Abstract
Inflammation plays a key role in formation and rupture of intracranial aneurysms. Because hepatocyte growth factor (HGF) protects against vascular inflammation, we sought to assess the role of endogenous HGF in the pathogenesis of intracranial aneurysms. Circulating HGF concentrations in blood samples drawn from the lumen of human intracranial aneurysms or femoral arteries were compared in 16 patients. Tissue from superficial temporal arteries (STA), and ruptured or unruptured intracranial aneurysms collected from patients undergoing clipping (n=10) were immunostained with antibodies to HGF and its receptor c-Met. Intracranial aneurysms were induced in mice treated with PF-04217903 (a c-Met antagonist) or vehicle. Expression of inflammatory molecules was also measured in cultured human endothelial, smooth muscle cells and monocytes treated with LPS in presence or absence of HGF and PF-04217903. We found that HGF concentrations were significantly higher in blood collected from human intracranial aneurysms (1076 ± 656 pg/ml) than in femoral arteries (196 ± 436 pg/ml, p<0.001). HGF and c-Met were detected by immunostaining in STA, and in both ruptured and unruptured human intracranial aneurysms. A c-Met antagonist did not alter the formation of intracranial aneurysms (P>0.05), but significantly increased the prevalence of subarachnoid hemorrhage and decreased survival in mice (P<0.05). HGF attenuated expression of VCAM-1 (P<0.05) and E-Selectin (P<0.05) in human aortic endothelial cells. In conclusion, plasma HGF concentrations are elevated in intracranial aneurysms. HGF and c-Met are expressed in STA and in intracranial aneurysms. HGF signaling through c-Met may decrease inflammation in endothelial cells and protect against intracranial aneurysm rupture.
Keywords: Intracranial aneurysm, Inflammation, Hepatocyte Growth Factor, Subarachnoid hemorrhage, C-met, E-selectin, VCAM-1
Introduction
Inflammation appears to play a key role in the formation and rupture of intracranial aneurysms.1, 2 Various constituents of the inflammatory response appear to be increased in intracranial aneurysms including cytokines, chemokines, growth factors, reactive oxygen species, leukocytes, matrix metalloproteinases, and vascular smooth muscle cells.2–4 Therapies targeting the inflammatory cascade have also shown promising results in humans and experimental animals.2, 5–8
Hepatocyte Growth Factor (HGF), initially discovered as a growth factor of hepatocytes, was shown to have mitogenic, motogenic, morphogenic, antifibrotic, and antiapoptotic activities in several tissues.9–14 The biological responses to HGF are mediated through a tyrosine kinase receptor, the c-Met protooncogene.15 Emerging data suggest that HGF modulates the cytokine profile and protects various tissues including arterial walls from inflammatory damage.10, 13, 16–20 A recent study found that HGF promotes an anti-inflammatory cytokine profile in abdominal aortic aneurysm tissue and concluded that pharmacological interventions enhancing endogenous HGF secretion could have efficacy in prevention and treatment of these aneurysms.13 There have been no reports regarding the role of HGF in intracranial aneurysms. The purpose of this study was to assess the role of endogenous HGF in the pathogenesis of intracranial aneurysms. Specifically, we sought to determine: 1) whether HGF concentrations were higher in blood samples drawn from the lumen of human intracranial aneurysms as compared to femoral arteries of the same patients; 2) whether HGF and c-Met were expressed in the wall of human intracranial aneurysms; 3) whether a c-Met antagonist increases the risk of aneurysm rupture in a mouse model; and 4) whether HGF modulates the expression of inflammatory molecules in cultured endothelial, smooth muscle cells, and monocytes.
Methods
Human studies
The human study protocol was approved by the University of Iowa Institutional Review Board (IRB). The nature, benefits and risks of the study were explained to all patients prior to the study, and all participants read and signed written the IRB-approved informed consent.
Measurement of HGF concentrations in plasma
All patients presented to the Department of Neurosurgery at the University of Iowa Hospitals and Clinics between November 2012 and December 2012. Consecutive patients harboring saccular intracranial aneurysms (ruptured or unruptured) who were candidates for coil embolization were enrolled in the study. Patients taking corticosteroids, aspirin, or immunosuppressant therapy were excluded. A total of 16 patients harboring 18 aneurysms were enrolled. Other findings in the cohort (12 with unruptured and 4 with ruptured CA) were described previously.16
In each patient, arterial access was obtained through femoral puncture by use of the Seldinger technique, and a 7-French arterial sheath was inserted. A blood sample was subsequently drawn from the femoral artery. The guiding catheter was navigated into the studied vessel and the aneurysm was identified. A microcatheter was subsequently advanced over a micro-wire and placed in the aneurysm lumen. A blood sample (5 ml) was taken from the aneurysm lumen prior to coil deployment. Blood was centrifuged, and the plasma was stored at −80° C until analysis. Serum concentrations of HGF in aneurysm and femoral samples were quantified with Luminex-based immunoassay.
