Kidney disease afflicts 33 million in the United States and chronic kidney disease (CKD) accounts for over $60 billion in Medicare costs.1,2 Hypertension afflicts 75 million in the US and significant portions of those patients develop CKD and progress to end stage renal disease (ESRD).1-5 Interestingly, resistant hypertension which is defined as uncontrolled hypertension despite three anti-hypertensive medication classes increases the risk for cardiovascular diseases and ESRD.6 These recent findings in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) highlights the fact that current treatments only slow the loss of kidney function, or have no benefit at all.5,6 New therapeutic approaches are urgently needed.
Development of drugs to increase a novel class of fatty acids, epoxyeicosatrienoic acids (EETs), represents a unique approach to treat hypertension and kidney disease. EETs are generated from the substrate arachidonic acid by cytochrome P450 (CYP) epoxygenase enzymes.7,8 There are four regioisomeric EETs formed, 5,6-EET; 8,9-EET; 11,12-EET; and 14,15-EET. These regioisomeric EETs are further metabolized to less active or inactive diols by the soluble epoxide hydrolase (sEH; Ephx2) enzyme. For clarity, EETs will be used as a general term and regioisomers mentioned when actions can be attributed to a specific regioisomeric EET. In the majority of circumstances, the primary EETs evaluated for cardiovascular and renal function have been 11,12-EET and 14,15-EET.7 Once formed EETs act in an autocrine or paracrine manner to elicit biological responses. Vascular endothelial and renal epithelial cells are major sites for EET production.7,8 This localized EET generation aligns with the biological actions and contribution of EETs to cardiovascular and renal function. Prominent biological actions of EETs include their role as endothelial derived hyperpolarizing factors (EDHFs) and regulation of tubular sodium reabsorption by inhibiting epithelial sodium channel (ENaC) in the kidney.8-11 These actions position EETs to increase blood flow to organs, decrease peripheral vascular resistance, and enhance sodium excretion. EETs also have anti-inflammatory actions that are beneficial in cardiovascular and renal diseases.7,12 The focus of this brief review is to discuss changes in EETs that contribute to hypertension and kidney injury and to discuss EET-based therapeutics being developed to combat cardiovascular and renal diseases.
Kidney Renal Disease
The link between decreased EETs and hypertension, especially salt-sensitive hypertension, has been strongly established.8,11,13-16 Decreased renal epoxygenase activity and decreased renal EET levels have been associated with angiotensin-dependent hypertension, salt-sensitive hypertension, and Lyon hypertensive rats.14-18 Transgenic rats overexpressing both human renin and angiotensinogen genes (dTGR) develop hypertension and renal failure that is associated with decreased kidney epoxygenase enzymatic activity and CYP2C11 and CYP2C23 protein levels.17 Likewise, we have found that an inability to increase renal cortical and vascular rat CYP2C11 and CYP2C23 or mouse Cyp2c44 protein expression contributes to salt-sensitive hypertension.14,18 These CYP2C enzymes are primarily responsible for 11,12-EET and 14,15-EET formation in the rat and mouse kidneys.19 Rat CYP2C23 and mouse Cyp2c44 are the predominant kidney epoxygenases which are up regulated by a high K+ (2.5%) or high Na+ (8%) salt diet.8,20 Another potential epoxygenase is the CYP2J5 protein that is abundantly expressed in the mouse kidney.21 However, the ability of CYP2J5 to generate EETs is questionable and Cyp2j5 (-/-) mice have demonstrated that CYP2J5 appears to contribute to blood pressure control by regulating estrogen rather than EET synthesis.21 On the other hand, genetic manipulation of CYP2C epoxygenase expression has provided additional support to the concept that CYP2C-derived EETs are essential in renal sodium handling and blood pressure regulation. Cyp2c44(-/-) mice develop hypertension when fed a high K+ or high Na+ salt diet.8,11,22 Similarly, Cyp4a10(-/-) mice have decreased renal Cyp2c44 epoxygenase activity in response to high Na+ salt and develop salt-sensitive hypertension.23 Differences in renal