Abstract
Botswana was one of the first African countries to transition from WHO Option A to Option B for prevention of mother-to-child HIV transmission (MTCT). We evaluated the impact of this transition on projected MTCT risk through review of 10,681 obstetric records of HIV-infected women delivering at 6 maternity wards. Compared with Option A, women receiving antenatal care under Option B were more likely to receive combination antiretroviral therapy (ART), adjusted odds ratio (aOR) 2.59 (95% confidence interval [CI] 2.25-2.98), but they were also more likely to receive no antenatal antiretrovirals, aOR 2.10 (95% CI 1.74-2.53). Consequently, initial implementation of Option B was associated with increased projected MTCT at 6 months of age, 3.79% under Option A and 4.69% under Option B (P<0.001). Successful implementation of Option B or B+ may require that ART can be initiated within antenatal clinics, and novel strategies to remove barriers to rapid ART initiation.
Keywords: PMTCT, Option B, implementation science, sub-Saharan Africa, ART initiation, HIV-1
Introduction
Expanded access to maternal combination antiretroviral therapy (ART) has the potential to nearly eliminate pediatric HIV infection.1 In 2010, the WHO recommended that one of two strategies be adopted for prevention of mother-to-child transmission (PMTCT) for women who did not require treatment for their own health (CD4+ cell count >350 cells/μL and WHO clinical stage 1 or 2)— Option A, antenatal maternal zidovudine monotherapy, or Option B, maternal ART during pregnancy and breastfeeding.2 However, recognizing a number of implementation challenges with Option A,3 and observing encouraging results from expanded maternal ART in Malawi,4 in 2013 the WHO recommended ART for all pregnant and breastfeeding women regardless of CD4 count (Option B), and that ART could be continued lifelong (Option B+). Option A was no longer recommended.5
Since 1999, Botswana has had one of the most successful PMTCT programs in Africa. The program achieves nearly universal HIV testing in pregnancy, and MTCT of Botswana citizens has been reported at less than 5% for nearly a decade.6 To further reduce transmission, in 2009 the Botswana government initiated a pilot of Option B in 5 districts and this strategy became national policy in 2011.7,8
We sought to evaluate the impact of the programmatic transition from Option A to Option B in Botswana on the risk of MTCT. Infant diagnosis is only available for the potentially biased subset that survives and presents for testing, so we utilized a large birth surveillance registry9 and projected the estimated risk of MTCT based on maternal disease stage and receipt of antiretrovirals.
Methods
Birth Surveillance
Active surveillance was conducted in public maternity wards in all regions of Botswana. Surveillance sites were selected to represent diverse settings and included the principal maternity wards in Gaborone (surveillance from May 2009 to October 2012), Molepolole, Mochudi, Francistown, Maun, and Ghanzi (surveillance from November 2009 to April 2011). Records for all deliveries occurring after 20 weeks gestation at these maternity wards were reviewed.
MTCT Prevention Program
At the time the birth surveillance started, pregnant women with CD4 count ≤ 250 cells/μL (or WHO clinical stage 3 or 4) were eligible for ART for their own health. HIV-infected women with CD4 count >250 cells/μL received zidovudine (provided by antenatal staff onsite) from 28 weeks gestation and single-dose nevirapine in labor.10 Specimens for CD4 were drawn at the antenatal clinic and analyzed in central laboratories; women eligible for treatment were referred to ART clinics, many offsite.
As part of the Option B pilot in 2009, women with CD4 count > 250 cells/μL were eligible to receive ART. When Option B became national policy in 2011, women with CD4 count ≤350 cells/μL were eligible for ART for life and women with CD4 count >350 cells/μL were eligible for ART during pregnancy and breastfeeding. Under these guidelines, ART was prescribed at ART clinics (not in antenatal clinics) following receipt of CD4 results and completion of adherence counseling. The national rollout of Option B occurred from early 2011 to early 2012. The birth surveillance included the entirety of two pilot sites and the first 12 months of the nationwide implementation of Option B. Throughout the surveillance period, replacement feeding was recommended for most women according to government guidelines and infant formula provided. Infant prophylaxis beyond single-dose nevirapine and 4-weeks of zidovudine was not provided.
The study was reviewed and approved by the Botswana Health Research Development Committee and the Institutional Review Board of the Harvard School of Public Health. A waiver for the requirement of informed consent was received to review health records.