Expression of HGF and c-Met in human intracranial aneurysms
Samples of intracranial aneurysms (5 ruptured and 5 unruptured) were taken from patients undergoing microsurgical clipping. A segment of the aneurysm was resected, fixed in formalin and embedded in paraffin. Superficial temporal arteries were used as controls. 4 μm sections were collected and immunostained with human HGF R/c-MET affinity purified polyclonal antibody (AF276), or human HGF affinity purified polyclonal Ab (AF-294-NA) from R&D systems (Minneapolis, MN). Antibodies were validated in sections of skin and liver at the Histology Research Laboratory of the University of Iowa (Figure S1). Images were collected using a 20x objective lens in an Olympus BX-61motorized microscope.
Mouse studies
Assessment of the role of endogenous HGF in a mouse model of intracranial aneurysms
Studies were performed in 41 adult (4.8±0.1 months old) c57/Bl6 mice, obtained from the Jackson Laboratory (Bar Harbor, ME). All experimental protocols and procedures conform to the National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Intracranial aneurysms were induced in mice according to previously published methods.4, 8, 21 Briefly, under anesthesia a longitudinal incision was made in the scalp and a 1 mm hole was drilled in the skull. A stereotactic injection of elastase (35 mU in 2.5 μl) was subsequently performed using the following coordinates: 2.7 mm posterior to the bregma, 1 mm to the right of the midline, depth of 6.3 mm from the skull. Then, an osmotic mini-pump that delivered a pressor dose of angiotensin II (1000 ng/Kg/min) was implanted subcutaneously. Sham mice underwent surgery, but received physiologic saline in both the intracranial injection and the mini-pump. Mice were allowed to recover and food and water were provided ad libitum.
The c-Met antagonist PF-04217903 22 (10 mg/kg per day, Selleckchem, Houston, TX), or its vehicle (100% dimethyl sulfoxide (DMSO) were given daily by gavage, from day 0 (when elastase was injected in the basal cistern and infusion of angiotensin II is begun), until the end of experiment (21 days) or until the mice died or were euthanized. The dose of PF-04217903 was chosen according to previously published pharmacokinetic data.22 Sham controls were given 50 μl of 100% dimethyl sulfoxide daily, by gavage.
Mice were monitored daily and euthanized if signs of neurological deficit, or weight loss (>20%) were observed. All asymptomatic mice were euthanized 3 weeks after aneurysm induction. Five mice were excluded from the study. One sham control died after complications of gavage. Four mice were euthanized because they did not recover completely after the surgical procedure (3 vehicle-treated mice and 1 mouse treated with PF04217903). Systolic blood pressure was recorded weekly using the tail-cuff method (Visitech Systems BP-2000).
After euthanasia, the chest and abdomen of the mouse were exposed and examined for major bleeding or aneurysms of the aorta. Then, mice were perfused transcardially with 10–15 ml of ice-cold physiologic saline solution containing papaverine (100 μM) to cause systemic vasodilation, followed by an infusion of a 8% gelatin- physiologic saline mix containing 2 mg/ml of bromophenol blue to facilitate visualization of the cerebral circulation. The brain was dissected, and inspected for the presence of intracranial aneurysms and/or subarachnoid hemorrhage (SAH). Aneurysms were defined as a localized outward bulging of the vascular wall with a diameter greater than 1.5 times the parent artery. A survival curve was made according to the time of euthanasia or death.
Effect of HGF on the expression of inflammatory molecules in cultured cells
Human aortic endothelial cells (HAECs, Lonza, Inc.) (3–7 passages) were preincubated in normal growth media with human recombinant HGF (10 ng/ml), with or without 6 μg/ml of c-met antagonist (PF-04217903)22 for 1 hour. Cells were then treated with 100 ng/ml of LPS (E coli 0111:B4 strain) for 3 hours. Real time quantitative PCR was performed following reverse transcription using TaqMan primers for gene of interest and house-keeping gene (β-actin) in the same reaction. ΔΔCt method was used to quantify gene expression normalized by β-actin. Intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), cyclooxygenase-1 (COX-1), COX-2, or transforming growth factor-β (TGF-β), were measured. All of the findings in tissue culture are based on measurements from n=3.
Similarly, human aortic smooth muscle cells (HASMCs) (3–7 passages) and THP-1, a human monocyte cell line, were preincubated with recombinant human HGF (10 ng/ml) or HGF + PF-04217903. HASMC and THP-1 were then treated with LPS for 3 hours, and the mRNA levels of inflammatory molecules were measured as described above.
Statistical analysis
Data are presented as mean ± SEM for continuous variables, and as frequency for categorical variables. Analysis was carried out using unpaired t-test, Chi-square, and one-way ANOVA as appropriate. Survival was analyzed with log rank (Mantel-Cox) test. P-values less than 0.05 were considered statistically significant. Statistical analysis was carried out with Prism 6 (Graphpad, La Jolla, CA) and Stata 10.0 (College Station, TX).