EET generation and blood pressure in response to dietary NaCl intake between the Cyp2c44 (-/-) mice and Cyp4a10(-/-) mice provide additional evidence for a critical contribution for EETs in blood pressure regulation. Interestingly, Cyp4a10 (-/-) mice have decreased urinary EET levels and an elevated blood pressure on a normal salt (0.3% NaCl) diet.23 Lowering dietary salt to 0.05% NaCl lowers blood pressure in Cyp4a10 (-/-) mice.23 In contrast, Cyp2c44 (-/-) mice do not have decreased urinary EET levels or elevated blood pressures on a normal salt diet.11 Both Cyp2c44 (-/-) and Cyp4a10 (-/-) mice demonstrate salt-sensitive hypertension in response to 8% NaCl feeding which is associated with an inability to increase renal EET generation. The fact that amiloride lowers blood pressure in Cyp2c44 (-/-) and Cyp4a10 (-/-) mice fed a high salt diet suggests a significant contribution for ENaC.11,22,23
A major cellular mechanism responsible for salt-sensitive hypertension that results from decreased renal EET levels appears to be increased ENaC activity (Figure 1).8,11,22 Actions of 11,12-EET on basolateral inwardly rectifying K+ channels and apical ENaC channels on the cortical collecting duct (CCD) epithelium can explain the salt-sensitive blood pressure regulation in response to high K+ or Na+ salt diets. Hypertensive Cyp2c44(-/-) mice show a hyperactive ENaC and reduction in ERK1/2 and ENaC subunit phosphorylation.8,11 In regards to EET regiosomeric actions on ENaC, 11,12-EET inhibits ENaC to a greater extent than 14,15-EET and 8,9-EET had no effect on ENaC activity.11 11,12-EET can inhibit basolateral inwardly rectifying K+ channels that results in cell membrane depolarization to reduce the driving force for Na+ entry across the apical membrane.20,24 Another renal epithelial cell action attributed to 11,12-EET is stimulation of apical large-conductance Ca2+-activated K+ epithelial channels that could contribute to renal K+ secretion in response to high K+ intake.8,25,26 Interestingly, 11,12-EET is the major product of the mouse Cyp2c44 and is generated in the CCD and increases in response to a high K+ or Na+ salt diet.11,20 The inability of Cyp2c44 -/- mice to increase 11,12-EET in response to either a high Na+ or K+ diet and the lack of actions on K+ channels and ENaC in the CCD results in salt-sensitive hypertension. Taken together these findings clearly demonstrate a critical role for renal CYP2C enzymes in fluid and electrolyte homeostasis and blood pressure control.
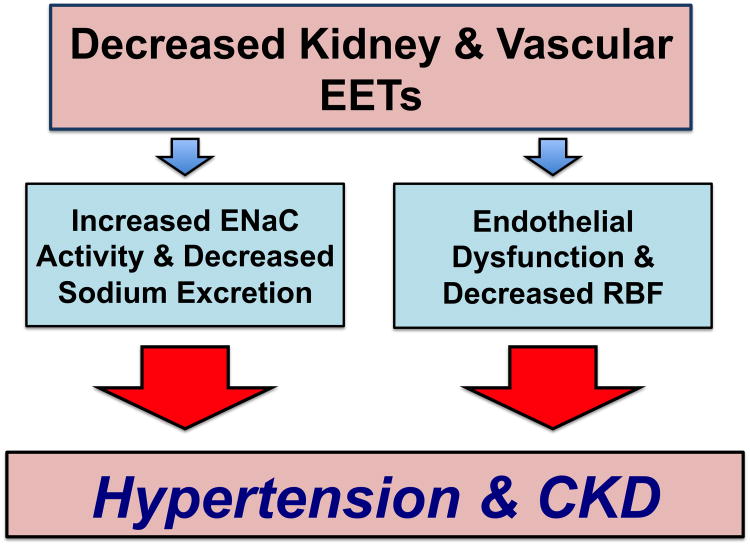
Figure 1. Cytochrome P450 epoxygenase metabolites, hypertension, and chronic kidney disease (CKD).
Decreased epoxyeicosatrienoic acids (EETS) contribute to enhanced epithelial sodium channel (ENaC) activity, endothelial dysfunction, and decreased renal blood flow (RBF). These changes in kidney and vascular function contribute to hypertension and CKD.
Vascular Endothelial Dysfunction
EETs also contribute importantly to endothelial function in the pathology of hypertension and cardiovascular diseases (Figure 1).7,8 Numerous studies have shown that EETs are an EDHF and are critical for proper regulation of resistance arteries and arterioles.7,9,10,27 EETs activate vascular smooth muscle cell large-conductance calcium-activated K+ channels (KCa) through a cAMP and protein kinase A dependent mechanism.28,29 Vascular expression of epoxygenase enzymes and generation of EETs is decreased in cardiovascular diseases.7,14,18,30 Decreased renal microvessel CYP2C11, CYP2C23, and CYP2J expression in the obese Zucker rat and in rats fed a high fat diet is thought to contribute to increased blood pressure.30 Vascular EET levels are further reduced by increased sEH expression in obese Zucker rats and this has been demonstrated to contribute to endothelial dysfunction.30 Likewise, endothelial dysfunction and inflammation are associated with decreased plasma EET levels and increased sEH activity in humans with atherosclerotic disease.31-34 Reactive oxygen species that are elevated in hypertension can also reduce EET bioavailability and vasodilation in human coronary arterioles.35,36 Thus, decreased vascular EET levels significantly contribute to the progression of cardiovascular disease and organ damage in hypertension.