Projected MTCT Risk
For each delivery record for an HIV-infected mother, an individual projected risk of MTCT was calculated conditional on maternal CD4 strata, type of maternal antiretroviral therapy received, and infant feeding method following published methodology.11 Infant feeding status was defined as the method used while on the maternity ward, and women opting to breastfeed were assumed to exclusively breastfeed for 6 months. We used the conditional MTCT estimates11 utilized in the base Spectrum model as our primary inputs, and in sensitivity analyses used high and low estimates and inputs conditional on duration of ART received from published estimates 12-20 (supplemental Table S1).
Statistical Analysis
As rollout of Option B occurred in a staged fashion, in order to compare clinical performance under the two PMTCT strategies we defined two exposure groups. Women registering for antenatal care at a clinic that had not yet implemented Option B were considered in the Option A group. Women registering after their clinic had implemented Option B were considered in the Option B group. The date of clinic transition from Option A to Option B was defined as the date for the first sustained use in that district of ART for women with CD4 count >350 cells/μL. PMTCT interventions actually received were determined by data abstracted from medical records. As non-citizens were not eligible for the free PMTCT program, only records for Botswana citizens were analyzed to assess impact of programmatic change.
We used LOESS regression to describe longitudinal trends. Multivariable linear and logistic models were fit to assess the impact of the implementation of Option B on the projected MTCT risk and the receipt of maternal antenatal antiretrovirals, respectively. Models accounted for clustering effects at the district and clinic level and adjusted for maternal age, marital status, employment, education, knowledge of HIV diagnosis prior to pregnancy, delivery hospital, presence of an onsite ART clinic, and antenatal clinic location (urban, large village, rural). Statistical analyses were performed with the use of the SAS statistical package, version 9.3 (SAS Institute, Cary, North Carolina). All tests were two-tailed and P-values of less than 0.05 were considered statistically significant.
Results
Surveillance cohort
During the surveillance period at the study maternity wards there were 35,281 live born deliveries by Botswana citizens. Of these, 10,681 (29.2%) women were HIV-infected, 24,600 (69.7%) were HIV-uninfected, and 368 (1.0%) had unknown HIV status. Women received antenatal care at 398 different clinics and health posts. Maternal and clinic characteristics were similar between women registering during Option A versus Option B (Table 1).
Table 1.
Participant and delivery characteristics for the study cohort.
| Option A (N=6731) |
Option B (N=3582) |
|
|---|---|---|
|
| ||
| Maternal Characteristics | ||
| Age at delivery (years), median (quartiles) | 29.0 (25.0, 33.0) | 29.0 (25.0, 33.0) |
| Secondary school education, no. (%) | 5280 (83.1) | 2790 (81.7) |
| Employed in formal sector, no. (%) | 3330 (55.2) | 1669 (60.7) |
| Nulliparous, no. (%) | 3060 (49.6) | 1086 (45.8) |
| Gestation age at ANC registration, median (quartiles) | 19.6 (15.4, 24.3) | 20.4 (16.0, 25.3) |
| HIV diagnosed before current pregnancy, no. (%) | 3152 (46.9) | 1814 (50.7) |
| Recent CD4+ cell count (cells/μL), median (quartiles) | 381 (251, 529) | 398 (264, 554) |
| No recorded CD4 results, no. (%) | 3308 (49.2) | 1802 (50.3) |
| HAART prior to conception, no (%) | 1760 (26.2) | 1008 (28.2) |
| Dates of LNMP not available, no. (%) | 175 (2.6) | 181 (5.1) |
| Breastfed infant at maternity, no. (%) | 509 (7.6) | 381 (10.7) |
| Antenatal Clinic Characteristics | ||
| Clinic location, no. (%) | ||
| Urban (population >50,000) | 4375 (67.8) | 1810 (51.6) |
| Large village (population 20,000-50,000) | 634 (10.8) | 364 (10.4) |
| Rural (population <20,000) | 1384 (21.5) | 1332 (38.0) |
| Delivery hospital, no. (%) | ||
| Princess Marina Hospital, Gaborone | 2753 (40.9) | 1983 (55.4) |
| Scottish Livingston Hospital, Molepolole | 280 (4.2) | 732 (20.4) |
| Deborah Retief Memorial Hospital, Mochudi | 758 (11.3) | 28 (0.8) |
| Nyangabgwe Referral Hospital, Francistown | 1714 (25.5) | 510 (14.2) |
| Letsholathebe Memorial Hospital, Maun | 1205 (17.9) | 39 (1.1) |
| Ghanzi Memorial Hospital, Ghanzi | 21 (0.3) | 290 (8.1) |
| Pregnancy potentially affected by strike, no. (%) a | 783 (11.6) | 487 (13.6) |
| Onsite ART clinic at antenatal clinic, no. (%) | 3272 (48.7) | 2393 (68.1) |
Note. ANC, antenatal clinic; LNMP, last normal menstrual period
Pregnancies in their second or third trimester during the period of the general public service strike (April to June 2011) that affected operation at most antenatal clinics.
