Abstract
Objective
The objective of this paper is to conduct a prospective, longitudinal study employing the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) to examine the baseline and follow-up association of prostate cancer (PCa)-specific anxiety, health-related quality of life (HRQOL), and PCa aggressiveness in men with newly-diagnosed PCa undergoing prostatectomy at our institution.
Methods
From our prospective PCa registry, we identified a total of 350 men with newly-diagnosed PCa who completed the MAX-PC and the Expanded Prostate Cancer Index Composite (EPIC) at baseline and one-year following surgery. Scores on both measures were compared with clinical measure and demographics using the Wilcoxon Rank Sum, Fisher's exact, and Cochran-Armitage Trend tests. Spearman test was used to assess correlation at between the MAX-PC and EPIC at baseline and one-year.
Results
Baseline overall MAX-PC measures were correlated with measures at one-year (r = 0.5479, p < 0.001). Those reporting high anxiety at one-year were more likely to have Gleason score > 6 (p = 0.004), T-Stage ≥ 2C disease (p = 0.004), and a postoperative prostate-specific antigen (PSA) > 0.1 (p = 0.002); however, this did not apply to all anxious patients. Baseline EPIC sexual function scores were predictive of follow-up EPIC sexual function scores as well (r = 0.5790, p < 0.001). Depression was noted as a problem in 16% of patients at follow-up.
Conclusions
Our data suggests that the MAX-PC could be used at baseline as a tool to determine who may benefit from psychological intervention pre-PCa and post-PCa treatment. In terms of individualized medicine, behavioral therapy may be the most beneficial in improving HRQOL for younger patients, those with advanced stage disease, and more specifically those whose anxiety outweighs their actual prognosis.
Keywords: anxiety, prostate cancer, cancer, oncology, surgery, quality of life
Due to advances in screening and surgical techniques, the past decade has seen dramatic increases in the number of men who are living for longer periods of time after surgical treatment for localized prostate cancer (PCa) [1, 2]. As a result, emphasis has grown in the field of PCa survivorship, particularly for psychosocial factors [3-5]. It has been established that men who undergo surgery for localized PCa can experience significant levels of cancer-specific anxiety before and after surgery [3-8]. Thus, there has been a growing interest in evaluating factors that affect the quality of life of men being treated for PCa [3, 5]. The ability to identify those who might benefit from psychological intervention at baseline could positively impact postsurgical quality of life outcomes.
Investigators have reported that cancer-specific anxiety represents the most common psychological reaction that men experience following the diagnosis and surgical treatment for PCa [9-13]. We previously reported that anxiety at one-year is associated with poor sexual satisfaction, depressive mood, and other psychosocial factors [3]. This suggests that interventions could be designed to lower anxiety in men, especially those with more indolent disease. The focus then becomes whether we could predict which men will be anxious at one-year on the basis of baseline scores and other metrics. In this way, we could identify men who would most benefit from early (i.e., from diagnosis to within three months of surgery) anxiety interventions, whereas sparring those whose anxiety will dissipate on its own. Although the existence of PCa-specific anxiety is well known, the development of PCa-specific anxiety and its association with survivorship remains undetermined [14-18].
Over the past decade, Roth et al. developed and subsequently validated a questionnaire, the Memorial Anxiety Scale for Prostate Cancer (MAX-PC), designed to measure several types of PCa-specific anxieties [9, 19]. This study provides the opportunity to explore unanswered questions in the literature regarding whether PCa-specific anxiety at baseline is associated with psychosocial and physical factors known to affect PCa survivorship at 1 year [5, 7, 8, 14-18, 20-24]. We utilized data from our ongoing PCa database to conduct a prospective, longitudinal study of PCa-specific anxiety in men undergoing treatment for PCa at our institution. Employing the MAX-PC, we examined the baseline and follow-up associations of PCa-specific anxiety, health-related quality of life (HRQOL), and PCa aggressiveness in men with newly diagnosed PCa undergoing prostatectomy at our institution.
