Macroautophagy, here referred to as ‘autophagy,' constitutes one of the most spectacular phenomena in cell biology beyond cell fusion, cell division, differentiation and demise. Metaphorically spoken, it constitutes a process in which the cell sequesters portions of itself in its stomach (the autophagosomes) and then assures their complete degradation in its digestive tract (the lysosomes).
Autophagy constitutes one of the most elementary reactions that a cell may have to adapt itself to a changing microenvironment. For instance, in conditions of dwindling external resources, be it nutrients, growth factors or oxygen, the cell may mobilize its stock of potentially energy-rich macromolecules by autophagy, thereby converting proteins and lipids into life-preserving fuel for bioenergetic reactions. In addition, the cell can take advantage of the autophagic machinery to remove damaged, dysfunctional and potentially harmful organelles such as uncoupled mitochondria from its cytoplasm or to destroy useless and even dangerous protein aggregates. Hence, autophagy constitutes an essential mechanism for the recycling of cytoplasmic material and in fine cleaning and rejuvenating extranuclear compartments, especially in non-dividing cells such as neurons or cardiomyocytes. Beyond its homeostatic function, autophagy also has a major role in hormetic reactions. Hormesis can be defined as a process in which the exposure of cells, organs or organisms to a mild stress allows them to mount an adaptive response that allow them to tolerate a later, stronger and normally lethal stress. One well-known example of hormesis is ischemic preconditioning in which a short episode of ischemia reduces the death of heart muscle cells to an otherwise fatal infarction. In this context, autophagy induction has a major role in increasing the robustness of the system, protecting it from deadly stress. Nonetheless, there are also specific situations in which an excess of autophagy may ultimately cause the death of cells by excessive self-digestion. This potentially lethal role of autophagy has received the name of ‘autosis'.
The present Special Issue of Cell Death & Differentiation deals with the physiological and pathological functions of autophagy in cell stress and disease. Liu and Levine1 provide an overview over the potential roles of autosis and autophagic cell death in health and disease. Filomeni et al.2 demonstrate the importance of autophagy regulation by reactive oxygen species and reactive nitrogen species in the context of cytoplasmic processes and DNA damage signaling. Orhon et al.3 insist on the important role of primary cilia, which are microtubule-based structures located at the cell surface of many cell types, as potential sensors of autophagy-inducing stimuli that are in turn affected in their biogenesis and function by autophagic responses. Nikoletopoulou et al.4 demonstrate the essential role of autophagy for the normal function of neurons in model organisms such as Caenorhabditis elegans, as well as the potential role of autophagy in the the unwarranted demise of neurons induced by pathological stimuli including overexcitation.
Two original articles deal with the complex regulation of mitophagy, a version of autophagy that assures the specific turnover of damaged mitochondria. Rossin et al.5 demonstrate that transglutaminase-2 has an unexpected essential role in mitophagy. Moreover, Strapazzon et al.6 show that AMBRA-1 can stimulate mitophagy through a novel pathway that does not require parkin nor STQM1/LC3. Menzies et al.7 reveal that inhibition of calpains with calpastatin increases autophagic turnover, thereby reducing neuronal damage in fly and mouse models of transgene-enforced expression of mutant huntingtin protein. In another differentiated cell type, Rožman et al.8 show that autophagy is not essential for neutrophil granulopoiesis, having instead a negative impact on the generation of neutrophils. Anding and Baehrecke9 reveal the broad implication of a specific protein, Vps15, which is part of the autophagy-regulatory Beclin 1 complex, in different cellular contexts and find that it is necessary for both stress-induced and developmentally programmed autophagy in various tissues in Drosophila melanogaster.9 Ber et al.10 present data on DAPK2 (death-associated protein kinase 2), a Ca2+-regulated serine/threonine kinase, that directly interacts with and phosphorylates the protein raptor within the mTORC1 (mammalian target of rapmaycin complex 1), which is one of the major autophagy regulators. Rodríguez-Muela et al.11 make a convincing case in favor of the cytoprotective role of autophagy in a mouse model of retinitis pigmentosa, an inherited, degenerative eye disease that causes severe vision impairment and often blindness. Similarly, Zhou et al.12 reveal the essential role of autophagy for maintaining the survival and normal function of rod photoreceptors. Finally, two papers deal with the mode of action of two autophagy-inducing longevity-extending comopounds. Pietrocola et al.13 show that spermidine, which has universal anti-aging effects across multiple model organisms including yeast, nematodes, flies and mice, induces autophagy owing to its capacity to inhibit the enzymatic activity of the EP300 acetyltransferase. Rockenfeller et al.14 describe the capacity of phosphatidylethanolamine to induce autophagy in yeast and mammalian cells, an effect that is echoed by a dramatic anti-aging effect in yeast.