Antiretroviral use
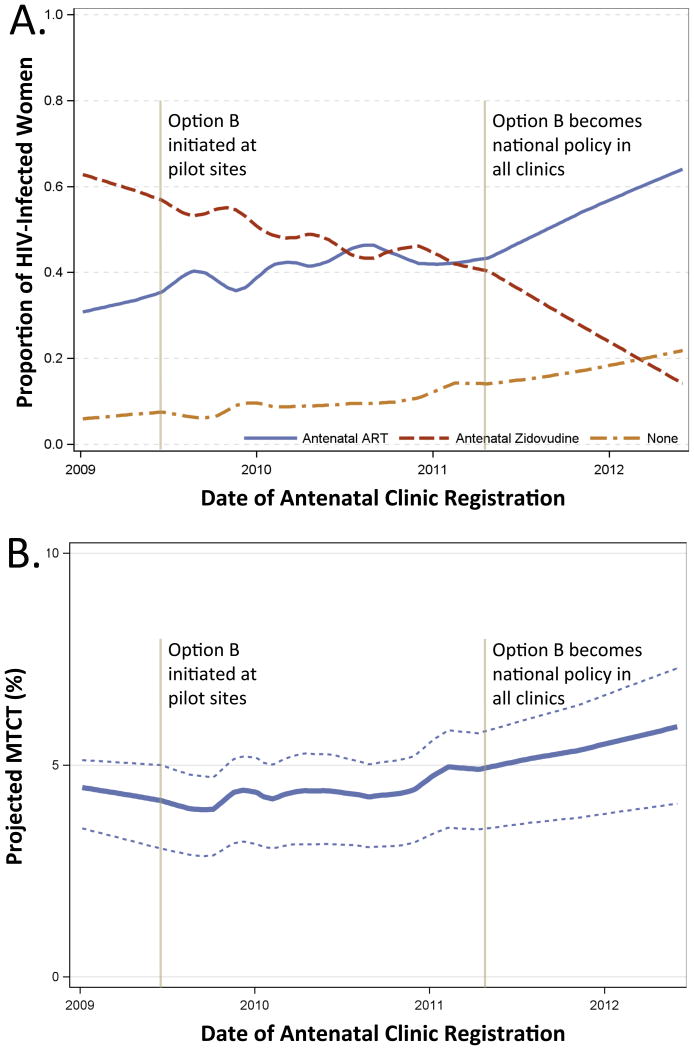
The proportion of HIV-infected women recorded as receiving ART at the time of delivery more than doubled during the surveillance period. Half of the increase in ART use during pregnancy was attributable to an increasing proportion of HIV-infected women already on ART at the time of conception. The remaining half of the increase was attributable to the shift to Option B for PMTCT, and the resulting increase in ART-eligible women. Following the adoption of Option B as national policy, ART initiation during pregnancy increased from 20% to 46% (Figure 1a).
Figure 1. Antiretroviral use and projected mother-to-child transmission, LOESS regression.
Trends in antiretroviral use among HIV-infected pregnant women during the surveillance period, demonstrating increases both in the proportion of women receiving combination ART and no antenatal antiretrovirals, Panel A. Corresponding increases in projected MTCT during in the initial implementation of Option B as demonstrated by solid line (Spectrum base inputs) and dotted lines (high and low Spectrum inputs), Panel B. Projected MTCT adjusted for delivery site.
The proportion of HIV-infected citizens receiving no antenatal antiretroviral treatment by delivery increased during the surveillance period. The increase was temporally correlated with the initial pilot and then the national adoption of Option B (when zidovudine was no longer regularly prescribed at antenatal clinics). Among ART-naïve women, 11.1% under Option A and 16.4% under Option B received no antenatal antiretrovirals.
