Abstract
Background
Very asymmetric papilledema in idiopathic intracranial hypertension (IIH) is rare, and few studies have dealt with this atypical presentation of IIH. Our aim was to describe the clinical and radiologic features of patients with IIH and very asymmetric papilledema.
Methods
We identified all adult patients from our IIH database with very asymmetric papilledema defined as a ≥2 modified Frisén grade difference between the two eyes. Demographic data and initial symptoms were collected, and all brain imaging studies performed at our institution were reviewed.
Results
Of the 559 adult patients with definite IIH, 20 (3.6%; 95%CI: 2.3-5.6%) had very asymmetric papilledema at initial evaluation. They were older (39 versus 30 years; p<0.001), had lower cerebrospinal opening pressure (35.5 versus 36 cm of water; p=0.03), and were more likely to be asymptomatic compared to patients with symmetric papilledema (27% versus 3%; p<0.001). Visual fields were worse on the side of the highest-grade papilledema (p=0.02). The bony optic canal was smaller on the side of the lowest-grade edema in all 8 patients (100%) in whom the imaging was sufficient for reliable measurements (p=0.008).
Conclusion
IIH with very asymmetric papilledema is uncommon. Very asymmetric papilledema may result from differences in size of the bony optic canals, supporting the concept of compartmentation of the peri-optic subarachnoid space.
Keywords: Idiopathic intracranial hypertension, papilledema, bony optic canal, compartmentation of the peri-optic subarachnoid spaces
Introduction
Very asymmetric papilledema in idiopathic intracranial hypertension (IIH) is an uncommon finding that can raise concern for alternate diagnoses, such as unilateral optic neuropathy. Few studies have systematically addressed the issue of asymmetric papilledema in IIH, and most are only case reports or small case series (1-11). The largest series of 38 patients focused specifically on visual function outcome (12), and only one study has detailed orbital imaging findings (13). Very asymmetric papilledema offers a unique opportunity to study factors proposed in the pathogenesis of papilledema. Although several mechanisms have been suggested to explain very asymmetric papilledema, such as optic nerve sheath defects and loss of lamina cribrosa compliance (1,6), its mechanism remains unclear.
Our aim was to describe the clinical and radiologic features of patients with IIH and very asymmetric papilledema and to characterize factors associated with this unusual presentation of IIH.
Methods
Clinical evaluation
Our study was approved by the Emory University Institutional Review Board. Using our established database of patients seen in our center between 1989 and 2013, we identified all adult patients (age 18 years or older) with definite IIH according to the most recent modified Dandy criteria (14). Although this study was a retrospective chart review, all patients were evaluated in a standardized fashion, and all had fundus photographs at presentation.
We first selected all IIH patients from our IIH database, who had been recorded as having a ≥1 Frisén grade difference in papilledema between the two eyes at initial presentation. All fundus photographs were then unpaired and graded independently using the modified Frisén scale (15) in random order by two neuro-ophthalmologists (SB and BBB), who were masked to the patients’ clinical data. In case of disagreement, a third neuro-ophthalmologist (VB) was asked to grade the papilledema. We defined very asymmetric papilledema as a ≥2 modified Frisén grade difference between the two eyes, and selected all IIH patients with at least 2 grades of difference in their papilledema.
Demographic data of all patients with very asymmetric papilledema, including age, gender, body mass index (BMI) and race were collected. Initial symptoms (decreased vision, transient visual obscurations, diplopia, tinnitus, headaches), Snellen visual acuity, Humphrey visual fields, and cerebrospinal fluid (CSF) opening pressure (OP) were recorded.
