Abstract
Rates and characteristics of intracerebral cavernous malformations (ICMs) after cranial radiation therapy (CRT) remain poorly understood. Herein we report on ICMs detected on follow+up imaging in pediatric cancer patients who received CRT at age ≤ 18 years from 1980 to 2009. Through chart reviews (n=362) and phone interviews (n=104) of a retrospective cohort we identified 10 patients with ICMs. The median latency time for detection of ICMs after CRT was 12 years (range 1+24 years) at a median age of 21.4 years (IQR 15+28). The cumulative incidence was 3% (95% CI 1+8%) at 10 years post CRT and 14% (95% CI 7+26%) at 15 years. Three patients underwent surgical resection. Two surgical specimens were pathologically similar to sporadically occurring ICMs; one was consistent with capillary telangiectasia. ICMs are common after CRT and can show a spectrum of histological features.
Keywords: intracerebral cavernous malformation, cranial radiation therapy, pediatric cancer survivors
Introduction
Intracerebral cavernous malformations (ICMs) are increasingly recognized as one of the long+term sequelae of cranial radiation therapy (CRT), especially in pediatric populations 1-5. While the majority of these ICMs are asymptomatic, symptomatic lesions can present with seizure, new headache, and focal neurologic deficits due to hemorrhagic stroke. Pathologically, ICMs are characterized by a circumscribed cluster of dilated vascular sinusoids lined by a single endothelial layer, with little to no intervening brain parenchyma 1-5. Intralesional or perilesional deposits, including thrombus, calcification, and cysts are common, and ICMs are often bordered by hemosiderin and reactive gliosis. On MRI, ICMs present as mixed signal lesions of low and high intensity surrounded by a complete rim of T1 hypointensity. This “popcorn” appearance corresponds to the mixture of blood products at various stages of degradation within and around the lesion.
The prevalence of ICMs has been reported to be 0.4+0.6% in the general population; however, the incidence of ICMs after CRT remains largely unknown 6-9. A few studies have reported that ICMs occur within the field of prior radiation as early as five months, with the majority occurring 3+10 years after CRT 1, 4. The precise relationship between CRT dose and ICM development, however, is still unclear. Some studies have found a dose+dependent relationship noting decreased latency to ICM development in those who receive higher radiation doses 1, 5, 10. Others report an inverse relationship, finding that ICMs occur more commonly at the lower dose rim of the radiation field 5, 11. Regardless, it appears that low+dose radiation is sufficient to induce ICMs, as children with leukemia, who are treated with relatively low doses of radiation (12+24 Gy), are also at increased risk for developing ICMs 11, 12.
There are conflicting reports as to whether radiation+induced ICMs differ pathologically from congenital/spontaneously occurring ICMs 1, 13-16. Clinically, radiation+ induced ICMs also appear to carry an increased risk of hemorrhage, reported at 3.9% per patient+year as compared to 0.25% in spontaneous ICMs 5, 7.
In this study, we assessed the incidence of radiographically detected ICMs in a single+center retrospective cohort of patients who received CRT during childhood. When available we reviewed pathology in order to further characterize the histologic features of radiation+induced ICMs.
Materials and Methods
We performed a retrospective cohort study of patients identified through the University of California, San Francisco (UCSF) Cancer Registry who received treatment at UCSF with CRT at age ≤ 18 years between 1980 and 2009. This cohort was previously described 17.