Materials and methods
HRQOL measures
Men with newly diagnosed PCa complete HRQOL questionnaires at baseline, at 6 months, and then annually following the date of their treatment as part of routine clinical practice at our institution. With approval from our local Institutional Review Board, we were able to conduct our study utilizing these clinical questionnaires. The questionnaires include the MAX-PC and the Expanded Prostate Cancer Index Composite (EPIC) [24]. The EPIC is comprised of 32 items divided into four domains of HRQoL including bowel, urinary, hormonal, and sexual functions. The EPIC was analyzed according to the standard procedures for each of the sexual function subscales. Four questions relating to depression were extracted from the hormone function subscale of the EPIC and were scored as individual items of psychosocial health. The MAX-PC consists of 18 questions grouped into subscales for PCa-Anxiety, Prostate-specific antigen Anxiety, Fear of Recurrence, and Overall Anxiety. Although the scores can range from 0 to 54, we considered a score of 27 to signify a clinically-significant level of anxiety, as is suggested by the literature [19].
Patients
We identified all cases (N = 765) who underwent radical retropubic (RRP) or robotic laparoscopic radical prostatectomy (RLAP) for newly diagnosed PCa at our institution from July 2006 to October 2010. Of those, a total of 350 patients returned voluntarily completed follow-up HRQoL questionnaire as part of clinical practice. Our analysis utilized all subscales of the MAX-PC, the Sexual Function (SF) domain, Sexual Bother domain, overall Sexual Summary (SS) scores, and four depression-related questions of the EPIC. Measures of PCa aggressiveness were obtained through our prospectively maintained PCa database. These data included co-morbidities, as well as the presurgical and postsurgical pathological features listed in Table 1. Demographic data were collected on age, martial status, race, history of erectile dysfunction, and family history of PCa.
Table 1.
Demographics for radical retropubic and laparoscopic radical prostatectomy patients who completed follow-up quality of life sexual function and anxiety questions (N=350)
| Variable | Overall N = 350a |
|---|---|
| 1. PCa, prostate cancer; PSA, prostate-specific antigen; RRP, Radical retropubic prostatecomy. | |
| 2. a | |
| The sample median (minimum, 25th percentile, 75th percentile, maximum) is given for numerical variables, n (%) for categorical variables. | |
| M‘Age at treatment (years) | 63.8 (42.7, 59.0, 67.8, 78.1) |
| Marital status | |
| Married | 311 (89%) |
| Single | 14 (4%) |
| Separated/divorced | 22 (6%) |
| Widowed | 3 (1%) |
| Race—White | 315 (90%) |
| History of erectile dysfunction (Unknown in 40) | 147/310 (47%) |
| Diabetes | 31 (9%) |
| Family history of PCa | 99 (28%) |
| Preoperative PSA (Unknown in 7) | |
| <4 | 70 (20%) |
| 4–10 | 245 (71%) |
| >10–20 | 23 (7%) |
| >20 | 5 (2%) |