Inflammation
Inflammation is considered a major player in hypertension and the associated progression of kidney disease. Kidney specific elevations in T-cells have also been implicated in numerous animal models of hypertension.37-39 Recent studies have implicated kidney selective increases in tumor necrosis factor-α (TNF-α) in the development of angiotensin II-dependent hypertension and associated kidney disease.37 Likewise, a contribution for increased sEH activity and decreased EET levels has been demonstrated for the inflammation and renal injury associated with hypertension.7,18,22 On the flip side, increasing EET levels by genetic disruption of Ephx2 decreased inflammation and attenuated the progression of renal damage associated with salt-sensitive hypertension.40 Interestingly, expression of human CYP2C8 or CYP2J2 to increase mouse endothelial cell EET generation decreased blood pressure, enhanced vasodilatory responses, and decreased renal injury in angiotensin high salt hypertension.41 These CYP2C8 and CYP2J2 transgenic miceor Ephx2 -/- mice also exhibited decreased vascular nuclear factor (NF)-κB signaling and inflammation in response to endotoxin.42 This is in agreement with the increasing amount of published data that EETs decrease vascular inflammation through inhibition of phospho-IKK-derived NF-κB activation.7,12,40,42 Therefore, evidence indicates that decreased EETs or increased sEH activity contribute to the vascular inflammation and pathogenesis of renal injury in hypertension and that increasing EET bioavailability can counteract disease progression.
Human Polymorphisms
There is also evidence in humans that decreased EET levels contribute to hypertension. Human CYP2C8 and CYP2C9 are the major epoxygenases whereas CYP2J2 has both epoxygenase and ω-1 hydroxylase activity.43 A number of CYP2C8 and CYP2C9 gene variants (2C8*2, 2C8*3, 2C9*2, and 2C9*3) demonstrate reduced arachidonic acid epoxidation rates.31,43 Analysis of Caucasian and African American cohorts failed to demonstrate an association between these variants and hypertension.44 On the other hand, the frequency of the CYP2C9*3 allele was lower in a subset of Chinese women with hypertension.45 A common polymorphism in the CYP2J2 gene, CYP2J2*7allele reduces CYP2J2 transcription, reduces plasma EET levels, and has been demonstrated to be associated with increased risk for essential hypertension in a Russian population.46 However, other studies have that the found CYP2J2*7allele associates with lower risk or no modification in the risk of developing hypertension.47 Although polymorphisms of the sEH gene EPHX2 have demonstrated associations to cardiovascular diseases, a majority of the studies have reported no association between EPHX2 variants and essential hypertension.31 Differences in the results of these genetic association studies could be attributed to factors including ethnicity of the population studied, small cohorts, gender effects, and environmental factors.
Despite the discrepancies in the genetic population studies there is more convincing evidence linking decreased EETs to hypertension when evaluating EET bio availablility and vascular responses. Genetic variations in EPHX2 have been demonstrated to affect the magnitude of human forearm vasodilator responses.48 There is a reduction in the forearm vasodilator response in Caucasian Americans that have the Arg55 variant allele which increases sEH activity and would be expected to decrease EET availability.48 Whereas, African Americans that that have the Gln287 variant allele that decreases sEH activity exhibit enhanced forearm bradykinin-mediated vasodilator responses.48 Healthy human volunteers exhibit slightly reduced basal forearm blood flow in the presence of the CYP inhibitor fluconazole whereas it did not alter radial artery blood flow in hypertensive patients in the presence or absence of nitric oxide inhibition.49 In addition, fluconazole decreased local plasma EET levels in control but not hypertensive individuals.49 Humans with hypertension also demonstrated decreased flow-mediated dilation an indicator of endothelial dysfunction that was associated with a reduced EET levels.50 These findings demonstrate that hypertensive patients where EET levels are genetically or pharmacological manipulated have vasodilator responses that differ from those of healthy volunteers. Thus in addition to nitric oxide, EET levels contribute importantly to endothelial function in hypertensive patients.
Overall, these experimental findings in rodents and humans have generated interest in developing pharmacological means to increase EETs that could potentially lower blood pressure and protect the kidney in hypertension.