We identified 385 eligible patients whom we contacted through mail and invited to participate in the study. Twenty+three patients declined and were excluded from the study. Chart review was conducted for the remaining 362 patients, and of those, 104 (or surrogates when necessary) consented/assented to telephone interviews. Interviews were carried out with either the patient (if alive and ≥ 18 years of age at the time of the study; n=71), or the legal guardian of the patients currently < 18 years of age (n=20), or deceased (n=13). Information gathered during chart review and/or interview included baseline demographics (age, sex, race), primary cancer diagnosis and cancer treatment, age and date of diagnosis, radiation dose and treatment time, date of initial MRI to detect primary cancer diagnosis, and date of last MRI follow+up at our institution. Primary cancer diagnosis was based on pathology report, and date of surgery was confirmed by operative report. ICM identified by chart review was defined as physician documentation of ICM and/or brain imaging report that indicated ICM. Nine ICM patients were identified by chart review. ICM identified by interview was defined as participant report of a healthcare provider communicating the diagnosis to the patient. One ICM patient was identified on interview, and the patient's diagnosis was subsequently confirmed by chart and imaging review. For all patients with a diagnosis of ICM, all relevant brain imaging was obtained and reviewed by a study neuroradiologist (CH), who confirmed presence or absence of ICM, and specified anatomic locations for each ICM. For patients with ICM, additional information gathered by chart review included interval timing of imaging follow+up after CRT, and ICM related symptoms or treatment. Pathology specimens were obtained and reviewed in detail by a trained pathologist (JC) for all patients who underwent resection of the ICM. Data from medical records and imaging for all patients, including those who did not have ICMs, were collected through September 2011.
Statistical Analysis
Survival analysis was used to determine cumulative incidence of radiographically defined ICM after CRT. The primary outcome was time to radiographic appearance of ICM, calculated from last day of CRT to the date of first MRI with evidence of ICM. Censoring criteria were death, evidence of ICM or last follow+up MRI without evidence of ICM. For all patients with evidence of ICM, we confirmed that initial diagnostic imaging and follow+up MRI after completion of CRT was negative for ICM until date of diagnosis. Most patients were followed with MRI scans at regular time intervals, every 1 to 2 years. To calculate incidence rates we used the midpoint of the interval between CRT/last normal MRI and first detection. For two patients who did not receive regular MRI follow+up, the median time between end of CRT and date of initial radiographic evidence of ICM was used as an estimate for our analysis. Because our primary goal was to estimate incidence and not to determine factors associated with time to occurrence of ICM, we did not account for the fact that our data were interval censored, given that actual time of ICM development cannot be known. Rather, we used detection date to calculate incidence rates.
Results
Patient characteristics
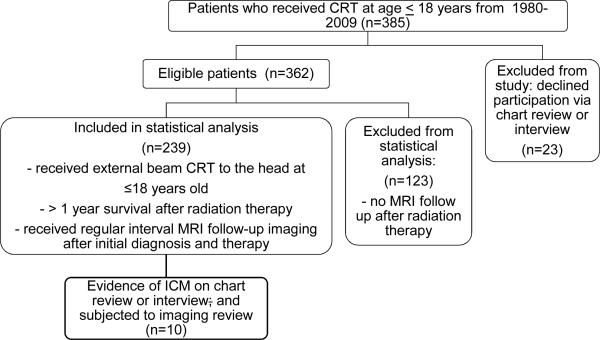
Of the 362 eligible patients, we excluded 123 given that these patients had no MRI after completion of radiation therapy (Figure 1, supplemental Table 1). For the 239 patients, median age at time of CRT was 8.7 years (IQR, 4.6+13.0), and median age at last follow up was 15.1 years (IQR, 9.6+19.3). The majority of the patients were alive (n=165; 69%) at time of interview and/or chart review (Table 1, 2).
Figure 1.
Flow diagram demonstrating the recruitment and participation of the 239 patients within the final study cohort.
Table 1.
Patient characteristics of cohort
| Characteristic | Patients with ICM (n=10) | Total cohort (n=229) |
|---|---|---|
| n (%) | n (%) | |
| Alive | 7 (70) | 158 (69) |
| Male gender | 8 (80) | 122 (53.3) |
| Race | ||
| African-American | 0 | 16 (7) |
| Caucasian | 8 (80) | 122 (53.3) |
| Asian American | 0 | 27 (11.8) |
| Latino | 2 (20) | 21 (9.2) |
| Other/unknown | 0 | 43 (18.8) |
| Age at cancer diagnosis, median (IQR) | 8.6 (7.5,13.3) | 8.4 (4.2,12.5) |
| Age at cranial radiation therapy, median (IQR) | 9.7 (7.9,13.7) | 8.7 (4.4,12.8) |
| Tumor type | ||
| Medulloblastoma | 2 (20) | 33 (14.4) |
| High-grade glioma | 3 (30) | 56 (24.5) |
| Low-grade glioma | 2 (20) | 43 (18.8) |
| Brainstem glioma | 0 | 3 (1.3) |
| Retinoblastoma | 0 | 19 (8.3) |
| ALL | 0 | 1 (0.44) |
| Others | 3 (30) | 74 (32.3) |
ALL: acute lymphoblastic anemia; SD: standard deviation
Table 2.