| Pretreatment Gleason score (missing in n = 1) | |
| 4–6 | 187 (54%) |
| 7 | 122 (35%) |
| 8–10 | 40 (11%) |
| Pathological Gleason score | |
| 6 | 127 (36%) |
| 7 | 191 (55%) |
| 8–10 | 32 (9%) |
| Treatment type | |
| RRP | 154 (44%) |
| Laproscopic | 196 (56%) |
| T stage (Unknown in 1) | |
| 1c | 2 (1%) |
| 2a,2b | 66 (19%) |
| 2c | 236 (68%) |
| 3a,3b | 45 (13%) |
| Prostatic capsule involvement (unknown in 4) | 69/346 (20%) |
| Positive margins (unknown in 1) | 99/349 (28%) |
| Seminal vesicle involvement (unknown in 2) | 20/348 (6%) |
| Nerve sparing | |
| No | 34 (10%) |
| Full | 291 (83%) |
| Partial | 25 (7%) |
| PSA 6–18 months post surgery (Missing in n = 49) | |
| 0–0.1 | 267 (89%) |
| >0.1–0.5 | 23 (8%) |
| >0.5–2 | 7 (2%) |
| >2–4 | 2 (1%) |
| >4–<10 | 1 (<1%) |
| >10 | 1 (<1%) |
Statistical analysis
Numerical variables were summarized with the sample median, minimum, and maximum. Categorical variables were summarized with number and percentage. Associations of the binary outcome of Anxiety on the MAX-PC at follow-up (mild to moderate vs. high anxiety) with pathological and demographic features were assessed using Wilcoxon Rank Sum, Fisher's exact, and Cochran-Armitage Trend tests. Associations of the binary outcome of surgery type (RRP vs. RLAP) on follow-up HRQOL scores were assessed using Wilcoxon Rank Sum test. Associations of the outcome across nerve-sparing procedures (unilateral, bilateral, or none) on follow-up HRQOL scores were assessed using the Kruskal–Wallis test. Associations of the ordinal outcomes of baseline MAX-PC and EPIC scores with their response scores at follow-up were assessed using Spearman's test of correlation, where Spearman's correlation coefficient r was estimated. A p-value of 0.05 or less was considered statistically significant. All analyses were performed using SAS [25].
Results
Of the 350 men completing a follow-up HRQOL questionnaire, 226 (65%) also had a baseline HRQOL prior to surgery. Follow-up was defined by HRQOL questionnaires returned six to 18 months from the date of surgery. Follow-up was completed and returned with a mean of 13.3 months (SD, 2.1) postsurgery.
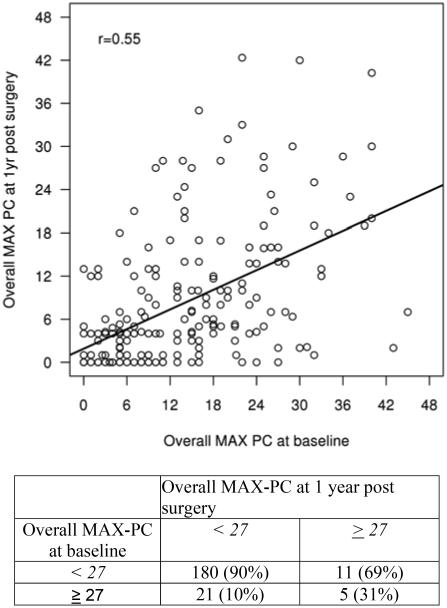
Summaries of the main outcome (MAX-PC scores) on the patient, clinical, and pathological characteristics are given in Tables 1 and 2. Those reporting high anxiety at follow-up were more likely to have a pathological Gleason score >6 (p = 0.004), T-Stage ≥ pT2C disease (p = 0.004), and a postoperative PSA > 0.1 (p = 0.002). There were no significant associations of surgery type (RLAP vs. RRP) or intraoperative nerve-sparing status with MAX-PC scores at follow-up. Baseline and follow-up scores on the MAX-PC were significantly correlated, particularly the overall MAX-PC score at baseline with the follow-up PCa Anxiety subscale (r = 0.5580, p < 0.001) and with the follow-up overall MAX-PC score (r = 0.5479, p < 0.001). Baseline and follow-up overall MAX-PC scores are shown in Figure 1.
Table 2.