Results
HGF levels in aneurysm lumens vs. femoral arteries
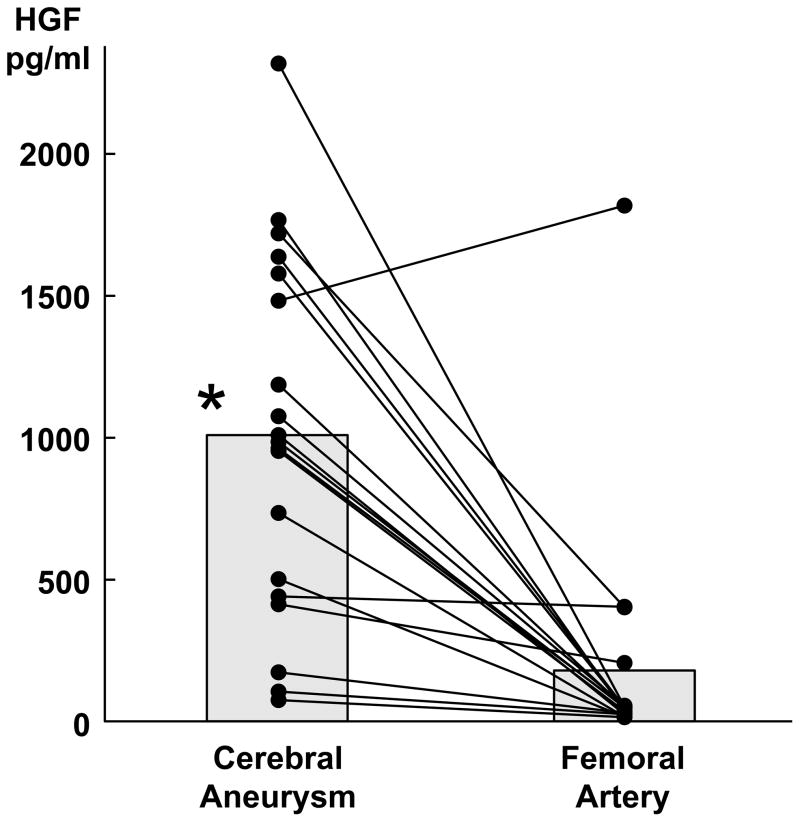
Of the 16 patients with intracranial aneurysms, 13 were women and 3 were men with a mean age of 55 ± 13 years. Aneurysm size was 10 ± 9 mm on average. Three of the 16 patients (18%) had ruptured aneurysms. The mean plasma concentration of HGF was significantly higher in samples taken from cerebral aneurysms (1076 ± 656 pg/ml) versus samples taken from femoral arteries (196 ± 436 pg/ml, 5-fold, p<0.001) (figure 1).
Figure 1.
Higher plasma levels of HGF in samples drawn from aneurysm lumens vs. femoral arteries. (* =p<0.05)
HGF and c-Met are expressed in human intracranial aneurysms
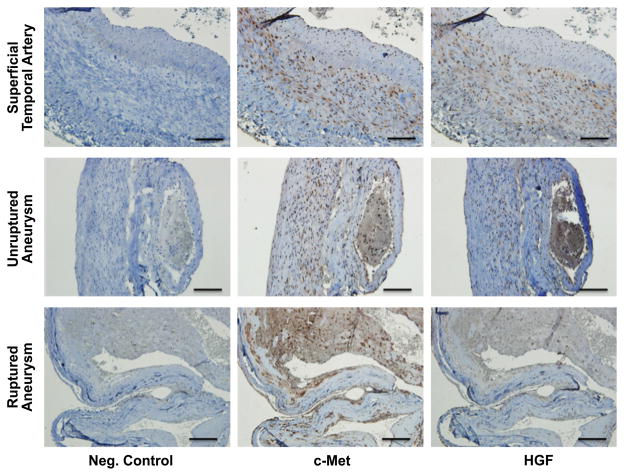
Samples from human STA and ruptured or unruptured intracranial aneurysms were positively stained for HGF and its receptor c-Met. HGF and c-Met localized to the endothelium and smooth muscle layers (Figure 2).
Figure 2.
Examples of expression of c-Met and HGF in human superficial temporal arteries and intracranial aneurysms (ruptured and unruptured). Positive immunostaining for C-Met and HGF was noted in the endothelium and smooth muscle layers of the examined samples. Negative control excluded the primary antibody for c-Met or HGF. Similar findings were observed in tissues from 10 other patients. Scale bar=100μm.