Patient characteristics of those with radiographically detected ICM
| Patient | Gender | Cancer Type | Age at Radiation Therapy | Radiation Dose (cGy) | Radiation Field | ICM location | Interval of f/u MRI (year) | Time to ICM detection† (years) | Age at ICM detection (years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | male | MB | 6 | 6100 | whole brain | left frontal periventricular | 1 | 1 | 7 |
| 2 | female | MB | 7 | 6055 | posterior fossa | left parietal | 1 | 6 | 13 |
| 3 | female | PA | 7 | 5800 | posterior fossa | left inferior temporal | 1 | 12 | 20 |
| 4* | male | PA | 8 | 5580 | right frontal | right frontoparietal | 1 | 19 | 28 |
| 5* | male | MG | 8 | 5400 | left temporal | left temporal lobe | 2 | 13 | 21 |
| 6 | male | AA | 10 | 5940 | whole brain | bilateral thalami | 1 | 6 | 16 |
| 7 | male | BL | 11 | 2400 | frontal | frontal subcortical | 0 | 14 | 26 |
| 8 | male | MEC | 14 | 5760 | parotid | right frontal lobe | 1 | 4 | 19 |
| 9 | male | PA | 15 | 5580 | whole brain | right periventricular | 0 | 24 | 39 |
| 10* | male | PA | 16 | 6600 | right temporal | right temporal parietal | 2 | 11 | 28 |
These patients received surgical resection for symptomatic ICM (Patient 4: seizures and headache; Patient 5: headache; Patient 10: L sided numbness, weakness and facial droop)
presented as years after cranial radiation therapy.
AA = Anaplastic astrocytoma, BL = Burkitt's lymphoma, PA = pilocytic astrocytoma, MB = medulloblastoma, MEC = mucoid epidermal carcinoma, MG = mixed germinoma
Radiographically detected ICM
In our cohort of 239 participants, we identified 10 patients with a diagnosis of ICM and all were confirmed radiographically. Figures 2 and 3 show representative MRI images of confirmed ICMs, revealing well+circumscribed lesions with heterogeneous T1 and T2 signal, with surrounding hemosiderin and varying degrees of calcification and gliosis. Of the 10 identified patients with ICMs, eight had had MRI follow+up every 1+2 years from the time of their CRT to their ICM diagnosis, while two patients were lost to follow+up after their initial CRT. These two patients underwent MRI more than 10 years after completion of CRT, at which time ICM was noted on imaging.
Figure 2.
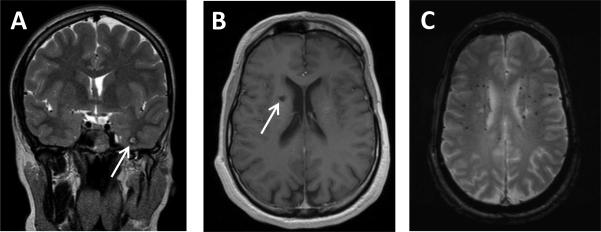
MRI appearance of vascular malformations: A: Example of classic MRI appearance of intracranial cavernous malformation (ICM) on T2+weighted imaging (Patient 3). Inferior left temporal lobe ICM showing complete low signal rim surrounding an area of mixed T2 signal. B,C: Radiographic spectrum of vasculopathies in the same patient (Patient 9): B gadolinium+enhanced T1+weighted image showing a right periventricular ICM with small area of hyperintensity surrounded by a hypointense signal, and C: T2*+weighted image showing small and scattered radiation related microbleeds shown as areas of decreased signal intensity.