Comparison of overall follow-up Memorial Anxiety Scale for Prostate Cancer scores for mild to moderate anxiety versus high anxiety (N = 343)
| Variablea | Mild to moderate Anxiety (N = 321) |
High anxiety (N = 22) |
p-Value | |
|---|---|---|---|---|
| 1. PCa, prostate cancer; PSA, prostate-specific antigen. | ||||
| 2. a | ||||
| The sample median (minimum, maximum) is given for numerical variables, n (%) for categorical variables. | ||||
| 3. b | ||||
| Comparison for marital status compared married versus not married, comparison for race compared white with non-white. | ||||
| 4. c | ||||
| p-values are based on Fisher's exact test | ||||
| 5. d | ||||
| p-values are based on Wilcoxon Rank Sum test | ||||
| 6. e | ||||
| p-value is based on the Cochran-Armitage Trend test | ||||
| Age at treatment (years) | 64.0 (42.7, 78.1) | 58.9 (46.9, 76.0) | 0.094 d | |
| Marital status b | 0.024 c | |||
| Married | 289 (90%) | 16 (73%) | ||
| Single | 10 (3%) | 3 (14%) | ||
| Separated/divorced | 19 (6%) | 3 (14%) | ||
| Widowed | 3 (1%) | 0 (0%) | ||
| Race—White b | 292 (91%) | 17 (77%) | 0.054 c | |
| History of erectile dysfunction (unknown in 38 mild; 2 high anxiety) |
133 (47%) | 10 (50%) | 0.82 c | |
| Diabetes | 30 (9%) | 1 (5%) | 0.71 c | |
| Family history of PCa | 89 (28%) | 8 (36%) | 0.46 c | |
| Preoperative PSA (unknown in 6 mild anxiety) |
0.10 d | |||
| <4 | 64 (20%) | 4 (18%) | ||
| 4–10 | 228 (72%) | 14 (64%) | ||
| >10–20 | 20 (6%) | 2 (9%) | ||
| >20 | 3 (1%) | 2 (9%) | ||
| Pretreatment Gleason score (missing in n = 1 mild anxiety) |
0.025 d | |||
| 4–6 | 178 (56%) | 6 (27%) | ||
| 7 | 105 (33%) | 13 (59%) | ||
| 8–10 | 37 (12%) | 3 (14%) | ||
| Pathological Gleason score | 0.004 d | |||
| 6 | 123 (38%) | 2 (9%) | ||
| 7 | 170 (53%) | 16 (73%) | ||
| 8–10 | 28 (9%) | 4 (18%) | ||
| Treatment type | 1.00 c | |||
| Radical retropubic prostatecomy | 142 (44%) | 10 (45%) | ||
| Robotic laparoscopic retropubic prostatectomy |
179 (56%) | 12 (55%) | ||
| T stage (Missing in n = 1 mild anxiety) | 0.004 e | |||
| 1c | 2 (1%) | 0 (0%) | ||
| 2a,2b | 64 (20%) | 2 (9%) | ||
| 2c | 218 (68%) | 12 (55%) | ||
| 3a,3b | 36 (11%) | 8 (36%) | ||
| Prostatic capsule involvement (unknown in 3 mild, 1 high) |
61 (19%) | 7 (33%) | 0.15 c | |
| Positive margins (unknown in 1 mild anxiety) | 87 (27%) | 12 (55%) | 0.013 c | |
| Seminal vesicle involvement (unknown in 2 mild anxiety) |
15 (5%) | 4 (18%) | 0.027 c | |
| Nerve sparing | 0.95 e | |||
| No | 30 (9%) | 3 (14%) | ||
| Full | 266 (83%) | 19 (86%) | ||
| Partial | 25 (8%) | 0 (0%) | ||
| PSA 6–18 months postsurgery (missing in 45 mild anxiety; 2 high) |
0.002 d | |||
| 0–0.1 | 249 (90%) | 13 (65%) | ||
| >0.1–0.5 | 20 (7%) | 3 (15%) | ||
| >0.5–2 | 5 (2%) | 2 (10%) | ||
| >2–4 | 1 (<1%) | 1 (5%) | ||
| >4–<10 | 1 (<1%) | 0 (0%) | ||
| >10 | 0 (0%) | 1 (5%) | ||
Figure 1.