Radiologic evaluation
We reviewed all brain imaging and selected those performed at our institution (either magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT)). MRI was performed at either 3.0-Tesla (Siemens Trio, Erlangen, Germany) or 1.5-Tesla (Siemens Avanto, Erlangen, Germany or GE Signa, Milwaukie, Wisconsin) using a standard head coil. The MRI protocol included a T2-weighted sequence at a slice thickness of 5-mm or 3-mm. Patients received intravenous gadolinium contrast material at a standard dose (0.1 mmol/kg Multihance, Bracco Diagnostics Inc.), followed by post-contrast axial T1-weighted and sagittal volumetric T1-weighted images. The MRV protocol included either a non-contrast MRV using an oblique sagittal 2D time-of-flight (TOF) technique or a contrast-enhanced MRV which protocol included an axial pre-contrast MRV mask, followed by repetition of the sequence after contrast administration. The pre-contrast MRV dataset was subtracted from the post-contrast dataset, and multiple oblique maximum intensity projections were generated from this subtracted dataset. The CT examination included orbital cuts and was performed on a 64 detector row scanner (GE Lightspeed VCT, Milwaukee, Wisconsin) using 70 cc iodinated contrast agent (Isovue 370, Bracco Imaging) with 5-mm slice reconstructions.
All MRI and CT examinations were reviewed by an experienced neuroradiologist (AS) blinded to the neuro-ophthalmic examination. Previously described orbital imaging findings associated with increased intracranial pressure were recorded on all patients with imaging: protrusion of the optic nerve head, flattening of the posterior sclera, increased peri-optic nerve CSF [0: none; 1: mild; 2: moderate; 3: severe (measured at the level of maximal dilation of the orbital optic nerve sheath)], and vertical tortuosity of the intra-orbital optic nerve. The presence of meningoceles, as defined by CSF containing structures outside the expected confines of the cranial vault, was recorded. The presence or absence and side of transverse sinus stenosis was evaluated from either the contrast-enhanced MRV, post-contrast T1-weighted (16), 2D TOF MRI, post-contrast CT, or transverse sinus flow voids on T2-weighted images when there was no contrast-enhanced image set. When possible, and before recording the aforementioned radiologic findings, cross-sectional area of both the right and left optic canal were measured using either pre-contrast volumetric 1 mm isotropic T1-weighted images, by reformatting into a coronal oblique plane orthogonal to the axis of each optic canal, or thin-section CT examination that was also reformatted in a similar fashion using 0.625 mm images to obtain cross sectional area (fig. 1).
Figure 1.
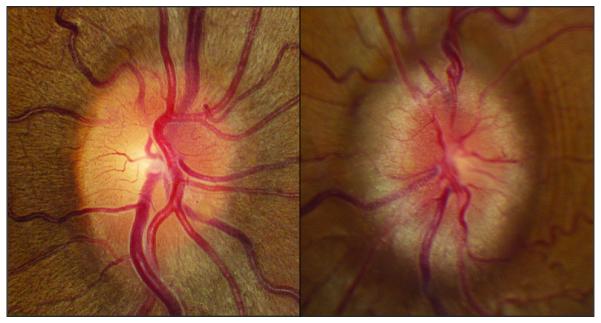
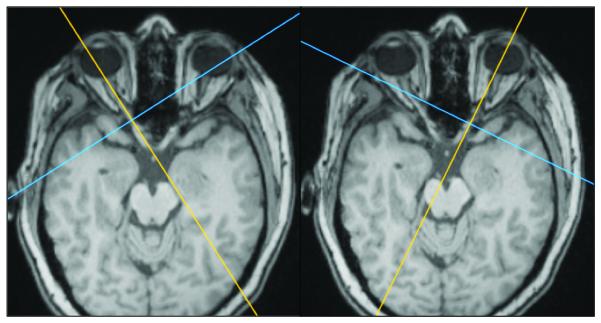
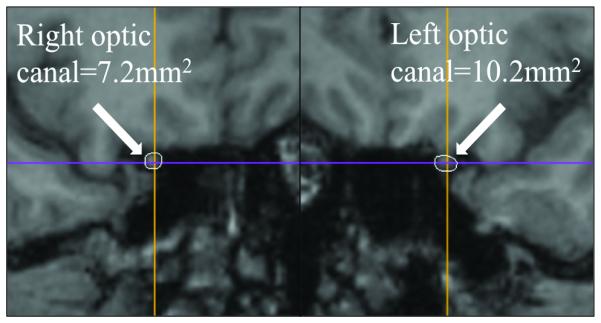
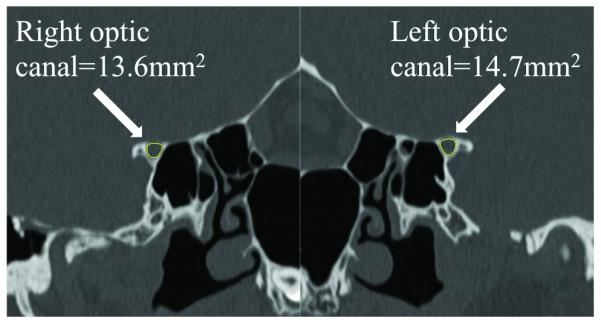
Cross-sectional area measurement technique. Figures on the right and left match with the right and left side respectively. Figures A, B, and C come from the same patient, and measurement was obtained by using precontrast volumetric T1-weighted MRI (B and C). Figure D come from another patient to illustrate the technique using thin-section CT scan reconstruction.