Figure 3.
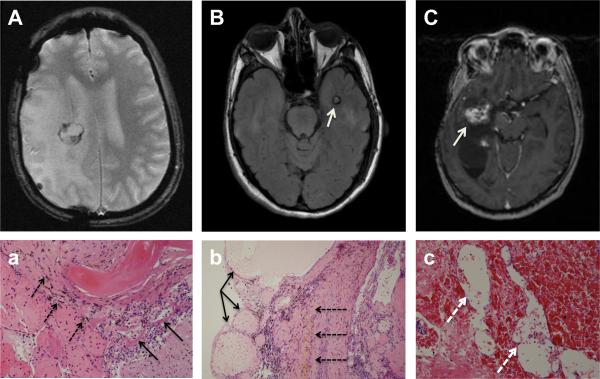
MRI images of three patients who underwent surgical resection for symptomatic ICMs and the corresponding pathology. MRI images of radiographically identified ICMs are shown in A+C. A (Patient 4): T2*+weighted, B (Patient 5): T2 FLAIR, C (Patient 10): T1+weighted MRI. A and B depict the typical appearance of ICM (white arrow) on MRI with a core of mixed increased and decreased signal intensity surrounded by low signal intensity from adjacent hemosiderin+laden parenchyma. Corresponding pathology (a,b) showing classic ICM with abnormal thin walled, dilated non+arterial blood vessels (black thin arrows) with little to no intervening brain tissue surrounded by a rim of hemosiderin+laden macrophages (black dashed arrows) in the adjacent parenchyma. C shows a right temporal+parietal ICM (white arrow) with high signal intensity and (c) corresponding pathology with dilated capillary+like vascular spaces (white dashed arrows) separated by intervening tissue.
Baseline demographics of the patients with ICMs show that most were male (n=8; 80%). Nine out of 10 patients with ICMs received CRT doses >50 Gy (range 54+ 66 Gy) and one patient received low dose CRT of 24 Gy. Seven out of the 10 ICMs were located within the brain region that received high+dose CRT whereas the remaining three were located in the periphery of the radiation field (Table 2). Median age at CRT was 9.7 years (IQR= 7.9+13.7) and median latency of ICM diagnosis (defined as time from CRT to midpoint between last cavmal+free MRI and cavmal diagnosis MRI) was 11.6 years post CRT (IQR= 4.3+12.8) (Figure 4). Median latency decreases slightly to 9.0 if the two children with long intervals between last cavmal+free MRI and diagnosis MRI are excluded, but IQRs for this latency period remain largely unchanged ((3.9+12.6). The overall rate of radiographically detected ICM was 584 per 100,000 person years (95% CI 538+633). The cumulative incidence of radiographically detected ICM was 2% (95% CI 0.6+6%) at 5 years, 3% (95% CI 1+8%) at 10 years, and 14% (95% CI 7+26%) at 15 years.
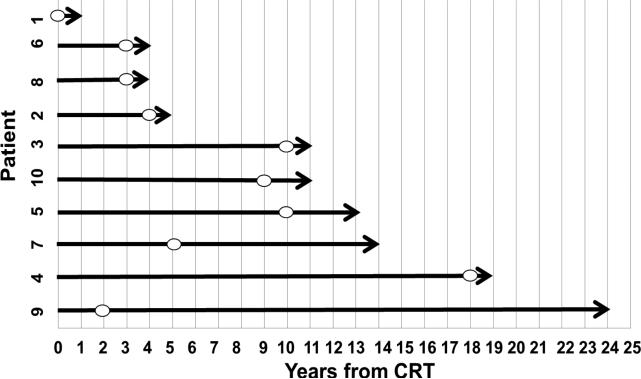
Figure 4.
Overview of time to detection of ICM on MRI per individual patient. The figure depicts the latency time from the end of CRT to the time ICM was first detected on MRI. The symbol (o) denotes each patient's last MRI follow+up free of evidence of any lesion meeting radiographic criteria of ICM. Of note, imaging was not systematically acquired at specific time points therefore latency to radiographic detection of ICM is an estimate.