A c-Met antagonist increases the risk of aneurysm rupture and decreases survival in mice
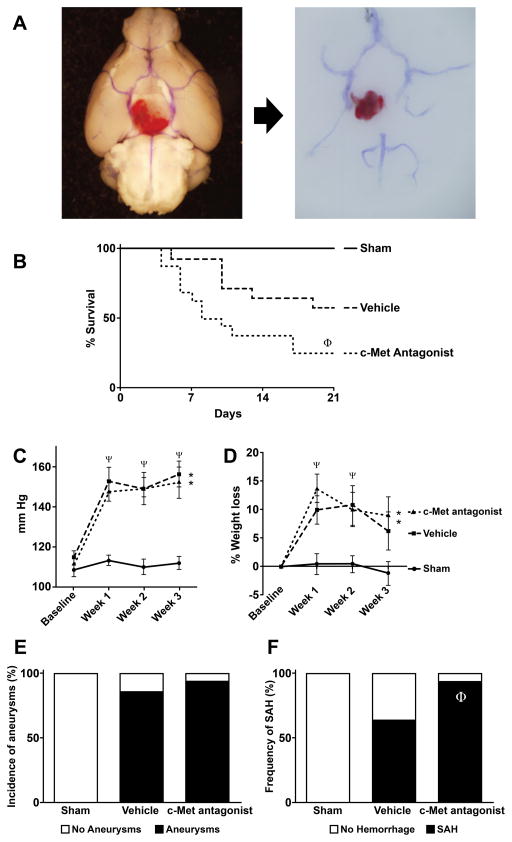
After induction of aneurysms, systolic pressure increased significantly (p<0.05) and similarly in response to infusion of angiotensin II in mice that received PF-04217903 or vehicle, compared to sham controls (Figure 3C). After induction of aneurysms, mice lost weight especially during the first 2 weeks of follow up (p<0.05). Weight loss was not observed in Sham controls (Figure 3D). PF04217903 did not have an effect on blood pressure or weight loss compared to vehicle.
Figure 3.
Effect of a c-Met antagonist on intracranial aneurysm formation and rupture in mice. (A) Cerebral arteries and intracranial aneurysm in situ (left) and after dissection (right). Note the hemorrhage surrounding the aneurysm. Survival (B), systolic blood pressure (C), weight loss (D), aneurysm formation (E) and prevalence of subarachnoid hemorrhage (F) in sham controls (n=5), and after induction of aneurysms in mice treated with PF-04217903 (n=16) or vehicle (n=14). *=p<0.05 vs sham; ψ=P<0.05 vs baseline. ϕ=P<0.05 vs. vehicle.
Compared to sham controls, more than 85% of mice that received elastase and angiotensin II displayed evidence of intracranial aneurysms and/or subarachnoid hemorrhage after euthanasia (Figure 3A). Intracranial aneurysms were found in 12/14 (86%) mice treated with vehicle vs. 15/16 (94%) mice treated with PF04217903 (Figure 3E). Prevalence of SAH was significantly higher in mice treated with PF04217903 (15/16 (94%)) than in vehicle treated mice (9/14 (64%), p<0.05) (Figure 3F). Survival was significantly lower in mice treated with PF04217903 than in vehicle treated mice (25 vs 57% respectively, p<0.05) (Figure 3B). Sham controls have 100% survival after surgery, and they did not have evidence of intracranial aneurysms or hemorrhage.
HGF attenuates inflammation in cultured human aortic endothelial cells
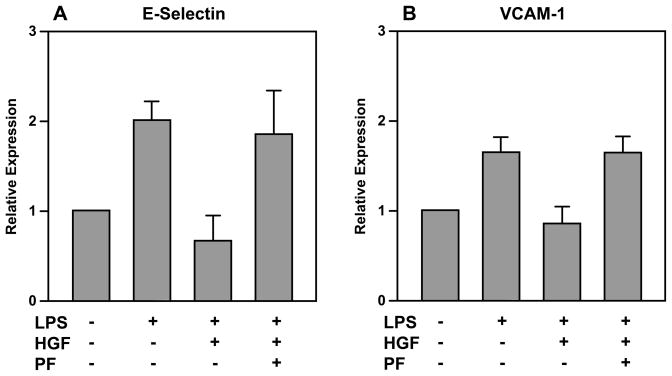
The expression of VCAM-1and E-selectin was lower in HAECs incubated with HGF+LPS vs. LPS alone (Figure 4). Treatment with c-Met antagonist and HGF abolished the protective effect of HGF on inflammatory markers; specifically levels of VCAM-1, and E-selectin were similar in HAECs treated with HGF + PF-665752+ LPS vs. LPS alone. There was no significant difference in levels of TNF-α, IL-1β, MCP-1, COX-1, COX-2, TGF-β, or ICAM-1 in HAEC cells treated with HGF+LPS, HGF+PF-04217903+ LPS, or LPS alone (data not shown).
Figure 4.
Lower levels of VCAM-1 and E-selectin in HAECs incubated with HGF+LPS vs. LPS alone. Addition of c-Met antagonist abolishes the effect of HGF on inflammatory markers. (One-way ANOVA analysis; P=0.01 for VCAM-1 and P=0.036 for E-selectin)
There was no significant difference in levels of TNF-α, IL-1β, MCP-1, COX-1, COX-2,TGF-β, VCAM-1, E-Selectin, or ICAM-1 in HASMCs or THP-1 cells treated with HGF+LPS, HGF+PF-04217903+ LPS, or LPS alone (data not shown).