Therapeutic Approaches – Hypertension and Kidney Diseases
Over the past decade epoxyeicosatrienoic acid and soluble epoxide hydrolase (sEH) enzyme based drugs have been developed with anti-hypertensive and kidney protective properties that will be particularly beneficial for hypertensive patients that develop chronic kidney disease (Figure 2).51,52 Carbamate urea sEH inhibitors were developed and demonstrated to lower blood pressure and decrease renal injury in animal models of hypertension.15,18,51 Further development of sEH inhibitors progressed rapidly and has resulted in clinical trials for hypertension, diabetes, and more recently, chronic obstructive pulmonary disease.51,53 This development of sEH inhibitors has been extensively chronicled in a number of excellent review articles.51,54,55
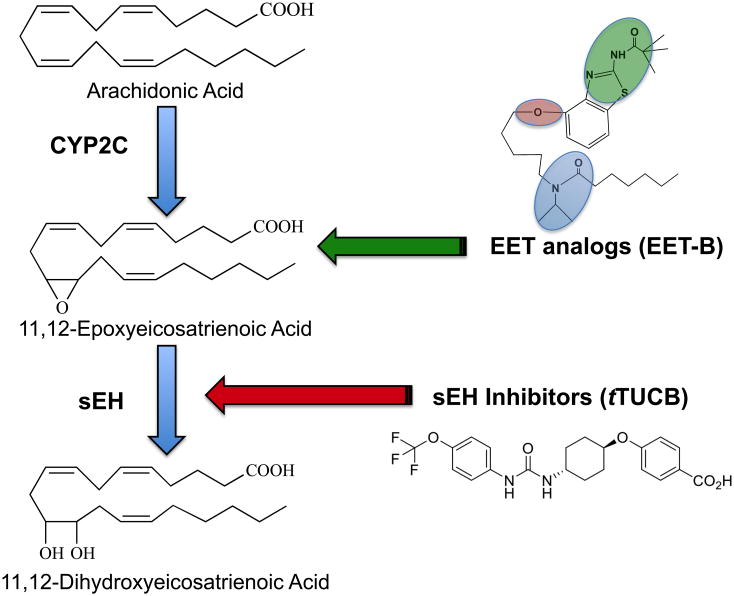
Figure 2. Therapeutic manipulation of epoxygenase metabolites.
Arachidonic acid is converted to epoxyeicosatrienoic acids (EETs) by cytochrome P450 (CYP2C) epoxygenase enzymes. EETs primary metabolic fate is conversion to dihydroxyeicosatrienoic acids (DHETs) by the soluble epoxide hydrolase (sEH) enzyme. EET analogs and sEH inhibitors are two therapeutic approaches being tested to combat hypertension and kidney injury. EET-B has three structural attributes: (1) an acidic or hydrogen bonding replacement (green) for the C(1)-carboxylate to avoid esterification and β-oxidation; (2) a cis-Δ8,9 -olefin or equivalent (red); (3) an epoxide isostere (mimetic) (blue) to obviate sEH metabolism.
More recent developments with sEH inhibitors are keeping enthusiasm for their potential use in hypertension and chronic kidney disease at a high level. In a recent controlled clinical trial with peripheral arterial disease participants that were fed flaxseed containing α-linolenic acid for six months had decreased blood pressure.56 α-Linolenic acid was demonstrated in an inhibitor screening assay to decrease sEH activity and the anti-hypertensive effects of flaxseed feeding were associated with a decrease in plasma sEH-derived oxylipins.56 As for chronic kidney disease a recently published study demonstrated that Ephx2 deficiency or sEH inhibition in mice decreased renal inflammation and fibrosis associated with unilateral ureteral obstruction.57 The anti-inflammatory and fibroprotective effects in unilateral ureteral obstruction kidneys was via PPAR activation and down regulation of NF-κB, TGFβ1/Smad3 inflammatory signaling.57 Another of the more recent findings is that dietary fatty acid composition can enhance the effectiveness of sEH inhibitors in cardiovascular diseases.58 Fish oil or ω-3 polyunsaturated fatty acid diet rich in eicosapentaenoic acid (EPA) and docosaheaenoic acid (DHA) coupled with sEH inhibitors lowers blood pressure and provides superior anti-inflammatory effects in angiotensin II-dependent hypertension.58 EPA-derived epoxyeicosatetraenoic acids (EEQs) and DHA-derived epoxydocosapentaenoic acids (EDPs)are of particular interest because these epoxygenase metabolites of ω-3 polyunsaturated fatty acid have been demonstrated to protect from coronary heart disease and a trial fibrillation.34,59,60 These newer findings suggest that other fatty acid epoxides could be beneficial and that sEH inhibitors still have promise for hypertension and kidney disease.