Characteristics of ICM on Pathology
Of the 10 patients with radiographically detected ICMs, three patients underwent surgical resection for symptoms attributed to these lesions. Symptoms included new onset of headache plus seizures (patient 4), worsening headache (patient 5) and left+ sided numbness, left facial droop, left visual field defect, and decreased strength on the left side (patient 10). Two patients had resection specimens that were morphologically similar to spontaneously occurring or congenital ICMs. These specimens demonstrated thin+walled dilated loops of non+arterial vessels with little to no intervening brain tissue, and adjacent hemosiderin+laden macrophages. The third specimen exhibited characteristics of a telangiectasia, with smaller, capillary+like vascular lumina separated by intervening brain parenchyma (Figure 3).
DISCUSSION
In this single+center retrospective cohort study, we found that children undergoing CRT for cancer therapy are at high risk for developing ICMs years after their initial therapy. Symptomatic radiation+induced+ICMs that are classified as such on imaging represent a spectrum of vascular disorders on histology ranging from capillary telangiectasia to a more classic ICM appearance. The cumulative incidence of ICMs after CRT has been estimated at 3.9% and 5.0% within 10 and 15 years post CRT, respectively 3. In our cohort of pediatric cancer patients treated with CRT, the cumulative incidence of post+CRT ICM was found to be slightly higher at 3% at 10 years and 14% at 15 years however due to our small sample number the 95% CI are relatively large and that difference might not be statistically significant. Not all patients in our cohort, however, underwent imaging most sensitive to ICM detection, such as gradient echo imaging at high field strengths. Some ICMs may therefore have remained undetected, and our results likely underestimate the true incidence in this cohort.
Our study period spanned 29 years, and most cases of ICM were documented in the last 10 years of the study, with the majority of ICMs documented in the last 5 years of the study. Although there is literature reporting radiographic identification of ICMs decades before the onset of our study period, the identified ICMs in the later years of our study could reflect the significant evolution in imaging technology, or even an increased awareness of ICM's among radiologists. Hence, these are additional factors that could have potentially contributed to underreporting of ICMs in the earlier years of the study.
Radiation+induced ICMs are known to develop over a long time period after CRT: in our cohort, 1 to 24 years after radiation. The median latency time to detection was 9 years; however, imaging was not done systematically at specific time points for all patients, so the exact latency could not be determined. Prior reports have estimated very similar latency times, ranging from 3 months to 22 years 11, 16, 18. These results suggest that pediatric cancer patients treated with CRT who present with new neurological findings should be also assessed for ICMs as a potential underlying diagnosis.
Young age at time of CRT has been associated with an increased risk of developing ICMs 1, 2, 5. Vinchon et al., however, reported that receiving CRT at an older age was a risk factor for developing ICMs 19. In our cohort, seven children were ≤ 12 years of age at time of CRT, while only three were treated with CRT at age >12 years. Although the rate of ICM detection per 100,000 person+years did not significantly differ between the <12 years age group (595, 95% CI 548+644, n=7) and the >12 years group (559, 95% CI 514+607, n=3), given the small sample size of the groups we cannot rule out the possibility that we lacked the power to detect a true difference.
Vascular malformations after CRT can exhibit heterogenous histopathology. A study by Baumgartner et al. found that radiation+induced ICMs are pathologically identical to sporadic and familial ICMs 16. However, as Pozzatti et al. note, naturally occurring ICMs usually display elements that suggest a prolonged development, including calcification 20. In our study, two of the three pathological specimens showed classic appearance of ICMs and had no evidence of calcifications, whereas the other specimen was consistent with telangiectasia with evidence of calcifications. Although the pathogenesis of radiation+induced ICMs is not well understood, it has been proposed that they result from radiation+induced injury to the vasculature that histologically manifests as fibrinoid necrosis, hyalinization, and edema. Such changes can cause narrowing of the vascular lumen, leading to ischemia and the release of angiogenic factors, thus inducing dilation and proliferation of thin+walled vessels in adjacent areas 21. While ICM and capillary telangiectasia are considered separate, pathologically defined entities, they can have a similar imaging appearance, and some authors have proposed that they may be part of the same disease spectrum, representing early and late phases of radiation+induced vasculopathy, respectively 22. Although our sample size is small, our results are consistent with prior observations of pathological variation among radiographically detected ICMs.