Discussion
Inflammation is a critical pathway underlying the development, progression, and rupture of intracranial aneurysms2, 3, 7, 8, 23, 24. Evidence suggests that proinflammatory and proliferative pathways are activated in endothelial cells in response to local hemodynamic stress25. This is followed by monocyte infiltration, activation, and release of several proinflammatory molecules in arterial walls.4, 26 A later final common pathway appears to involve the release of matrix metalloproteinases and apoptosis of cellular constituents of the vessel wall, leading to aneurysmal remodeling, progression and rupture.2, 4, 27–29
HGF may have a protective role in vascular disease and in the pathogenesis of intracranial aneurysms. HGF as a growth factor is regulated in a paracrine manner after tissue injury and it promotes organ regeneration and wound healing30. In the vascular system, HGF is involved in angiogenesis31, and it also regulates function of endothelial progenitors cells. For example, HGF decreases angiotensin II induced senescence and oxidative stress in endothelial progenitors18. At the cellular level, HGF signaling through the receptor c-Met protects cells against DNA damage, promotes DNA repair, and decreases apoptosis12. HGF also regulates other molecules involved in vascular biology and inflammation20. HGF modulates EGFR degradation during LPS-induced inflammation, by controlling translocation of phosphatases such as SHIP2 (Src homology domain 2-containing inositol 5′-phosphatase)19, 20. Several studies have reported that HGF and c-Met are involved in arterial repair11, 12, 30.
In this study, we found serum concentrations of HGF to be particularly high in the lumen of human intracranial aneurysms as compared to femoral arteries. Moreover, HGF and its receptor c-Met were expressed in the walls of both ruptured and unruptured human cerebral aneurysms. These findings provide the first evidence suggesting that HGF may be associated with human intracranial aneurysms (Figure 5). Thus, expression of HGF in the wall and lumen of human intracranial aneurysms may be a response to local cellular injury from hemodynamic stress and inflammation. Circulating HGF levels are changed in other cardiovascular diseases. Several studies have shown that HGF levels are increased in hypertensive patients compared to control subjects32–35. On the other hand, HGF levels are decreased in diabetic patients and animal models of diabetes34. HGF appears to have a protective role in vascular disease and its levels may be modulated by mechanical, inflammatory and metabolic stress.
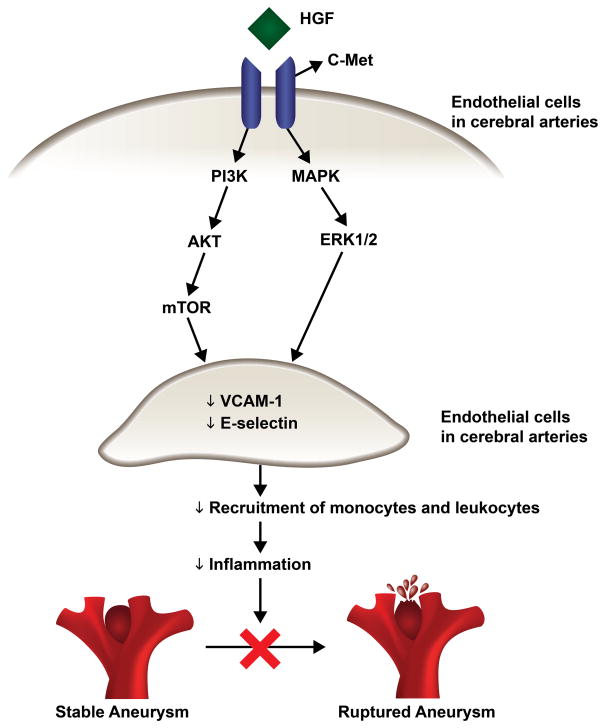
Figure 5.
Schematic figure of potential pathways by which HGF acts on cerebral endothelial cells to decrease the expression of adhesion molecules and inflammatory molecules such as E-selectin and VCAM-1. This attenuates the inflammatory response, stabilizes aneurysm walls, and prevents aneurysm rupture.
HGF levels may increase locally in aneurysms to protect against vascular damage. Spin et al also showed that HGF expression is increased in aneurysms of the aorta36. Our current studies suggest that HGF is upregulated in aneurysms and reduces inflammation and vascular damage, because PF-04217903, an antagonist of c-Met22, significantly increases aneurysm rupture and decreases survival in a mouse model of intracranial aneurysms. Importantly, protective effects of c-Met antagonism do not appear to modulate high blood pressure in this model of intracranial aneurysms.