Significant recent advancements in the development of robust EET analogs that mimic the actions of endogenous EETs position them as a potential therapeutic for renal and cardiovascular diseases. First generation EET analogs were methyl esters and sulfonimide substitutions of the carboxylic acid which obviated esterification and resisted β-oxidation.61 The next generation of EET analogs removed the 1,4-diene responsible for autoxidation and replaced the labile epoxide with bio-isosteres that resist metabolism (Figure 2).61,62 Studies of the second generation of EET analogs assessing vascular inflammation and dilation resulted in the following structural requirements: an acidic carboxyl group, Δ8 olefin bond, 20-carbon chain length, and a cis epoxide.61,62
EET analogs have substantial promise for the treatment of kidney and cardiovascular diseases. One such EET analog that has been successfully used in vivo in rodents is the aspartic amide of 11-nonyloxy-undec-8(Z)-enoic acid, NUDSA.63,64 NUDSA has been found to decrease blood pressure, improve metabolic status in metabolic syndrome, and provide cardio-protection in ischemic injury.63-65 Overall, the effects of NUDSA are linked to its ability to reduce inflammation and cell death, supporting the notion that EET analogs could be beneficial in renal pathologies. In support of this notion orally active EET analogs, EET-A and EET-B were found to protect the kidneys from cisplatin-induced nephrotoxicity.66 Attenuated nephrotoxicity correlated with reduced inflammation, oxidative stress, and decreased apoptosis through a reduction in Bcl-2 protein mediated proapoptotic signaling, reduced renal capase12 expression, and reduced renal caspase-3 activity.66 EET-A and EET-B have been shown to dramatically decrease blood pressure and prevent hypertensive renal injury.22,67 EET-A lowers blood pressure in angiotensin dependent hypertension and in Cyp2c44-/- mice with salt-sensitive hypertension.22 Additional findings demonstrated that EET-A inhibits ENaC activity in cultured CCD cells and reduced kidney expression of ENaC subunits in angiotensin II hypertension.22 Interestingly, kidney protection in Dahl SS rats independent of blood pressure lowering was demonstrated following two weeks of EET-B treatment. EET-B decreased renal injury by reducing oxidative stress, endoplasmic reticulum stress, and macrophage infiltration.67 Thereare two potential explanations for the lack of blood pressure by EET-B in the Dahl SS rats. First, EET-B does not inhibit ENaC in the same manner as EET-A.67 Although EET-B treated Dahl SS rats had decreased macrophage infiltration, EET-B failed to lower kidney T cell levels which is known to be a major contributor to the elevated blood pressure in this animal model of salt-sensitive hypertension.38,39,67 Taken together, these diverse biological actions and development of oral EET analogs demonstrate their therapeutic potential for hypertension and CKD.
Perspectives
It is now established that a reduction in EETs can contribute to hypertension and the associated renal injury and that approaches to increase EETs have therapeutic potential. As with every therapeutic approach there is always a down side that is of concern. In the case of EETs, that concern has been their angiogenic and tumorigenic actions.51,68,69 Although initial studies demonstrated that EETs or sEH inhibition enhanced angiogenesis, tumorigensis, and resulted in metastasis; recent studies have shown that sEH inhibition or Ephx2 gene deficiency inhibits inflammatory bowel tumor development and supports the notion that EETs can inhibit cancer by blocking inflammation.70,71 Interestingly dual inhibition of COX-2 and sEH synergistically inhibits primary tumor growth and metastasis by suppressing tumor angiogenesis.72 EET analogs also failed to increase cultured tumor cell proliferation and did not interfere with the ability of cisplatin to kill tumor cells.66 Although these findings do not eliminate the concern for unwanted tumorigenesis with EET based therapies, this concern appears to be considerably less than originally thought.
Other considerations for blood pressure regulation and hypertension are differences in sEH and EET levels between males and females and central nervous system effects. Cerebral vascular sEH expression is higher in male mice and females have increased EET-mediated protection from ischemic injury when compared to males.73,74 Furthermore, sEH inhibition abolishes sex-specific differences in endothelial cell survival and ischemic brain injury.73,74 Brain sEH inhibition via intracerbroventricular deliver of AUDA increases blood pressure and heart rate in spontaneously hypertensive rats (SHR).75 In contrast, neuronal specific expression of sEH to increase activity 3-fold failed to increase arterial blood pressure in mice.76 Sex differences have also been found with regards to blood pressure regulation. Basal blood pressure in Ephx2 -/- mice was lower in males but not females when compared to wild-type mice.77 This decrease basal blood pressure in male Ephx2 -/- mice has not been observed when other colonies on various genetic backgrounds were generated.51,78 More recently, renal vascular EET levels were higher in female SHR compared to males.79 In this study ten-day treatment with the sEH inhibitor AUDA increased EET levels but did not lower blood pressure in either male or female SHR.79 This finding is consistent with previous studies that have found variable effects of sEH inhibition on blood pressure in the SHR.51 These experimental findings highlight the need to consider brain actions of EETs and sex-specific actions of EETs when evaluating sEH inhibitors and EET analogs for hypertension and CKD.
The further development of EET analogs will be greatly enhanced if protein targets and receptors for EETs can be identified. Although the identity of EET binding sites/receptors remain elusive, EETs activate renal and coronary vascular smooth muscle cell KCa channels through G protein (Gαs) – dependent mechanism.9,10,27,28,80,81 Other investigations provide evidence that cAMP and protein kinase A (PKA) are key signaling molecules required for KCa channel activation.27-29 Likewise, endothelial cell action of 11,12-EET are PKA dependent and require the Gs protein.82 There are also differences in potency and activity when comparing 11,12-EET and 14,15-EET in various vascular tissues.7,9,10 11,12-EET is more potent than 14,15-EET in renal arterioles whereas rat mesenteric resistance arteries respond similarly to 11,12-EET and 14,15-EET.7 In addition, mesenteric resistance artery flow-induced dilation was inhibited bythe 14,15-EET antagonist, 14,15-DHE5ZE, but unchanged by the 11,12-EET antagonist, 11,12,20-THE8ZE.83 These findings suggest unique biological activities and the potential for multiple vascular EET binding sites/receptors.