Eight out of our 10 radiographically detected ICMs were found in male patients. This male predominance is supported by a review of the literature of radiation+induced ICMs and other small case series 4, 11. However, others have found no gender predilection in the development of ICMs after CRT 18. More studies with greater numbers are needed to assess whether a gender predilection is present in the risk of ICM development following CRT.
In our study, nine out of 10 patients with ICM had a CRT dose of >30 Gy, suggesting that higher CRT doses may confer an increased risk of developing ICM. Our results are supported by multiple prior studies reporting an association between CRT dose >30 Gy and increased ICM incidence 3, 4, 11. Similarly, our cohort reflects previously published data that most CRT+associated ICMs are asymptomatic 3, 12, as only three of our 10 radiographically detected ICMs required surgical intervention for symptom control.
It has been reported that risk of hemorrhage may be significant among pediatric patients with CRT+induced ICMs 11. However, no patients in our cohort presented with or subsequently developed a symptomatic hemorrhage during our follow up period. In one study, out of 419 patients who received CRT, 9 developed ICMs, and 3 of those developed symptomatic hemorrhagic ICMs requiring surgical intervention 11. By contrast, the rate of symptomatic hemorrhage in sporadically occurring ICMs in the general population is only 2.4% per patient year 6. Studies showing a higher bleeding risk for CRT+induced ICMs had small cohorts of six to nine patients with ICMs; therefore larger, prospective studies are needed to further clarify this risk.
Conclusion
Radiation induced ICMs are frequent in patients who underwent CRT and occur on a histopathological spectrum.
Supplementary Material
Acknowledgements
This work was presented at the Child Neurology Society 41st Annual Meeting, October 31 – November 3 2012, Huntington Beach, CA.
Funding
The work was supported by a donation from the LaRoche family (HF), the Hellman Fellow Fund (SM), and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF+CTSI Grant Number KL2TR000143 (SM).
Footnotes
Author Contributions
EG, KS, HF and SM conceived and designed the study and also drafted the manuscript. EG, KS, ER, DR, NC, CH, JC, DHK, SM acquired the data. EG, KS, NH, JC, CH, HF and SM analyzed and interpreted the data. EG, KS, NH, ER, CH, JC, DHK, HF and SM critically revised the manuscript for important intellectual content. SM and HF supervised the study. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by the Institutional Review Board (# 10+01049) of the University of California, San Francisco.
References
- 1.Larson JJ, Ball WS, Bove KE, Crone KR, Tew JM., Jr. Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. Journal of neurosurgery. 1998;88(1):51–6. doi: 10.3171/jns.1998.88.1.0051. [DOI] [PubMed] [Google Scholar]
- 2.Heckl S, Aschoff A, Kunze S. Cavernomas of the skull: review of the literature 1975-2000. Neurosurgical review. 2002;25(1-2):56–62. doi: 10.1007/s101430100180. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 3.Strenger V, Sovinz P, Lackner H, et al. Intracerebral cavernous hemangioma after cranial irradiation in childhood. Incidence and risk factors. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2008;184(5):276–80. doi: 10.1007/s00066-008-1817-3. [DOI] [PubMed] [Google Scholar]
- 4.Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurgical focus. 2006;21(1):e4. doi: 10.3171/foc.2006.21.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Keezer MR, Del Maestro R. Radiation-induced cavernous hemangiomas: case report and literature review. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2009;36(3):303–10. doi: 10.1017/s0317167100007022. [DOI] [PubMed] [Google Scholar]