We also explored, in cultured cells challenged with LPS, potential mechanisms through which HGF may prevent intracranial aneurysm rupture. We found that HGF mainly exerts its effects on endothelial cells (as opposed to smooth muscle cells or monocytes), where it attenuates the expression of VCAM-1 and E-selectin. The finding that HGF decreases the expression of adhesion molecules selectively in endothelial cells is particularly relevant because the infiltration of inflammatory cells in the intracranial aneurysm tissue is a hallmark of intracranial aneurysms1–3. Aoki et al26 demonstrated that attenuation of macrophage migration through inhibition of MCP-1, halted intracranial aneurysm formation in mice. In addition, VCAM-1 and E-selectin were increased in experimental aortic aneurysm37 and human ruptured cerebral aneurysm tissue38 respectively. Collectively, these data suggest that attenuation of adhesion molecules (VCAM-1 and E-selectin) by HGF, and decreased infiltration of inflammatory cells in intracranial aneurysm tissue, may attenuate the inflammatory response associated with aneurysm formation, progression, and rupture.
Anti-inflammatory actions of HGF have also been reported in several other studies. Shintani et al13 demonstrated in ex vivo cultures of human abdominal aortic aneurysm tissue that exogenous HGF enhanced the secretion of anti-inflammatory cytokine (IL-10), and suppressed the secretion of proinflammatory monocyte chemotactic protein-1 (MCP-1). Likewise, Coudriet et al39 noted a decrease in production of the pro-inflammatory cytokine IL-6, along with an increase in the anti-inflammatory cytokine IL-10 by bone marrow-derived macrophages in the presence of HGF. Along similar lines, Rutella et al40 found that monocytes cultured with HGF released low amounts of IL-12 and upregulated IL-10. In other studies, HGF suppressed MCP-1, IL-6, IL- 1, and interferon-gamma,9, 10, 41, 42 all of which have been implicated in the pathogenesis of intracranial aneurysms1, 2. Additionally, the antiapoptotic effect of HGF is relevant with regard to intracranial aneurysms, because apoptosis of endothelial and smooth muscle cell is also found in the pathogenesis of aneurysm progression and rupture.2, 43
Limitations
A relatively small number of patients were enrolled in this study. However, the observation of markedly higher HGF concentrations in the aneurysm lumen compared with a systemic blood within the same patients is novel and supports the idea of local production of HGF in the aneurysm tissue. Although aneurysm rupture and survival were significantly affected by a c-met antagonist, further experiments are needed to demonstrate whether treatment with exogenous HGF or c-met receptor agonists can decrease aneurysm formation and rupture. Despite these limitations, the present study provides data from both humans and an animal model supporting a key role for endogenous HGF in the pathogenesis of intracranial aneurysms.
Perspectives
We demonstrated that plasma hepatocyte growth factor (HGF) is elevated in human intracranial aneurysms. HGF and c-Met are expressed in the wall of intracranial aneurysms and STA. In addition, c-Met antagonist did not alter the formation of intracranial aneurysms, but significantly increased the prevalence of SAH and decreased survival rate in a mouse model of intracranial aneurysms. A potential mechanism by which HGF confers protection is by attenuation of the expression of VCAM-1 and E-Selectin evident in human aortic endothelial cells.
In conclusion, this study implies a potential novel therapeutic strategy for medical management of intracranial aneurysms.
Additional studies may explore pharmacological strategies to modulate HGF signaling in human intracranial aneurysms.
Conclusion
In this study, we found that the concentration of HGF is higher in the lumen of human intracranial aneurysms than in peripheral blood, and that HGF and its receptor, c-Met, are expressed in the wall of intracranial aneurysms. We also provide evidence that inhibition of endogenous HGF signaling increases the risk of intracranial aneurysm rupture and decreases survival in mice. HGF inhibition, in vitro, also attenuated the expression of inflammatory and adhesion molecules in cultured human endothelial cells. Collectively, these findings point to a novel role for endogenous HGF in the pathogenesis of human intracranial aneurysms that might be exploited therapeutically.
Supplementary Material
Novelty and Significance.
What is new?
This is the first report to suggest a protective role of endogenous HGF in cerebral aneurysms.
We found increased levels of HGF in the lumen of intracranial aneurysms, and found that HGF and its receptor c-Met are expressed in superficial temporal arteries and in ruptured or unruptured intracranial aneurysms.
HGF decreased mortality and frequency of subarachnoid hemorrhage in a mouse model of intracranial aneurysms.
HGF decreased the inflammatory response of cultured endothelial cells exposed to LPS.
What is relevant?
HGF could have a therapeutic use by halting progression of cerebral aneurysms, and preventing rupture of unstable aneurysms.
Summary
Plasma HGF concentrations are elevated in intracranial aneurysms. HGF and c-Met are expressed in STA and in intracranial aneurysms. HGF signaling through c-Met may decrease inflammation in endothelial cells and protect against intracranial aneurysm rupture
Acknowledgments
Funding/financial support:
This work was supported by K08NS082363 to David Hasan.