Association of Memorial Anxiety Scale for Prostate Cancer overall score at baseline versus follow-up postsurgery using a cutoff of ≥27 (n = 201)
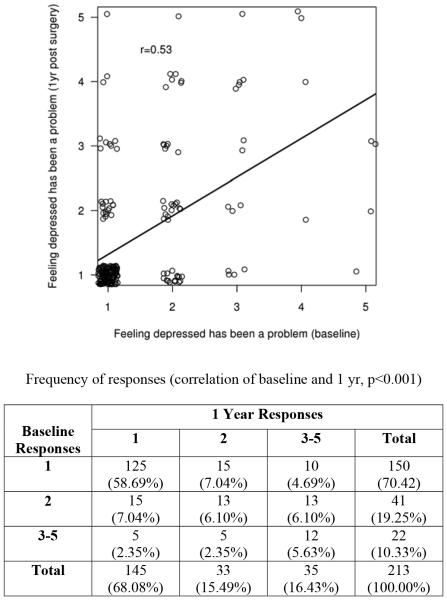
Scores on the EPIC subscales at baseline were predictive of EPIC scores at follow-up. Baseline SS and follow-up SF scores (r = 0.5871, p < 0.001), baseline and follow-up SF scores (r = 0.5790, p < 0.001), and baseline and follow-up SS scores were positively correlated (r = 0.5676, p < 0.001). Of note, baseline Q31d, ‘How much of a problem has feeling depressed been for you over the last 4 weeks?’ was significantly correlated to the same question at follow-up (r = 0.5340, p < 0.001). Spearman's rank correlation coefficient was used to measure the association between depression as a problem response at baseline and at follow-up post-surgery. This association resulted in a correlation coefficient of 0.53 (p-value <0.001), suggesting statistical dependence of the follow-up post-surgery response on the baseline response. At baseline, 22 patients (10%) found depression to be at least a small problem ranging to a big problem, increasing to 35 (16%) at follow-up post-surgery (Figure 2). The feeling of depression being a problem at baseline was not related to clinical measures of PCa aggressiveness. Depression as a problem at follow-up was moderately associated with overall MAX-PC scores (r = 0.380, p < 0.001) and weakly associated with the pathological measure of T stage (r = 0.128, p = 0.017). Age, race, and urinary function (p = 0.083) were not significantly associated with anxiety or problem depression in this study.
Figure 2.
Association of Expanded Prostate Cancer Index Composite Q31d response at baseline versus follow-up postsurgery (1 = no problem, 2 = very small problem, 3 = small problem, 4 = moderate problem, 5 = big problem)
Discussion
This is the first study to our knowledge that has examined the predictive nature of the baseline MAX-PC on levels of PCa-specific anxiety, HRQOL, and PCa aggressiveness at follow-up following surgical treatment for PCa. Our findings indicate that the MAX-PC can be used to screen patients for levels of anxiety that may require intervention prior to or following PCa surgery. High anxiety was correlated with pathological measures of PCa aggressiveness at follow-up, suggesting that patients with more pathologically aggressive disease could benefit from interventions aimed at aiding the patients to cope with the effect of PCa after initial treatment. These results were somewhat anticipated, given the nature of the prognoses and PSA screenings following treatment. However, some patients who reported high anxiety at baseline and follow-up did not have clinically significant disease.
Korfage et al. [18] reported similar findings in their 2006 study evaluating 5-year anxiety outcomes in Dutch men undergoing PCa treatment via radical prostatectomy or radiotherapy using the generalized State Trait Anxiety Inventory. Of note, anxiety levels remained high for some patients during follow-up despite a favorable prognosis, though the author suggests that more data needs to be collected on the sensitivity of the State Trait Anxiety Inventory on PCA-specific anxiety. Our findings using the MAX-PC support integrated psychological or behavioral services to address clinically-significant, PCa-specific anxiety for any patients in need of intervention regardless of prognosis.
Scores on the EPIC were likewise moderately predictive of follow-up scores of HRQOL. Better sexual function at baseline indicated better sexual function after surgery, controlling for nerve-sparing procedure. RRP or RLAP did not influence scores on any measure of HRQOL. This notably indicates that a patient's HRQOL is not determined by the type of surgery offered.