A. Optic disc appearance. Asymmetric papilledema graded 2 in the right eye, and grade 4 in the left eye.
B. Positioning of the guides according to axial plane.
C and D. Measurement of the cross-sectional area of each optic canal using MRI and CT scan (D).
Statistical analysis
All statistical analyses were performed with R: A language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org). Continuous and ordinal variables were compared between groups using the Mann-Whitney U test. Pearson’s Chi Square with Yates’ continuity correction or Fisher's exact test, as appropriate, were used to compare categorical variables. The eyes of patients with very asymmetric papilledema were compared using the Wilcoxon signed rank test for continuous variables and paired proportion test for categorical variables. These tests were two tailed, and significance was set at 5%.
Results
Of the 559 adult patients with definite IIH seen over 24 years, 20 (3.6%; 95%CI: 2.3-5.6%) had very asymmetric papilledema defined as a ≥2 modified Frisén grade difference between two the eyes at initial evaluation. Patients with very asymmetric papilledema were significantly older (39 versus 30 years; p<0.001), had significantly lower CSF-OP (35.5 versus 36 cm of water; p=0.03), and were significantly more likely to be asymptomatic compared to patients with more symmetric papilledema (27% versus 3%; p<0.001) (Table 1).
Table 1.
Demographics, initial symptoms, and CSF opening pressure in IIH patients with asymmetric and symmetric papilledema
| Papilledema | Asymmetric, n=20 | Symmetric, n=539 | |
|---|---|---|---|
|
|
|||
| n [%] or median [IQR] | p | ||
| Demographics | |||
| Age, years | 39 [33-45] | 30 [25-36] | <0.001 |
| Gender, men | 3 [15%] | 39 [7%] | 0.20 |
| Race, white | 13 [65%] | 292 [54%] | 0.35 |
| BMI, kg/m2 | 37.8 [32.5-45.1] | 38.0 [32.2-44.0] | 0.83 |
| Initial symptoms | |||
| Headache | 7 [35%] | 417 [77%] | <0.001 |
| TVOs | 7 [35%] | 80 [15%] | <0.001 |
| Decreased vision* | 2 [9%] | 127 [24%] | <0.001 |
| Diplopia | 0 [0%] | 30 [6%] | 0.62 |
| Tinnitus | 0 [0%] | 37 [7%] | 0.64 |
| None | 6 [27%] | 17 [3%] | <0.001 |
| CSF-OP, cm | 35.5 [27.0-37.0] | 36.0 [31.0-44.0] | 0.03 |
-BMI: body mass index, TVO: transient visual obscurations, CSF OP: cerebrospinal fluid opening pressure, IQR: interquartile range
The highest-grade edema was on the right side in 9/20 patients (45%). Papilledema was strictly unilateral in 8/20 patients (40%), and was located on the right side in 3/8 patients (38%). When comparing the eye with the highest-grade edema to the fellow eye, visual fields were significantly worse (mean deviation, −3.0 versus −2.1 decibels; p=0.02), but visual acuities, and intraocular pressures were not different (Table 2).
