and Immunofluorescence Staining
Tissue was dissected, fixed in 10% phosphate-buffered formalin and then processed for paraffin sections. Five-micrometer sections were stained with hematoxylin and eosin. Immunofluorescence staining of frozen or paraffin-embedded tissues and list of antibodies are described in Supplementary Procedures.
Laser-capture microdissection
Frozen tissue sections (10 μm in thickness) were cut under RNase-free conditions. Samples of intestine tissue were captured from the stained slides on Arcturus CapSure Macro LCM caps by using an ArcturusXT Microdissection microscope (Applied Biosystems) (for details, see Supplementary Procedures).
DNA extraction, 16S rDNA amplification, and multiplex sequencing
DNA was obtained from whole gut of P0 mice using the DNeasy Blood and Tissue Kit (QIAGEN). Bacterial 16S rRNA genes were amplified using the primers as described in Caporaso et al. 50. Sample preparation and analysis of 16S DNA sequence were done as previously described51.
Barrier function assay
The barrier function assay based on sulfo-NHS-biotin was performed as described previously 43(for details, see Supplementary Procedures). Mean fluorescence intensity in the PP anlagen (WT mice) or erosive lesions (V23 mice) and corresponding adjacent tissues were analyzed on images set to a 5% pixel saturation using ImageJ v1.49a. The data were expressed as relative fluorescence intensity representing subtraction of mean fluorescence intensity in adjacent tissue from PP anlagen (WT mice) or erosive lesions (V23 mice).
Statistical Analysis
Differences between groups were analyzed with nonparametric Mann-Whitney test. Survival curves were analyzed by a log-rank test. All statistical analyses were performed with GraphPad Prism 5 software.
Supplementary Material
Acknowledgments
We thank Juan Lafaille, Daniel Mucida and Andrea Cerutti for discussions. We thank Claudia Canasto-Chibuque for help with genotyping and Parinati Kharel for help with the antibiotic treatment of mice.
We thank the Flow Cytometry, Mouse Genetics and Histology Shared Resource Facilities at Mount Sinai for technical advice and support.
This study was supported by the NIH grant P01 DK072201 and by the SUCCESS grant to S.A.L.
Footnotes
Disclosure:
The authors declare no conflict of interest.
References
- 1.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 3.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, et al. Interleukin-23 Drives Intestinal Inflammation through Direct Activity on T Cells. Immunity. 2010;33(2):279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, et al. The Transcription Factor T-bet Regulates Intestinal Inflammation Mediated by Interleukin-7 Receptor(+) Innate Lymphoid Cells. Immunity. 2012;37(4):674–684. doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5(1):99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 9.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. Journal of Experimental Medicine. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 2012;12(4):445–457. doi: 10.1016/j.chom.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 12.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 13.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 15.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3-LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7(4):493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 16.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330(6004):665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 17.Rankin LC, Groom JR, Chopin M, Herold MJ, Walker JA, Mielke LA, et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat Immunol. 2013;14(4):389–395. doi: 10.1038/ni.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nature Reviews Immunology. 2010;10(9):664–U624. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 19.Cupedo T. Human lymph node development: An inflammatory interaction. Immunol Lett. 2011;138(1):4–6. doi: 10.1016/j.imlet.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawa S, Lochner- M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, et al. ROR gamma t(+) innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12(4):320–U371. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 23.Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial Flora Drives Interleukin 22 Production in Intestinal NKp46(+) Cells that Provide Innate Mucosal Immune Defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118(2-3):137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal Vgamma9Vdelta2 T cells expressing high levels of cytotoxic mediators and producing IFN-gamma and IL-17. J Leukoc Biol. 2011;89(5):743–752. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 26.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 27.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal Innate TLR-Mediated Responses Are Distinct from Those of Adults. J Immunol. 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36(1):21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 29.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39(1):26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 30.Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35(2):469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 31.Sun CM, Fiette L, Tanguy M, Leclerc C, Lo-Man R. Ontogeny and innate properties of neonatal dendritic cells. Blood. 2003;102(2):585–591. doi: 10.1182/blood-2002-09-2966. [DOI] [PubMed] [Google Scholar]
- 32.Dakic A, Shao QX, D'Amico A, O'Keeffe M, Chen WF, Shortman K, et al. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172(2):1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 33.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 34.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182(10):5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto D, Robine S, Jaisser F, El Marjou F, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274(10):6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 36.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33(5):752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 39.Marchesi F, Martin AP, Thirunarayanan N, Devany E, Mayer L, Grisotto MG, et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2(6):486–494. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 40.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3(4):292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 41.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 42.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33(5):736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei Z, Maeda T, Tamura A, Nakamura T, Yamazaki Y, Shiratori H, et al. EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins. Dev Biol. 2012;371(2):136–145. doi: 10.1016/j.ydbio.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philip NH, Artis D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol Cell Biol. 2013;91(3):225–231. doi: 10.1038/icb.2013.2. [DOI] [PubMed] [Google Scholar]
- 46.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197(1):111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coursodon-Boyiddle CF, Snarrenberg CL, Adkins-Rieck CK, Bassaganya-Riera J, Hontecillas R, Lawrence P, et al. Pomegranate seed oil reduces intestinal damage in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G744–751. doi: 10.1152/ajpgi.00248.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4(1):22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135(2):529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bongers G, Pacer ME, Geraldino TH, Chen L, He Z, Hashimoto D, et al. Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J Exp Med. 2014;211(3):457–472. doi: 10.1084/jem.20131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.