In adjusted analyses, ART-naïve women registering at a clinic implementing Option B were significantly more likely to receive ART during pregnancy compared to women registering at an Option A clinic, adjusted odds ratio (aOR) 2.59 (95% confidence interval [CI] 2.25-2.98, P<0.001). However, they were also significantly more likely to receive no antenatal antiretrovirals by the time of delivery, aOR 2.10 (95% CI 1.74-2.53, P<0.001). Registration under Option B was also associated with an increased odds of receiving no antiretrovirals among the subset of women with CD4 <250 cells/μL, OR 2.58 (95%CI 1.97-3.38, P<0.001), although these women were eligible for ART throughout the surveillance period.
Projected MTCT
During the surveillance period, overall projected MTCT increased (Figure 1b). This projected increase was greatest (58%) among women with CD4 <250 cells/μL. In multivariable analyses, registering for antenatal care at a clinic implementing Option B was associated with an absolute increase of 0.90% in projected MTCT (95% CI 0.62-1.18% increase, P<0.001) compared with Option A. Holding other factors at their population means, projected MTCT was 3.79% for women registering under Option A and 4.69% under Option B. Using these estimates, nationwide 528 annual infant infections are projected to have occurred under Option A and 653 under Option B (increase of 125 annual infections, 24%, 95% CI 16-31%). Sensitivity analyses varying risk of MTCT and utilizing inputs that included gestational age of antiretroviral initiation resulted in similar findings (supplemental Table S2).
Registration for antenatal care earlier in pregnancy, older maternal age, diagnosis of HIV infection prior to pregnancy, ART at the time of conception, and increased education were associated with decreased projected MTCT (supplemental Table S3). Presence of an onsite ART clinic (co-located with antenatal clinic but generally with separate staff and record systems) was also associated with decreased projected MTCT, but impact was limited. Among ART-naïve women under Option B, 28.3% with offsite ART clinics and 33.5% with onsite ART clinics successfully started ART (P = 0.011). Under Option A, where zidovudine was prescribed and dispensed by antenatal clinic staff, 89.1% received antiretrovirals.
Discussion
In this observational study under operational conditions in Botswana, the initial phase of programmatic Option B rollout was associated with a 24% increase in projected MTCT, from 3.79% to 4.69%. While antenatal ART use increased sharply with implementation of Option B, rates of women receiving no antiretroviral therapy unfortunately also increased, offsetting the gains resulting from expanded access to ART. To our knowledge, this is the first study to investigate the programmatic transition from a well-implemented Option A strategy to Option B.
Discussions with patients, midwives, ART clinicians, and program officers offer several possible explanations for unexpected poor coverage during Option B implementation. Under Option A, the antenatal clinic midwife managed the entirety of the pregnancy, including PMTCT. Women with low CD4 cell counts were referred to ART clinics to initiate ART, but started zidovudine at 28 weeks gestation while awaiting ART. Consequently, women unable to access ART prior to delivery did receive zidovudine. Under Option B, all HIV-infected women were referred to ART clinics and antenatal clinics no longer prioritized dispensing zidovudine. Women referred to the ART clinics, and the lay counselors who aided them, reported encountering barriers to ART initiation that included lack of transportation, repeat laboratory testing, and perceived requirements for adherence counseling and an adherence partner. Additionally, clinicians reported that more women were reluctant to accept ART than zidovudine alone.
The surveillance period only captures the first 12 months of national implementation of Option B. The surveillance does not reflect current program performance following actions by the program to address encountered challenges. Preliminary findings from ongoing surveillance studies [Botswana-Harvard AIDS Institute Partnership, unpublished data, 2014] and aggregate government reports21 indicate subsequent improvement. However, these data from the first year in Botswana may reflect the challenges of many programs beginning to make the transition from Option A to Option B now, and so it is critical to understand the initial period of rollout.
There are a number of limitations that should be considered when interpreting the results of this study. We utilized projected MTCT risk rather than observed infant infections. While the projections were based on observed risks in high quality studies, it is uncertain how accurately they reflect actual transmission. Analyses are based on antiretroviral use as documented in the record. In this and prior work we have not observed frequent discrepancies, but cannot exclude the possibility of bias.