Scores on depression questions were also correlated at follow-up. Those reporting at least some level of problem with depression on Q31d at baseline (small, moderate, or big problem) were likely to report some level of problem with depression at follow-up. In fact, patients were more likely to report depression at follow-up than at baseline. Problems with depression were not related to age and were only slightly associated with T stage at follow-up. Depression was moderately correlated with Overall MAX-PC at follow-up as well, suggesting that both depression and anxiety are consistent for a portion (16%) of this patient population despite favorable outcomes for PCa treatment in the literature.
From a clinical perspective, selecting a treatment plan for PCa is a complex process with numerous therapeutic choices that rely on the patient's age and health in combination with cancer grade and stage. Following treatment for PCa, the patient is faced with the anxiety of cancer recurrence. Reporting to the urology clinic for repeat PSA testing every 4 to 6 months to assess for cancer can lead to pretest anxiety and depression. Stress over fluctuations in PSA levels can lead to uncertainty of disease progression and is a key reason for the high dropout rate of men from active surveillance protocols [26]. Men with PCa tend to have lower anxiety and depression compared with patients with breast, lung, or gastrointestinal cancers, whereas younger patients and those with advanced disease stage tend to report higher levels of anxiety. If the treating urologist is able to describe a PCa diagnosis that is not life-threatening, then one can provide a sense of relief to patients [27]. However, visits to the urologist regarding psychosocial issues may not fully address the psychological needs of a patient.
There is a shift in HRQOL following surgical treatment for PCa. Preoperative anxiety levels are predictive of follow-up postsurgical anxiety levels on the MAX-PC. Furthermore, patients reported an increased problem with the feeling of depression at follow-up. Levels of PCa-specific anxiety indicate that patients are lacking coping strategies to deal with the consequences of a diagnosis of PCa. Sexual function, anxiety, and feelings of depression are at least moderately correlated at follow-up, suggesting that HRQOL is a complex issue for patients that may require more attention from healthcare providers. Instruments like the distress thermometer are also useful and support the notion that routine screening and follow-up on cancer-specific distress can aid providers in caring for their patient in the most beneficial and timely ways possible [28]. Ultimately, psychological and behavioral interventions offered throughout treatment and follow-up may better suit the needs of the patient, with the goal of reducing distress and improving QoL [29].
Limitations and future research
This study had several limitations of note. A primary limitation is that the data was collected as part of a clinical urological practice. As such, the primary focus of the clinical questionnaires was on urological functioning and PCa-specific anxiety. Psychological instruments used in the clinical registry, like the MAX-PC, were not compared with other gold standard psychological instruments such as the Hospital Anxiety and Depression Scale. However, we felt that the data collected within the EPIC as well as the MAX-PC, were important indicators of a patient's quality of life, although we had limited information on levels of depression it his study. Although did not focus on the EPIC's urinary function scale specifically, one should note that urinary function could be related to PCa-specific anxiety and may be an area for future research. Therefore, it is important to emphasize that a future studies on psychosocial effects of PCa should include validated depression and anxiety scales as part of the data collection. Keeping in mind that we are trying to establish a prognostic use for the MAX-PC, a comparative anxiety scale with established prognostic features would strengthen the study efforts. This is certainly a focus for future research.
A second limitation was the inability to randomize patients to either RLAP or RRP surgical groups. Surgical technique was dictated by the urological practice. The data collected was part of a prospective, observational database, and the questionnaires were returned voluntarily. This also made it more challenging to increase the response rates for each group. Diversity was also lacking in this population. However, responses were somewhat evenly distributed across the two groups and showed no significant difference in rates of anxiety between RRP or RLAP. Future research might explore how these two groups did not differ in patient outcomes.