- 6.Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurgical focus. 2011;30(6):E24. doi: 10.3171/2011.3.FOCUS1165. [DOI] [PubMed] [Google Scholar]
- 7.Del Curling O, Jr., Kelly DL, Jr., Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. Journal of neurosurgery. 1991;75(5):702–8. doi: 10.3171/jns.1991.75.5.0702. [DOI] [PubMed] [Google Scholar]
- 8.Otten P, Pizzolato GP, Rilliet B, Berney J. [131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies]. Neuro-Chirurgie. 1989;35(2):82–3. 128–31. [PubMed] [Google Scholar]
- 9.Sarwar M, McCormick WF. Intracerebral venous angioma. Case report and review. Archives of neurology. 1978;35(5):323–5. doi: 10.1001/archneur.1978.00500290069012. [DOI] [PubMed] [Google Scholar]
- 10.Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002;94(12):3285–91. doi: 10.1002/cncr.10596. [DOI] [PubMed] [Google Scholar]
- 11.Duhem R, Vinchon M, Leblond P, Soto-Ares G, Dhellemmes P. Cavernous malformations after cerebral irradiation during childhood: report of nine cases. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2005;21(10):922–5. doi: 10.1007/s00381-004-1120-2. [DOI] [PubMed] [Google Scholar]
- 12.Faraci M, Morana G, Bagnasco F, et al. Magnetic resonance imaging in childhood leukemia survivors treated with cranial radiotherapy: a cross sectional, single center study. Pediatric blood & cancer. 2011;57(2):240–6. doi: 10.1002/pbc.22923. [DOI] [PubMed] [Google Scholar]
- 13.Alexander MJ, DeSalles AA, Tomiyasu U. Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma. Case report. Journal of neurosurgery. 1998;88(1):111–5. doi: 10.3171/jns.1998.88.1.0111. [DOI] [PubMed] [Google Scholar]
- 14.Gewirtz RJ, Steinberg GK, Crowley R, Levy RP. Pathological changes in surgically resected angiographically occult vascular malformations after radiation. Neurosurgery. 1998;42(4):738–42. doi: 10.1097/00006123-199804000-00031. discussion 42-3. [DOI] [PubMed] [Google Scholar]
- 15.Maraire JN, Abdulrauf SI, Berger S, Knisely J, Awad IA. De novo development of a cavernous malformation of the spinal cord following spinal axis radiation. Case report. Journal of neurosurgery. 1999;90(2 Suppl):234–8. doi: 10.3171/spi.1999.90.2.0234. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner JE, Ater JL, Ha CS, et al. Pathologically proven cavernous angiomas of the brain following radiation therapy for pediatric brain tumors. Pediatric neurosurgery. 2003;39(4):201–7. doi: 10.1159/000072472. [DOI] [PubMed] [Google Scholar]
- 17.Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. International journal of radiation oncology, biology, physics. 2013;86(4):643–8. doi: 10.1016/j.ijrobp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burn S, Gunny R, Phipps K, Gaze M, Hayward R. Incidence of cavernoma development in children after radiotherapy for brain tumors. Journal of neurosurgery. 2007;106(5 Suppl):379–83. doi: 10.3171/ped.2007.106.5.379. [DOI] [PubMed] [Google Scholar]
- 19.Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011;27(3):445–53. doi: 10.1007/s00381-011-1390-4. [DOI] [PubMed] [Google Scholar]
- 20.Pozzati E, Giangaspero F, Marliani F, Acciarri N. Occult cerebrovascular malformations after irradiation. Neurosurgery. 1996;39(4):677–82. doi: 10.1097/00006123-199610000-00004. discussion 82-4. [DOI] [PubMed] [Google Scholar]
- 21.Poussaint TY, Siffert J, Barnes PD, et al. Hemorrhagic vasculopathy after treatment of central nervous system neoplasia in childhood: diagnosis and follow-up. AJNR American journal of neuroradiology. 1995;16(4):693–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Humpl T, Bruhl K, Bohl J, Schwarz M, Stoeter P, Gutjahr P. Cerebral haemorrhage in long-term survivors of childhood acute lymphoblastic leukaemia. European journal of pediatrics. 1997;156(5):367–70. doi: 10.1007/s004310050616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.