Footnotes
Disclosures
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–3622. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 2.Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Biology of intracranial aneurysms: Role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalouhi N, Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Cigarette smoke and inflammation: Role in cerebral aneurysm formation and rupture. Mediators Inflamm. 2012;2012:271582. doi: 10.1155/2012/271582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pena Silva RA, Kung DK, Mitchell IJ, Alenina N, Bader M, Santos RA, Faraci FM, Heistad DD, Hasan DM. Angiotensin 1–7 reduces mortality and rupture of intracranial aneurysms in mice. Hypertension. 2014;64:362–368. doi: 10.1161/HYPERTENSIONAHA.114.03415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan DM, Chalouhi N, Jabbour P, Magnotta VA, Kung DK, Young WL. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced mri: Preliminary results. J Neuroradiol. 2013;40:187–191. doi: 10.1016/j.neurad.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasan DM, Mahaney KB, Brown RD, Jr, Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan DM, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, Young WL, Hashimoto T, Winn HR, Heistad D. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: Preliminary results. Journal of the American Heart Association. 2013:2. doi: 10.1161/JAHA.112.000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Futamatsu H, Suzuki J, Mizuno S, Koga N, Adachi S, Kosuge H, Maejima Y, Hirao K, Nakamura T, Isobe M. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: A potential role for induction of t helper 2 cytokines. Circ Res. 2005;96:823–830. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
- 10.Homsi E, Janino P, Amano M, Saraiva Camara NO. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am J Nephrol. 2009;29:283–291. doi: 10.1159/000159275. [DOI] [PubMed] [Google Scholar]
- 11.McKinnon H, Gherardi E, Reidy M, Bowyer D. Hepatocyte growth factor/scatter factor and met are involved in arterial repair and atherogenesis. Am J Pathol. 2006;168:340–348. doi: 10.2353/ajpath.2006.050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26(Suppl 1):188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 13.Shintani Y, Aoki H, Nishihara M, Ohno S, Furusho A, Hiromatsu S, Akashi H, Imaizumi T, Aoyagi S. Hepatocyte growth factor promotes an anti-inflammatory cytokine profile in human abdominal aortic aneurysm tissue. Atherosclerosis. 2011;216:307–312. doi: 10.1016/j.atherosclerosis.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Kosai K, Matsumoto K, Funakoshi H, Nakamura T. Hepatocyte growth factor prevents endotoxin-induced lethal hepatic failure in mice. Hepatology. 1999;30:151–159. doi: 10.1002/hep.510300102. [DOI] [PubMed] [Google Scholar]
- 15.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 16.Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, Shokei K, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin ii blockade in cardiomyopathic hamsters. Circulation. 2000;102:246–252. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- 17.Kaido T, Yamaoka S, Seto S, Funaki N, Kasamatsu T, Tanaka J, Nakamura T, Imamura M. Continuous hepatocyte growth factor supply prevents lipopolysaccharide-induced liver injury in rats. FEBS Lett. 1997;411:378–382. doi: 10.1016/s0014-5793(97)00744-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanada F, Taniyama Y, Azuma J, Iekushi K, Dosaka N, Yokoi T, Koibuchi N, Kusunoki H, Aizawa Y, Morishita R. Hepatocyte growth factor, but not vascular endothelial growth factor, attenuates angiotensin ii-induced endothelial progenitor cell senescence. Hypertension. 2009;53:77–82. doi: 10.1161/HYPERTENSIONAHA.108.120725. [DOI] [PubMed] [Google Scholar]
- 19.Sanada F, Taniyama Y, Iekushi K, Azuma J, Okayama K, Kusunoki H, Koibuchi N, Doi T, Aizawa Y, Morishita R. Negative action of hepatocyte growth factor/c-met system on angiotensin ii signaling via ligand-dependent epithelial growth factor receptor degradation mechanism in vascular smooth muscle cells. Circ Res. 2009;105:667–675. 613. doi: 10.1161/CIRCRESAHA.109.202713. following 675. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu K, Taniyama Y, Sanada F, Azuma J, Iwabayashi M, Iekushi K, Rakugi H, Morishita R. Hepatocyte growth factor inhibits lipopolysaccharide-induced oxidative stress via epithelial growth factor receptor degradation. Arterioscler Thromb Vasc Biol. 2012;32:2687–2693. doi: 10.1161/ATVBAHA.112.300041. [DOI] [PubMed] [Google Scholar]
- 21.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou HY, Li Q, Lee JH, Arango ME, Burgess K, Qiu M, Engstrom LD, Yamazaki S, Parker M, Timofeevski S, Cui JJ, McTigue M, Los G, Bender SL, Smeal T, Christensen JG. Sensitivity of selected human tumor models to pf-04217903, a novel selective c-met kinase inhibitor. Molecular cancer therapeutics. 2012;11:1036–1047. doi: 10.1158/1535-7163.MCT-11-0839. [DOI] [PubMed] [Google Scholar]