Lastly, we chose to focus on surgical interventions in this study. Focusing on surgical patients allowed us to better control for age as a variable, given that older men may be given less invasive options. Although radiation, cryotherapy, seed therapy, chemotherapy, and active surveillance are among other treatment options available to men with PCa, these types of treatments were not as readily collected within the surgical urology practice. Future studies would benefit from including other treatment types when evaluating adjustment outcomes following diagnosis and treatment for PCa.
Conclusion
These data suggest that the MAX-PC could be a useful baseline tool for identifying those who could benefit from psychological intervention prior to and following treatment for PCa. Men with PCa may benefit from a referral for therapy from an experienced mental health care professional who is familiar with the needs of PCa patients. In terms of individualized medicine, behavioral therapy may be most beneficial to improve HRQOL for younger patients, those with advanced stage disease, and more specifically, those whose anxiety outweighs their actual prognosis.
References
- 1.American Cancer Society . Cancer Facts and Figures 2010. American Cancer Society; Atlanta, GA: 2010. [Google Scholar]
- 2.Zincke H, Osterling JE, Blute ML, Bergstralh EJ, Myers R, Barrett DM. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152:1850–1857. doi: 10.1016/s0022-5347(17)32399-6. [DOI] [PubMed] [Google Scholar]
- 3.Tavlarides AM, Parker AS, Thiel DD, Diehl NN, Ames SC. Prostate cancer-specific anxiety in men one year follow surgical treatment for newly diagnosed prostate cancer. Psycho-Oncology. 2012;22(6):1328–1335. doi: 10.1002/pon.3138. [DOI] [PubMed] [Google Scholar]
- 4.Khan NF, Ward AM, Watson E, Rose PW. Consulting and prescribing behaviour for anxiety and depression in long-term survivors of cancer in the UK. Eur J Cancer. 2010;46(18):3339–3344. doi: 10.1016/j.ejca.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Johansson E, Bill-Axelson A, Holmberg L, Onelov E, Johansson JE, Steineck G. Scandinavian Prostate Cancer Group Study No. 4. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: The randomized Scandinavian Prostate Cancer Group Study Number 4 (SPGG-4) clinical trial. Eur Urol. 2009;55(2):422–430. doi: 10.1016/j.eururo.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Kotwal AA, Schumm P, Mohile SG, Dale W. The influence of stress, depression, and anxiety on PSA screening rates in a nationally representative sample. Med Care. 2012;50(12):1037–1044. doi: 10.1097/MLR.0b013e318269e096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consedine NS, Adjei BA, Ramirez PM, McKiernan JM. An object lesson: source determines the relations that trait anxiety, prostate cancer worry, and screening fear hold with prostate screening frequency. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1631–1639. doi: 10.1158/1055-9965.EPI-07-2538. [DOI] [PubMed] [Google Scholar]
- 8.Nelson GJ, Weinberger MI, Balk E, Holland J, Breitbart W, Roth AJ. The chronology of distress, anxiety, and depression in older prostate cancer patients. Oncologist. 2009;14(9):891–899. doi: 10.1634/theoncologist.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth AJ, Rosenfeld B, Kornlith AB, et al. The memorial anxiety scale for prostate cancer: validation of a new scale to measure anxiety in men with prostate cancer. Cancer. 2003;97:2910–2918. doi: 10.1002/cncr.11386. [DOI] [PubMed] [Google Scholar]
- 10.Pietrow PK, Parekh DJ, Smith JA, Jr., Shyr Y, Cookson MS. Health related quality of life assessment after radical prostatectomy in men with prostate specific antigen only recurrence. J Urol. 2001;166(6):2286–2290. [PubMed] [Google Scholar]