Altogether, this special issue of Cell Death & Differentiation reflects the stunning role of autophagy in multiple cell types, its induction by a myriad of different stressful stimuli, as well as its essential role in maintaining homeostatic and hormetic functions. Beyond these life-preserving and anti-aging effects of autophagy, recent evidence suggest that this phenomenon also has a dark side, causing pathological cell death in specific circumstances (Figure 1). Hence, the present series of articles will incite cell death researchers all over the world to study autophagy in its multiple positive and negative aspects with respect to cellular and organismal survival. No reader can remain indifferent.
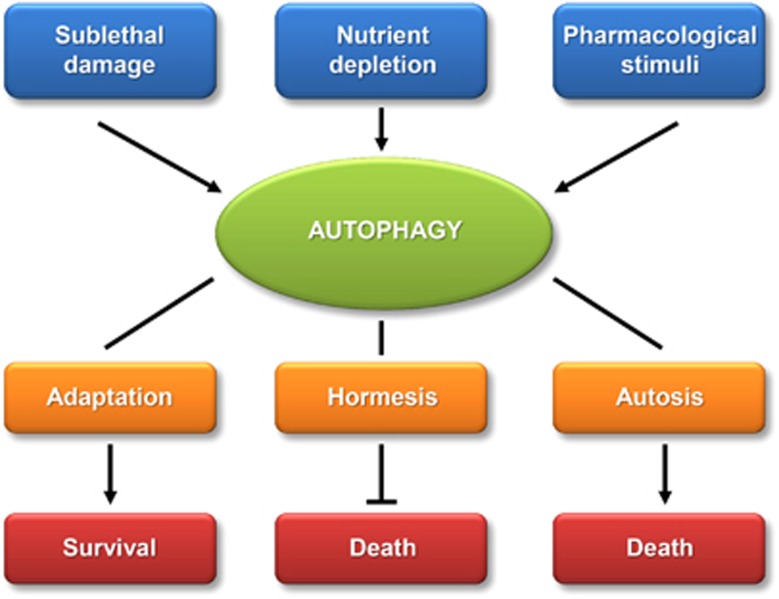
Figure 1.
Physiological roles of autophagy. Autophagy can be induced by damage, starvation or pharmacological cues. Autophagy then favors the adaptation of cells, hormetic reactions (protection against otherwise lethal stimuli) or autosis (cell death mediated by autophagy)
References
- Liu Y, Levine B.Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed]
- Filomeni G, De Zio D, Cecconi F.Oxidative stress and autophagy: the clash between damage and metabolic needs Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhon I, Dupont N, Pampliega O, Cuervo AM, Codogno P.Autophagy and regulation of cilia function and assembly Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou V, Papandreou M-E, Tavernarakis N.Autophagy in the physiology and pathology of the central nervous system Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin F, et al. Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed]
- Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM, et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1 Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Garcia-Arencibia M, Imarisio S, O'Sullivan NC, Ricketts T, Kent BA.Calpain inhibition mediates autophagy-dependent protection against polyglutamine toxicity Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rožman S, Yousefi S, Oberson K, Kaufmann T, Benarafa C, Simon HU.The generation of neutrophils in the bone marrow is controlled by autophagy Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anding AL, Baehrecke EH.Vps15 is required for stress induced and developmentally triggered autophagy and salivary gland protein secretion in Drosophila Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ber Y, et al. Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed]
- Rodríguez-Muela N, et al. Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed]
- Zhou Z, Doggett TA, Sene A, Apte RS, Ferguson TA.Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed]
- Pietrocola F, Lachkar S, Enot DP, Niso-Santano M, Bravo-San Pedro JM, Sica V, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300 Cell Death Differ 201522(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenfeller P, Koska M, Pietrocola F, Minois N, Knittelfelder O, Sica V, et al. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015;22:(this issue). doi: 10.1038/cdd.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]