Table 2.
Comparison of ophthalmic and radiologic features between the eye with the highest-grade edema versus the eye with the lowest-grade edema in patients with definite IIH and asymmetric papilledema.
| Eye with papilledema of the: |
|||
|---|---|---|---|
| Highest grade | Lowest grade | ||
| median [IQR] or n [%] | p | ||
|
|
|||
| Ophthalmic findings (n=20) | |||
| Modified Frisén grade | 3 [2; 4] | 1 [0; 2] | NA |
| VA, logMAR1 | 0.0 [−0.12; 0.7] | 0.0 [−0.12; 2.0] | 0.39 |
| IOP | 15.5 [13; 17] | 15.5 [13;18] | 0.90 |
| HVF, mean deviation2 (n=19) | −3.0 [−5.1; −2.1] | −2.1 [−2.9; −0,7] | 0.02 |
| Radiologic findings (n=12) | |||
| Optic canal measurement, mm2 (n=8) |
18.5 [13.6-29.8] | 15.8 [12.1-27.8] | 0.008* |
| Prominent peri-ON CSF | 6 [50%] | 0 [0%] | 0.01 |
| Scleral flattening | 8 [67%] | 3 [25%] | 0.07 |
| Vertical tortuosity of the ON | 1 [8%] | 1 [8%] | 1.0 |
| ON head protrusion | 9 [75%] | 3 [25%] | 0.07 |
| Meningocele | 5 [42%] | 5 [42%] | 1.0 |
| Transverse sinus stenosis | 11 [92%] | 10 [92%] | 1.0 |
Range provided instead of IQR,
All patients (100%) had a smaller optic canal on the side of the lowest grade papilledema.
Mean deviation in decibels
NA = not applicable, VA = visual acuity, IOP = intraocular pressure, ON = optic nerve, HVF= Humphrey visual field. CSF = cerebrospinal fluid
Neuro-imaging (11 MRIs, 1 CT) was performed at our institution for 12/20 patients. Nine out of 11 patients with MRI received intravenous gadolinium contrast material and 3/11 patients had an MRV (contrast-enhanced MRV: 2/3). The optic canal was smaller on the side of the lowest-grade edema in all 8 patients (100%, 7 MRIs, 1 CT) in whom it could be reliably evaluated (table 2). The cross-sectional optic canal measurement was 14.9% smaller on average on the side of the lowest-grade edema [range: 2.5%-31.0%; p=0.008] (fig. 1). Asymmetric peri-optic nerve CSF was reported in 6/12 of patients (50%), and was always less prominent on the side of the lowest-grade edema (p=0.01). Optic nerve protrusion into the globe and scleral flattening, both trended toward being more common on the side of the highest-grade edema (p=0.07).
Discussion
Our study confirms that very asymmetric papilledema is very rare in IIH, occurring in less than 4% of patients with definite IIH. Interestingly, we showed that the bony optic canal was always smaller on the side of the lowest-grade edema.
Few studies (1-13) have dealt with asymmetric papilledema in IIH. Three main studies reported its prevalence in a tertiary neuro-ophthalmic setting (1,11,12), and found heterogeneous results. Using the same definition of asymmetric papilledema, Wall and White (12) and the Idiopathic Intracranial Hypertension Treatment Trial (11) have shown that IIH with very asymmetric papilledema was uncommon (≈7.5%), but a smaller series of 6 patients (1) found a higher prevalence (23%). Surprisingly, our study found a much lower prevalence of 3.6%. Several factors may have contributed to these discrepancies. First, we believe that the high prevalence found by Lepore (1) should be interpreted cautiously, because the sample was small (6/27 patients; 95%CI: 7.1%-38.9%) and no details regarding the definition of asymmetric papilledema were provided. Second, we had a very strict definition of very asymmetric papilledema to emphasize the potential differences between symmetric and asymmetric papilledema in term of demographics, clinical presentation and radiologic features; therefore, we might have underestimated its prevalence.