- 11.Clark JA, Bokhour BG, Inui TS, Silliman RA, Talcott JA. Measuring patients' perceptions of the outcome of treatment for early prostate cancer. Med Care. 2003;41(8):923–936. doi: 10.1097/00005650-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Van den Bergh RC, Essink-bot ML, Roobol MJ, Schröder FH, Bangma CH, Steyerberg EW. Do anxiety and distress increase during active surveillance for low risk prostate cancer? J Urol. 2012;183(5):1786–1791. doi: 10.1016/j.juro.2009.12.099. [DOI] [PubMed] [Google Scholar]
- 13.Kazar MW, Psutka SP, Latini DM, Bailey DE., Jr. Psychosocial aspects of active surveillance. Urology. 2013;23(3):273–277. doi: 10.1097/MOU.0b013e32835eff24. [DOI] [PubMed] [Google Scholar]
- 14.Nelson CJ, Mulhall JP, Roth AJ. The association between erectile dysfunction and depressive symptoms in men treated for prostate cancer. J Sex Med. 2011;8(2):560–566. doi: 10.1111/j.1743-6109.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- 15.Kahana B, Kahana E, Deimling G, Sterns S, VanGunten M. Determinants of altered life perspectives among older-adult long-term cancer survivors. Cancer Nurs. 2011;34(3):209–218. doi: 10.1097/NCC.0b013e3181fa56b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Beuken-van EMH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Quality of life and non-pain symptoms in patients with cancer. J Pain Symptom Manage. 2009;38(2):216–233. doi: 10.1016/j.jpainsymman.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Messaoudi R, Menard J, Ripert T, Parquet H, Staerman F. Erectile dysfunction and sexual health after radical prostatectomy: Impact of sexual motivation. Int J Impot Res. 2011;23(2):81–86. doi: 10.1038/ijir.2011.8. [DOI] [PubMed] [Google Scholar]
- 18.Korfage IJ, Essink-Bot ML, Janssens AC, Schröder FH, de Koning HJ. Anxiety and depression after prostate cancer diagnosis and treatment: 5-year follow up. Br J Cancer. 2006;94(8):1093–1098. doi: 10.1038/sj.bjc.6603057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth A, Nelson CJ, Rosenfeld B, et al. Assessing anxiety in men with prostate cancer: Further data on the reliability and validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) Psychosomatics. 2006;47(4):340–347. doi: 10.1176/appi.psy.47.4.340. [DOI] [PubMed] [Google Scholar]
- 20.Gore JL, Gollapudi K, Bergman J, Kwan L, Krupski TL, Litwin MS. Correlates of bother following treatment for clinically localized prostate cancer. J Urol. 2010;184(4):1309–1315. doi: 10.1016/j.juro.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Martin AD, Nakamura LY, Nunez RN, Wolter CE, Humphreys MR, Castle EP. Incontinence after radical prostatectomy: a patient centered analysis and implications for preoperative counseling. Urology. 2011;186(1):204–208. doi: 10.1016/j.juro.2011.02.2698. [DOI] [PubMed] [Google Scholar]
- 22.Gerbershagen HJ, Ozgur E, Straub K, et al. Prevalence, severity, and chronicity of pain and general health-related quality of life in patients with localized prostate cancer. Eur J Pain. 2008;12(3):339–350. doi: 10.1016/j.ejpain.2007.07.006. Direct Link: [DOI] [PubMed] [Google Scholar]
- 23.Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–1669. [PubMed] [Google Scholar]
- 24.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. J Urol. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute Inc . SAS® 9.2 Companion for Windows. 2nd SAS Institute Inc; Cary, NC: 2010. [Google Scholar]
- 26.Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369–374. doi: 10.1016/j.pec.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Vodermaier A, Linden W, MacKenzie R, Creig D, Marshall C. Disease stage predicts post-diagnosis anxiety and depression only in some types of cancer. Brit J Cancer. 2011;105:1814–1817. doi: 10.1038/bjc.2011.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers SK, Zajdlewicz L, Youlden DR, Holland JC, Dunn J. The validity of the distress thermometer in prostate cancer populations. Psycho-Oncology. 2013;2:195–203. doi: 10.1002/pon.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers SK, Smith DP, Berry M, et al. A randomized controlled trial of mindfulness intervention for men with advanced stage prostate cancer. BMC Cancer. 2013;13:1–5. doi: 10.1186/1471-2407-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]