Recent studies on the contribution of EETs to inflammation, kidney function, and blood pressure regulation in hypertension have shed light on their potential as a target for therapeutic intervention. Thus, there is a bright future for sEH inhibitors and EET analogs as novel therapies to effectively treat hypertension and stop the progression of CKD to renal failure.
Acknowledgments
Sources of Funding: These studies were supported by NIH grant DK38226, and an American Heart Association Midwest Affiliate Grant-in-Aid.
Footnotes
Disclosures: Dr. Imig has patents and patent applications that cover the composition of matter for EET analogs.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Collins AJ. End-stage renal disease in the United States: and update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–2648. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association. High blood pressure statistics. Available at: http://www.americanheart.org/presenter.jhtml?identifier=4621.
- 4.Crook ED. the role of hypertension, obesity, and diabetes in causing renal vascular disease. American Journal of Medical Sciences. 1999;317:183–188. doi: 10.1097/00000441-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Barri YM. Hypertension and kidney diseases: a deadly connection. Curr Hypertens Rep. 2008;10:39–45. doi: 10.1007/s11906-008-0009-y. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M ALLHAT Collaborative Research Group. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Hypertension. 2014;64:1012–1021. doi: 10.1161/HYPERTENSIONAHA.114.03850. [DOI] [PubMed] [Google Scholar]
- 7.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiological Reviews. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capdevila J, Wang W. Role of cytochrome P450 exoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens. 2013;22:163–169. doi: 10.1097/MNH.0b013e32835d911e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 10.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 11.Capdevila JH, Pidkovka N, Mei S, Gong Y, Falck JR, Imig JD, Harris RC, Wang WH. The Cyp2c44 epoxygenase regulates epithelial sodium channel activity and the blood pressure responses to increased dietary salt. J Biol Chem. 2014;289:4377–4386. doi: 10.1074/jbc.M113.508416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94:2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with salt-sensitive hypertension. Hypertension. 2003;41:709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 15.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 16.Messer-Létienne I, Bernard N, Roman RJ, Sassard J, Benzoni D. Cytochrome P-450 arachidonate metabolite inhibition improves renal function in Lyon hypertensive rats. Am J Hypertens. 1999;12:398–404. doi: 10.1016/s0895-7061(98)00256-8. [DOI] [PubMed] [Google Scholar]
- 17.Kaergel E, Muller DN, Honeck H, Theuer J, Shagdarsuren E, Mullally A, Luft FC, Schunck WH. P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension. 2002;40:273–279. doi: 10.1161/01.hyp.0000029240.44253.5e. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 19.Karara A, Makita K, Jacobson HR, Falck JR, Guengerich FP, DuBois RN, Capdevila JH. Molecular cloning, expression, and enzymatic characterization of the rat kidney cytochrome P-450 arachidonic acid epoxygenase. J Biol Chem. 1993;268:13565–13570. [PubMed] [Google Scholar]
- 20.Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH. Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension. 2012;59:339–347. doi: 10.1161/HYPERTENSIONAHA.111.178475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450epoxygenases lowers blood pressure and attenuates hypertension-induced renalinjury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan MA, Pavlov TS, Christian SV, Neckar J, Staruschenko A, Gauthier KM, Capdevila JH, Falck JR, Campbell WB, Imig JD. Epoxyeicosatrienoic acid (EET) analog lowers blood pressure through vasodilation and sodium channel inhibition. Clinical Science. 2014;127:463–474. doi: 10.1042/CS20130479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa K, Holla VJ, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capdevila J, Wang W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens. 2013;22:163–169. doi: 10.1097/MNH.0b013e32835d911e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct. J Am Soc Nephrol. 2009;20:513–523. doi: 10.1681/ASN.2008040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun P, Lin DH, Yue P, Jiang H, Gotlinger KH, Schwartzman ML, Falck JR, Goli M, Wang WH. High potassium intake enhances the inhibitory effect of 11,12-EET on ENaC. J Am Soc Nephrol. 2010;21:1667–1677. doi: 10.1681/ASN.2009111110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci. 2007;28:448–452. doi: 10.1016/j.tips.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11, 12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 29.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Dey A, Romanko O, Stepp DW, Wang MH, Jin L, Pollock JS, Webb RC, Imig JD. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol RegulIntegr Comp Physiol. 2005;288:R188–R196. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 31.Bellien J, Joannides R. Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol. 2013;61:188–196. doi: 10.1097/FJC.0b013e318273b007. [DOI] [PubMed] [Google Scholar]