In conclusion, initial implementation in Botswana of WHO Option B was associated with a 24% increase in projected MTCT due to increased proportion of women receiving no antenatal antiretrovirals. Options B and B+ have many advantages for PMTCT delivery. However, successful implementation may require removal of barriers to rapid ART initiation (such as by integrating ART into antenatal clinics), and support for early engagement in antenatal care. Programs may also benefit from introducing novel strategies to improve acceptance of timely ART by pregnant women. Botswana is now supporting initiation of ART within antenatal clinics and ART initiation prior to receipt of CD4 results, and these are important steps towards eliminating MTCT.
Supplementary Material
Table S1: Duration of ARV model inputs.
Estimates of of risk of mother-to-child transmission by duration of maternal antiretrovirals and CD4 strata utilized in sensitivity analysis.
Table S2: Sensitivity analyses of projected MTCT and impact of Option B.
Estimates of risk of mother-to-child transmission and effect estimates of impact of Option B implementation under the Spectrum model inputs and estimates utilizing duration of ARV receipt.
Table S3: Estimates of effect from multivariate model of PMTCT strategy and other factors on probability of MTCT.
Acknowledgments
Source of Funding: Work supported by research grants from the Centers for Disease Control and Prevention (U2GPS000941) and the National Institutes of Health (3R01HD044391-06S1, 5K23AI091434).
Footnotes
Presented in part: 21st Conference on Retroviruses and Opportunistic Infections, Boston, MA, March 3-6, 2014.
Conflicts of Interest: The authors declare that they do not have commercial or other association that might pose a conflict of interest.
References
- 1.Mofenson LM. Protecting the next generation--eliminating perinatal HIV-1 infection. N Engl J Med. 2010 Jun 17;362(24):2316–2318. doi: 10.1056/NEJMe1004406. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Recommendations for a public heath approach. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 3.World Health Organization. Use of Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: World Health Organization; 2012. [Google Scholar]
- 4.Centers for Disease C, Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV--Malawi, July 2011-September 2012. MMWR Morb Mortal Wkly Rep. 2013 Mar 1;62(8):148–151. [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treatingand preventing HIV infection: Recommendations for a public health approach. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 6.Tlale J, Keapoletswe K, Anderson MG, de la Hoz Gomez F, Mmelesi M, Seipone K. Mother-to-child HIV transmission rate in Botswana - analysis of dried blood spot (DBS) results from the national PMTCT programme. Paper presented at: XVII Internataionl AIDS Conference; Mexico City. 2008. [Google Scholar]
- 7.Botswana Ministry of Health. Prevention of Mother-to-Child Transmission (PMTCT) of HIV: Pocket Guide. Ministry of Health, Botswana; 2011. [Google Scholar]
- 8.Botswana Ministry of Health. 2012 Botswana National HIV & AIDS Treatment Guidelines. Ministry of Health, Botswana; 2012. [Google Scholar]
- 9.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012 Dec 1;206(11):1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botswana Ministry of Health. Botswana 2008 National HIV/AIDS Guidelines, 1 November 2008 Edition. Ministry of Health, Botswana; 2008. [Google Scholar]
- 11.Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect. 2012 Dec;88(Suppl 2):i44–51. doi: 10.1136/sextrans-2012-050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010 Jun 17;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryden-Peterson S, Jayeoba O, Hughes MD, et al. Highly active antiretroviral therapy versus zidovudine for prevention of mother-to-child transmission in a programmatic setting, Botswana. J Acquir Immune Defic Syndr. 2011 Nov 1;58(3):353–357. doi: 10.1097/QAI.0b013e31822d4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. Jama. 2006 Aug 16;296(7):794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 15.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005 Feb 18;19(3):309–318. [PMC free article] [PubMed] [Google Scholar]
- 16.Dabis F, Msellati P, Meda N, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet. 1999 Mar 6;353(9155):786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 17.Kesho Bora Study, G. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011 Mar;11(3):171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 18.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009 Nov 1;52(3):406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008 Jul 10;359(2):130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petra Study, T. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002 Apr 6;359(9313):1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health. Botswana Country Update. Paper presented at: Regional EMTCT Stock-Taking Meeting; Johannesburg, South Africa. 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Duration of ARV model inputs.
Estimates of of risk of mother-to-child transmission by duration of maternal antiretrovirals and CD4 strata utilized in sensitivity analysis.
Table S2: Sensitivity analyses of projected MTCT and impact of Option B.
Estimates of risk of mother-to-child transmission and effect estimates of impact of Option B implementation under the Spectrum model inputs and estimates utilizing duration of ARV receipt.
Table S3: Estimates of effect from multivariate model of PMTCT strategy and other factors on probability of MTCT.