Regarding patient demographics, we found that IIH patients with very asymmetric papilledema were older compared to patients without asymmetric papilledema, which was also reported previously by Lepore (1). Lepore (1) suggested a possible loss of compliance in the aging lamina cribrosa, buffering the effect of the peri-optic CSF pressure. However, he did not address how this would result in asymmetric papilledema. Wall et White (12) found an over-representation of men (29%); we also found a higher frequency of men (15%) among patients with very asymmetric papilledema but this did not reach significance. In our study, the race and BMI of patients with very asymmetric papilledema were similar to those with symmetric papilledema.
The clinical presentation of IIH patients with very asymmetric papilledema seems to differ from that of IIH patients with symmetric papilledema, with a high proportion of asymptomatic cases (27%) and a lower proportion of patients with headaches (35%) in the asymmetric papilledema group. In a previous series including patients from the same IIH database (17), we emphasized that men with IIH experience headache less often. Our higher frequency of men with very asymmetric papilledema, none of whom had headache, may account partially for this difference. In addition, although no correlation between headaches and CSF-OP in IIH has been shown to our knowledge, the significantly Iower CSF-OP we found among patients with very asymmetric papilledema might have contributed to the difference in headache frequency. Regarding the visual function, as previously reported (12), visual fields were significantly worse on the side of the highest-grade papilledema.
The most interesting result from our study is that the bony optic canals were consistently smaller on the side of the lowest-grade papilledema. The pathophysiology of CSF dynamics in IIH is not fully understood (18). The lack of a clear relationship between the degree of papilledema and the CSF-OP (14,19) suggests that an underlying mechanism may sometimes prevent the optic disc from swelling in some cases of intracranial hypertension. The pathogenesis of papilledema (20) depends upon the translaminar pressure gradient at the optic nerve head. Either high peri-optic CSF pressure or low intraocular pressure cause identical microscopic changes and axonal flow stasis (20). However, our study and others (2,7,13) have shown that asymmetric intraocular pressure, although reported in anecdotal cases (3,5,10), is not the primary mechanism of asymmetric papilledema. Therefore, we believe that, in the absence of optic atrophy (20), asymmetric papilledema is most likely related to asymmetric transmission of the CSF pressure to the lamina cribrosa (21). Two mechanisms for asymmetric transmission of the CSF pressure along the peri-optic subarachnoid spaces have been previously proposed, namely asymmetric structural changes in the either lamina cribrosa (1) or along the optic nerve sheath (6). However, these mechanisms remain debated. Our study shows compelling data regarding the role of asymmetry in the bony optic canals in the genesis of very asymmetric papilledema. It is well-known that the orbital part of the peri-optic subarachnoid spaces shows distension in IIH (22,23). However, a previous study of 15 patients with intracranial hypertension in whom 10 had IIH (13) failed to demonstrate asymmetric distension of the peri-optic subarachnoid spaces in patients with unilateral papilledema, but no details regarding the grading of papilledema were provided. We included only very asymmetric papilledema in order to identify obvious potential differences between the two eyes. We showed that asymmetric distension of the peri-optic subarachnoid spaces occurred in half of our cases, always less prominent on the side of the lowest-grade edema, suggesting that the CSF pressure may be lower in the peri-optic subarachnoid spaces on the side with the lowest-grade edema. It is possible that our imaging or grading system might not have been sensitive enough to capture subtle asymmetry in the remaining 50% of cases with symmetric peri-optic CSF.