- 23.Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, Young WL, Hashimoto T, Winn HR, Heistad D. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: A pilot study. Stroke. 2012;43:3258–3265. doi: 10.1161/STROKEAHA.112.673400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (m1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. Journal of neuroinflammation. 2012;9:222. doi: 10.1186/1742-2094-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Aziz HA, Shono M, Satoh K. Endothelial injury and inflammatory response induced by hemodynamic changes preceding intracranial aneurysm formation: Experimental study in rats. J Neurosurg. 2007;107:405–411. doi: 10.3171/JNS-07/08/0405. [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40:942–951. doi: 10.1161/STROKEAHA.108.532556. [DOI] [PubMed] [Google Scholar]
- 27.Takemura Y, Hirata Y, Sakata N, Nabeshima K, Takeshita M, Inoue T. Histopathologic characteristics of a saccular aneurysm arising in the non-branching segment of the distal middle cerebral artery. Pathol Res Pract. 2010;206:391–396. doi: 10.1016/j.prp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Marchese E, Vignati A, Albanese A, Nucci CG, Sabatino G, Tirpakova B, Lofrese G, Zelano G, Maira G. Comparative evaluation of genome-wide gene expression profiles in ruptured and unruptured human intracranial aneurysms. J Biol Regul Homeost Agents. 2010;24:185–195. [PubMed] [Google Scholar]
- 29.Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and-9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38:162–169. doi: 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
- 30.Funakoshi H, Nakamura T. Hepatocyte growth factor: From diagnosis to clinical applications. Clin Chim Acta. 2003;327:1–23. doi: 10.1016/s0009-8981(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 31.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. Hgf/c-met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 32.Morishita R, Moriguchi A, Higaki J, Ogihara T. Hepatocyte growth factor (hgf) as a potential index of severity of hypertension. Hypertens Res. 1999;22:161–167. doi: 10.1291/hypres.22.161. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura S, Moriguchi A, Morishita R, Aoki M, Yo Y, Hayashi S, Nakano N, Katsuya T, Nakata S, Takami S, Matsumoto K, Nakamura T, Higaki J, Ogihara T. A novel vascular modulator, hepatocyte growth factor (hgf), as a potential index of the severity of hypertension. Biochem Biophys Res Commun. 1998;242:238–243. doi: 10.1006/bbrc.1997.7800. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura S, Morishita R, Moriguchi A, Yo Y, Nakamura Y, Hayashi S, Matsumoto K, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Hepatocyte growth factor as a potential index of complication in diabetes mellitus. J Hypertens. 1998;16:2019–2026. doi: 10.1097/00004872-199816121-00025. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, Morishita R, Nakamura S, Aoki M, Moriguchi A, Matsumoto K, Nakamura T, Higaki J, Ogihara T. A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension. 1996;28:409–413. doi: 10.1161/01.hyp.28.3.409. [DOI] [PubMed] [Google Scholar]
- 36.Spin JM, Hsu M, Azuma J, Tedesco MM, Deng A, Dyer JS, Maegdefessel L, Dalman RL, Tsao PS. Transcriptional profiling and network analysis of the murine angiotensin ii-induced abdominal aortic aneurysm. Physiol Genomics. 2011;43:993–1003. doi: 10.1152/physiolgenomics.00044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan J, Li X, Zhong L, Hao T, Di J, Liu F, Zhao HH, Bai SL. Mcp-1, icam-1 and vcam-1 are present in early aneurysmal dilatation in experimental rats. Folia Histochem Cytobiol. 2010;48:455–461. doi: 10.2478/v10042-010-0042-y. [DOI] [PubMed] [Google Scholar]
- 38.Jia W, Wang R, Zhao J, Liu IY, Zhang D, Wang X, Han X. E-selectin expression increased in human ruptured cerebral aneurysm tissues. Can J Neurol Sci. 2011;38:858–862. doi: 10.1017/s0317167100012439. [DOI] [PubMed] [Google Scholar]
- 39.Coudriet GM, He J, Trucco M, Mars WM, Piganelli JD. Hepatocyte growth factor. modulates interleukin-6 production in bone marrow derived macrophages: Implications for inflammatory mediated diseases. PloS one. 2010;5:e15384. doi: 10.1371/journal.pone.0015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, Danese S, Pessina G, Pandolfi S, Natoni F, Di Febo A, Scambia G, Manfredini R, Salati S, Ferrari S, Pierelli L, Leone G, Lemoli RM. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (il)-10++il-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 41.Kamimoto M, Mizuno S, Nakamura T. Reciprocal regulation of il-6 and il-10 balance by hgf via recruitment of heme oxygenase-1 in macrophages for attenuation of liver injury in a mouse model of endotoxemia. Int J Mol Med. 2009;24:161–170. doi: 10.3892/ijmm_00000219. [DOI] [PubMed] [Google Scholar]
- 42.Yamaura K, Ito K, Tsukioka K, Wada Y, Makiuchi A, Sakaguchi M, Akashima T, Fujimori M, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Suzuki J, Amano J, Isobe M. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation: Induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation. 2004;110:1650–1657. doi: 10.1161/01.CIR.0000143052.45956.71. [DOI] [PubMed] [Google Scholar]
- 43.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/met via the phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.