- 32.Yang T, Peng R, Guo Y, Shen L, Zhao S, Xu D. The role of 14,15-dihydroxyeicosatrienoic acid levels in inflammation and its relationship to lipoproteins. Lipids Health Dis. 2013;12:151. doi: 10.1186/1476-511X-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellien J, Remy-Jouet I, Iacob M, Blot E, Mercier A, Lucas D, Dreano Y, Gutierrez L, Donnadieu N, Thuillez C, Joannides R. Impaired role of epoxyeicosatrienoic acids in the regulation of basal conduit artery diameter during essential hypertension. Hypertension. 2012;60:1415–1421. doi: 10.1161/HYPERTENSIONAHA.112.201087. [DOI] [PubMed] [Google Scholar]
- 34.Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, Luft FC, Weylandt K, Schunck WH. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J Lipid Res. 2014;55:1150–1164. doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension. 2014;64:1275–1281. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL PhysGen Knockout Program. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–F448. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, DeGraff LM, Lih FB, Foley J, Bradbury JA, Graves JP, Tomer KB, Falck JR, Zeldin DC, Lee CR. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, Mugge A, Lindpaintner K, Huesing A, Maisch B, Zeldin DC, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–2136. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreisbach AW, Japa S, Sigel A, Parenti MB, Hess AE, Srinouanprachanh SL, Rettie AE, Kim H, Farin FM, Hamm LL, Lertora JJ. The Prevalence of CYP2C8, 2C9, 2J2, and soluble epoxide hydrolase polymorphisms in African Americans with hypertension. Am J Hypertens. 2005;18:1276–1281. doi: 10.1016/j.amjhyper.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Yu BN, Luo CH, Wang D, Wang A, Li Z, Zhang W, Mo W, Zhou HH. CYP2C9 allele variants in Chinese hypertension patients and healthy controls. Clin Chim Acta. 2004;348:57–61. doi: 10.1016/j.cccn.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 46.Polonikov AV, Ivanov VP, Solodilova MA, Khoroshaya IV, Kozhuhov MA, Ivakin VE, Katargina LN, Kolesnikova OE. A common polymorphism G-50T in cytochrome P450 2J2 gene is associated with increased risk of essential hypertension in a Russian population. Dis Markers. 2008;24:119–126. doi: 10.1155/2008/626430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King LM, Gainer JV, David GL, Dai D, Goldstein JA, Brown NJ, Zeldin DC. Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet Genomics. 2005;15:7–13. doi: 10.1097/01213011-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Lee CR, Pretorius M, Schuck RN, Burch LH, Bartlett J, Williams SM, Zeldin DC, Brown NJ. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension. 2011;57:116–122. doi: 10.1161/HYPERTENSIONAHA.110.161695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C, Joannides R. Crucial role of NO and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension. 2006;48:1088–1094. doi: 10.1161/01.HYP.0000246672.72188.bd. [DOI] [PubMed] [Google Scholar]
- 50.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, Gutierrez L, Vendeville C, Dreano Y, Mercier A, Thuillez C, Joannides R. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation. 2012;125:1266–1275. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 51.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;256:329–335. doi: 10.1097/FJC.0b013e3181e96e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A Study to Assess the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Doses of GSK2256294 in Healthy Volunteers, and Single and Repeat Doses of GSK2256294 in Adult Male Moderately Obese Smokers. http://clinicaltrials.gov/ct2/show/NCT01762774?term=epoxide+hydrolase&rank=2.
- 54.Morisseau C, Hammock BD. Gerry Brooks and epoxide hydrolases: four decades to a pharmaceutical. Pest Manag Sci. 2008;64:594–609. doi: 10.1002/ps.1583. [DOI] [PubMed] [Google Scholar]
- 55.Marino JP., Jr Soluble epoxide hydrolase, a target with multiple opportunities for cardiovascular drug discovery. Curr Top Med Chem. 2009;9:452–463. doi: 10.2174/156802609788340805. [DOI] [PubMed] [Google Scholar]
- 56.Caligiuri SP, Aukema HM, Ravandi A, Guzman R, Dibrov E, Pierce GN. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an α-linolenic acid-induced inhibition of solubleepoxide hydrolase. Hypertension. 2014;64:53–59. doi: 10.1161/HYPERTENSIONAHA.114.03179. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Imig JD, Yang J, Hammock BD, Padanilam BJ. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol. 2014;307:F971–F980. doi: 10.1152/ajprenal.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, Dong H, Iosif AM, Liu JY, Weiss RH, Chiamvimonvat N, Imig JD, Hammock BD. Anti-inflammatory effects of omega-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin II dependent hypertension. J Cardiovasc Pharmacol. 