Our data, along with that of other investigators (24), continue to challenge the concept of free circulation of the CSF. The concept of compartmentation of the peri-optic subarachnoid spaces (4,24,25), in which the peri-optic subarachnoid spaces are partially separated from the suprasellar cisternal spaces, appears more likely to explain asymmetric papilledema. Whereas the orbital portion of the peri-optic subarachnoid spaces is loose enough to distend under increased CSF pressure, the intracanalicular portion, the narrowest (4,19), is characterized by tight relationships between the bone and the optic nerve (24). Because of its unique anatomy, the region of the optic canal plays a crucial role in CSF flow dynamics between the suprasellar cistern and the peri-optic subarachnoid spaces. We showed that the bony optic canal was always smaller on the side of the lowest-grade edema. This peculiar anatomical configuration of the bony optic canals probably allows CSF pressure to be less easily transmitted along the optic nerve on the side of the smaller canal, thereby resulting in lower local intraorbital CSF pressure and less optic disc edema. However, longitudinal data on the optic canal diameter in IIH patients is needed to better understand whether this asymmetry in size is congenital or results from bony erosion related to longstanding CSF hypertension, as described in other skull base locations with chronic intracranial hypertension (26,27). The fact that we and others (1) have found that IIH patients with asymmetric papilledema are older than other IIH patients may support the latter.
Conclusion
IIH with very asymmetric papilledema is uncommon. Despite our small sample, there is a definite relationship between the severity of papilledema and the cross-sectional area of the optic canal, suggesting that asymmetric papilledema may result from asymmetries in the bony optic canal. Our study lends further support to the concept of compartmentation of the peri-optic subarachnoid spaces developed by Killer et al (24) and suggests that the bony optic canal may be a “bottleneck” interfering with the CSF flow between the peri-optic subarachnoid spaces and the suprasellar cistern. However, whether the asymmetry in size of the optic canal is congenital or acquired requires further study.
Acknowledgments
Supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). Dr. Bidot receives research support from Berthe Fouassier Foundation (Paris, France) and Philippe Foundation (New York, New York, USA). Dr. Bruce receives research support from the NIH/NEI (K23-EY019341). Dr. Newman received the Research to Prevent Blindness Lew R. Wasserman Merit Award; and has provided expert testimony on the topic of papilledema.
Footnotes
Disclosures
Dr. Biousse reports no disclosures.
Conflict of interest
None
References
- 1.Lepore FE. Unilateral and highly asymmetric papilledema in pseudotumor cerebri. Neurology. 1992;42:676–678. doi: 10.1212/wnl.42.3.676. [DOI] [PubMed] [Google Scholar]
- 2.Brosh K, Strassman I. Unilateral papilledema in pseudotumor cerebri. Semin Ophthalmol. 2013;28:242–243. doi: 10.3109/08820538.2013.768677. [DOI] [PubMed] [Google Scholar]
- 3.Abegg M, Fleischhauer J, Landau K. Unilateral papilledema after trabeculectomy in a patient with intracranial hypertension. Klin Monatsbl Augenheilkd. 2008;225:441–442. doi: 10.1055/s-2008-1027307. [DOI] [PubMed] [Google Scholar]
- 4.Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130:514–520. doi: 10.1093/brain/awl324. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield DS, Wanichwecharungruang B, Liebmann JM, Ritch R. Pseudotumor cerebri appearing with unilateral papilledema after trabeculectomy. Arch Ophthalmol. 1997;115:423–426. doi: 10.1001/archopht.1997.01100150425022. [DOI] [PubMed] [Google Scholar]
- 6.Moster ML, Slavin M, Wall M. Unilateral disk edema in a young woman. Surv Ophthalmol. 1995;39:409–416. doi: 10.1016/s0039-6257(05)80097-9. [DOI] [PubMed] [Google Scholar]
- 7.Strominger MB, Weiss GB, Mehler MF. Asymptomatic unilateral papilledema in pseudotumor cerebri. J Clin Neuroophthalmol. 1992;12:238–241. [PubMed] [Google Scholar]