2013;62:285–297. doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westphal C, Konkel A, Schunck WH. CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011;96:99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Falck JR, Wallukat G, Puli N, Goli M, Arnold C, Konkel A, Rothe M, Fischer R, Müller DN, Schunck WH. 17(R),18(S)-epoxyeicosatetraenoic acid, a potent eicosapentaenoic acid (EPA) derived regulator of cardiomyocyte contraction: structure-activity relationships and stable analogues. J Med Chem. 2011;54:4109–4118. doi: 10.1021/jm200132q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic Acid Analogs and Vascular Function. Curr Med Chem. 2010;17:1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falck JR, Koduru SR, Mohapatra S, Manne R, Atcha R, Manthati VL, Capdevila JH, Christian S, Imig JD, Campbell WB. 14,15-Epoxyeicosa-5,8,11-trienoic acid(14,15-EET) surrogates:carboxylate modifications. J Med Chem. 2014;57:6965–6972. doi: 10.1021/jm500262m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR, Sangra B, Anjaiah S, Manthati VL, Reddy DS, Falck JR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Frontiers in Vascular Physiology. 2010;1:157. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol ExpTher. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batchu SN, Lee SB, Qadhi RS, Chaudhary KR, El-Sikhry H, Kodela R, Falck JR, Seubert JM. Cardioprotective effect of a dual acting epoxyeicosatrienoic acid analogue towards ischaemia reperfusion injury. Br J Pharmacol. 2011;162:897–907. doi: 10.1111/j.1476-5381.2010.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan MAH, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27:2946–2956. doi: 10.1096/fj.12-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan MAH, Manthati V, Errabelli R, Pavlov TS, Staruschenko A, Falck JR, Imig JD. An orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl salt sensitive rat. Hypertension. 2013;62:905–913. doi: 10.1161/HYPERTENSIONAHA.113.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnés CM, Mammoto A, Mammoto T, Luria A, Benny O, Chaponis DM, Dudley AC, Greene ER, Vergilio JA, Pietramaggiori G, Scherer-Pietramaggiori SS, Short SM, Seth M, Lih FB, Tomer KB, Yang J, Schwendener RA, Hammock BD, Falck JR, Manthati VL, Ingber DE, Kaipainen A, D'Amore PA, Kieran MW, Zeldin DC. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122:178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pozzi A, Popescu V, Yang S, Mei S, Shi M, Puolitaival SM, Caprioli RM, Capdevila JH. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor alpha are arachidonic acid epoxygenase-mediated. J Biol Chem. 2010;285:12840–12850. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, 1, Li H, Dong H, Liao J, Hammock BD, Yang GY. Soluble epoxide hydrolase deficiency inhibits dextran sulfate sodium-induced colitis and carcinogenesis in mice. Anticancer Res. 2013;33:5261–5271. [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang W, 1, Liao J, Li H, Dong H, Bai H, Yang A, Hammock BD, Yang GY. Reduction of inflammatory bowel disease-induced tumor development in IL-10 knockout mice with soluble epoxide hydrolase gene deficiency. Mol Carcinog. 2013;52:726–738. doi: 10.1002/mc.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang G, Panigrahy D, Hwang SH, Yang J, Mahakian LM, Wettersten HI, Liu JY, Wang Y, Ingham ES, Tam S, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci U S A. 2014;111:11127–11132. doi: 10.1073/pnas.1410432111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arterioscler Thromb Vasc Biol. 2012;32:1936–1942. doi: 10.1161/ATVBAHA.112.251520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. JCereb Blood Flow Metab. 2009;29:1475–1481. doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sellers KW, Sun C, Diez-Freire C, Waki H, Morisseau C, Falck JR, Hammock BD, Paton JF, Raizada MK. Novel mechanism of brain soluble epoxide hydrolase-mediatedblood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19:626–628. doi: 10.1096/fj.04-3128fje. [DOI] [PubMed] [Google Scholar]
- 76.Bianco RA, Agassandian K, Cassell MD, Spector AA, Sigmund CD. Characterizationof transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res. 2009;1291:60–72. doi: 10.1016/j.brainres.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressureregulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 78.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282:2891–2898. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moulana M, Hosick K, Stanford J, Zhang H, Roman RJ, Reckelhoff JF. Sex differences in blood pressure control in SHR: lack of a role for EETs. Physiol Rep. 2(5) doi: 10.14814/phy2.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 81.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol ExpTher. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 82.Ding Y, Frömel T, Popp R, Falck JR, Schunck WH, Fleming I. The biological actions of 11,12-epoxyeicosatrienoic acid in endothelial cells are specific to the R/S-enantiomer and require the G(s) protein. J Pharmacol Exp Ther. 2014;350:14–21. doi: 10.1124/jpet.114.214254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bukhari IA, Shah AJ, Gauthier KM, Walsh KA, Konduru SR, Imig JD, Falck JR, Campbell WB. 11,12,20-Trihydroxy-eicosa-8(Z)-enoic acid: a selective inhibitor of 11,12-EET induced relaxations of bovine coronary and rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H1574–H1583. doi: 10.1152/ajpheart.01122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]