- 8.To KW, Warren FA. Unilateral papilledema in pseudotumor cerebri. Arch Ophthalmol. 1990;108:644–645. doi: 10.1001/archopht.1990.01070070030014. [DOI] [PubMed] [Google Scholar]
- 9.Sher NA, Wirtschafter J, Shapiro SK, See C, Shapiro I. Unilateral papilledema in “benign” intracranial hypertension (pseudotumor cerebri) JAMA. 1983;250:2346–2347. [PubMed] [Google Scholar]
- 10.Kawasaki A, Purvin V. Unilateral optic disc edema following trabeculectomy. J Neuroophthalmol. 1998;18:121–123. [PubMed] [Google Scholar]
- 11.Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, McDermott MP. The Idiopathic Intracranial Hypertension Treatment Trial: clinical profile at baseline. JAMA Neurol. 2014;71:693. doi: 10.1001/jamaneurol.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall M, White WN. Asymmetric papilledema in idiopathic intracranial hypertension: prospective interocular comparison of sensory visual function. Invest Ophthalmol Vis Sci. 1998;39:134–142. [PubMed] [Google Scholar]
- 13.Huna-Baron R, Landau K, Rosenberg M, Warren FA, Kupersmith MJ. Unilateral swollen disc due to increased intracranial pressure. Neurology. 2001;56:1588–1590. doi: 10.1212/wnl.56.11.1588. [DOI] [PubMed] [Google Scholar]
- 14.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 15.Scott CJ, Kardon RH, Lee AG, Frisén L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128:705–711. doi: 10.1001/archophthalmol.2010.94. [DOI] [PubMed] [Google Scholar]
- 16.Saindane AM, Mitchell BC, Kang J, Desai NK, Dehkharghani S. Performance of spin-echo and gradient-echo T1-weighted sequences for evaluation of dural venous sinus thrombosis and stenosis. AJR Am J Roentgenol. 2013;201:162–169. doi: 10.2214/AJR.12.9095. [DOI] [PubMed] [Google Scholar]
- 17.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, Preechawat P, Corbett JJ, Newman NJ, Biousse V. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batra R, Sinclair A. Idiopathic intracranial hypertension; research progress and emerging themes. J Neurol. 2013;261:451–460. doi: 10.1007/s00415-013-7019-7. [DOI] [PubMed] [Google Scholar]
- 19.Hayreh SS. Pathogenesis of oedema of the optic disc (papilloedema). A preliminary report. Br J Ophthalmol. 1964;48:522–543. doi: 10.1136/bjo.48.10.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol. 1977;95:1553–1565. doi: 10.1001/archopht.1977.04450090075006. [DOI] [PubMed] [Google Scholar]
- 21.Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. III. A pathologic study of experimental papilledema. Arch Ophthalmol. 1977;95:1448–1457. doi: 10.1001/archopht.1977.04450080158022. [DOI] [PubMed] [Google Scholar]
- 22.Bäuerle J, Nedelmann M. Sonographic assessment of the optic nerve sheath in idiopathic intracranial hypertension. J Neurol. 2011;258:2014–2019. doi: 10.1007/s00415-011-6059-0. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann J, Schmidt C, Kunte H, Klingebiel R, Harms L, Huppertz HJ, Ludemann L, Wiener E. Volumetric assessment of optic nerve sheath and hypophysis in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2014;35:513–518. doi: 10.3174/ajnr.A3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killer HE, Subramanian PS. Compartmentalized cerebrospinal fluid. Int Ophthalmol Clin. 2014;54:95–102. doi: 10.1097/IIO.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 25.Killer HE. The optic nerve: a new window into cerebrospinal fluid composition? Brain. 2006;129:1027–1030. doi: 10.1093/brain/awl045. [DOI] [PubMed] [Google Scholar]
- 26.Pérez MA, Bialer OY, Bruce BB, Newman NJ, Biousse V. Primary spontaneous cerebrospinal fluid leaks and idiopathic intracranial hypertension. J Neuroophthalmol. 2013;33:327–334. doi: 10.1097/WNO.0b013e318299c292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bialer OY, Pérez MA, Bruce BB, Newman NJ, Biousse V, Saindane AM. Meningoceles in idiopathic intracranial hypertension. AJR Am J Roentgenol. 2014;202:608–613. doi: 10.2214/AJR.13.10874. [DOI] [PMC free article] [PubMed] [Google Scholar]


