Abstract
Astrocytes are the most abundant non-neuronal glial cells in the brain. Once relegated to a mere supportive role for neurons, contemporary dogmas ascribe multiple active roles for these cells in central nervous system (CNS) function, including maintenance of optimal glutamate levels in synapses. Regulation of glutamate levels in the synaptic cleft is crucial for preventing excitotoxic neuronal injury. Glutamate levels are regulated predominantly by two astrocytic glutamate transporters, glutamate transporter 1 (GLT-1) and glutamate aspartate transporter (GLAST). Indeed, the dysregulation of these transporters has been linked to several neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD) and Parkinson's disease (PD), as well as manganism, which is caused by overexposure to the trace metal, manganese (Mn). Although Mn is an essential trace element, its excessive accumulation in the brain as a result of chronic occupational or environmental exposures induces a neurological disorder referred to as manganism, which shares common pathological features with Parkinsonism. Mn decreases the expression and function of both GLAST and GLT-1.Astrocytes are commonly targeted by Mn, and thus reduction in astrocytic glutamate transporter function represents a critical mechanism of Mn-induced neurotoxicity. In this review, we will discuss the role of astrocytic glutamate transporters in neurodegenerative diseases and Mn-induced neurotoxicity.
Keywords: Astrocytes, Glutamate, GLAST, GLT-1, glutamate transporters, Manganese, Neurotoxicity
Introduction
Astrocytes, which have been typically relegated as structural and nutritional support for neurons, exert several essential functions to maintain optimal neuronal activity [1]. One of their major functions is to maintain low levels of extracellular glutamate to prevent excitotoxic neuronal injury. The maintenance of optimal synaptic glutamate levels is achieved predominantly by two glutamate transporters which are preferentially expressed in astrocytes: glutamate aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1), known respectively as excitatory amino acid transporter (EAAT)1 and EAAT2 in humans [2]. The decreased expression and function of these transporters has been linked to multiple neurodegenerative disorders including amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD), Parkinson disease (PD) and Huntington's disease (HD) [3]. Manganese (Mn) neurotoxicity has been implicated in the development of these neurological disorders [4] along with manganism. Mn induces mitochondrial impairment, oxidative stress, and dysfunction of astrocytic glutamate transporters [5-7].
Astrocyte functions on neuronal survival and protection in the CNS
Astrocytes comprise greater than half of the brain volume, and carry out multiple essential functions, playing an active role in regulating CNS physiology. Astrocytes participate in a wide range of functions from synaptic transmission [8] to adult neurogenesis [9]. Consequently, dysfunction of astrocytes has been associated with a number of neurological disorders, including epilepsy, ALS, schizophrenia and manganese neurotoxicity, to name a few [10]. Astrocytes exert several important roles which are closely related to the prevention of neurotoxicity including antioxidant action, production of growth factors, and regulation of glutamate neurotransmission. For example, neurons are more vulnerable to injury due to their limited anti-oxidant capacity, and rely on astrocytes for productions of anti-oxidant enzymes and substances against neuronal oxidative stress. Astrocytes are a rich source of various anti-oxidants, including glutathione (GSH), ascorbate and superoxide dismutases [11, 12]. Astrocytes are also critically involved in neuronal survival and neuroprotection by producing various neurotrophic factors including brain- and glial- derived neurotrophic factors, nerve growth factor, insulin-like growth factor, basic fibroblast growth factor and transforming growth factor-α and -β [13-16]. Astrocytes are essential in the regulation of neurotransmitter homeostasis, particularly glutamate. During glutamate neurotransmission, astrocytes remove ~80% of glutamate from extracellular synaptic clefts via the glutamate transporters, GLT-1 and GLAST [17, 18].
Astrocytic glutamate transporters
In humans, there are five subtypes of excitatory amino acid transporters (EAATs) that have ~50% sequence identity and ~60% structural similarity among them [19]. These five subtypes also show distinct expression profiles; EAAT1 (GLAST in rodents) and EAAT2 (GLT-1 in rodents) are predominantly expressed in astrocytes, while the other three members, EAAT3, EAAT4 and EAAT5, are expressed in neurons with EAAT3 abundantly expressed throughout the CNS, EAAT4 localized in the cerebellum and EAAT5 in retina [20]. These transporters also show variation in expression and activity during developmental stages, as GLAST is mostly active in the developing CNS, while GLT-1 is predominant in the adult CNS [21]. Glutamate transport via astrocytic glutamate transporters is energy-dependent, coupled with uptake of three Na+ ions and one H+ ion, and the counter transport of one K+ ion [22, 23]. These transporters normally exist as oligomers, especially trimers, in the plasma membranes with each monomer acting as an independent functional unit [24-26].
Astrocytic glutamate transporters, GLT-1 and GLAST, play a major role in preventing excitotoxic neuronal injury. It has been shown that deletion of GLT-1 increases neuronal susceptibility to cortical injury as well as seizure activity in GLT-1 knockout mice [27]. Mutation of GLAST induces loss of motor coordination and increases the susceptibility of cerebellar neurons to neurotoxic insults in GLAST mutant mice [28]. Increased levels of glutamate in the extracellular space contribute to the development of these pathological symptoms in both studies. Moreover, knock down of GLT-1 and GLAST by their respective antisense oligonucleotides in both in vitro and in vivo settings induces excitotoxicity-related neurodegeneration and progressive paralysis, secondary to elevated extracellular glutamate [18].
Dysfunctions of astrocytic glutamate transporters in neurological diseases
Given the critical role of the glutamate transporters in maintaining glutamate levels below the toxic threshold, impairment of expression and function of these transporters leads to excitotoxic neuronal injury and is linked to numerous neurological disorders.
A decrease in glutamate uptake as well as expression of GLT-1 and GLAST has been noted in cultured cortical astrocytes derived from AD patients [29]. GLT-1 expression is also decreased in animal models of AD [30]. Amyloid beta (Aβ), whose excessive deposition as amyloid plaques in the brain is considered to play a key role in the etiology of AD, has been lined to a reduction in the expression of both GLT-1 and GLAST, as well as glutamate uptake in cultured astrocytes [31, 32]. Moreover, a reduction of GLT-1 expression and glutamate uptake has been noted in ALS patients [33, 34]. The impaired GLT-1 function in ALS is secondary to inflammation and oxidative stress [35]. Expression and function of both GLT-1 and GLAST are also decreased in animal models of seizure [36, 37].
In PD, impairment in astrocytic glutamate transporter function is associated with damage to nigrostriatal dopaminergic neurons [38]. In a PD animal model employing unilateral 6-hydroxydopamine, it has been shown that GLT-1 and GLAST proteins are significantly reduced in the striatum of the lesioned rats [39]. Similarly, N-methyl-4-phenylpyridinium (MPP+), a chemical known to mimic PD-like symptoms in humans and animal models, has been shown to induce dysregulation of glutamate uptake in both in vitro and in vivo experimental models [40, 41]. Clinically, the role of glutamate transporters in PD is further supported by observations on decreased glutamate uptake in platelets from PD patients [42]. However, the role on glutamate transporters in PD animal models remain controversial as some studies have failed to note changes in mRNA/protein levels of these transporters in 6-hydroxydopamine-lesioned rats [43, 44].
Heavy metal-induced dysfunction of glutamate transporters
Astrocytic glutamate transporters are susceptible to heavy metals, such as lead (Pb) and methylmercury (MeHg), in addition to Mn. Pb has been shown to decrease expression and function of both GLAST and GLT-1 [45]. The effects of Pb on glutamate transporters were region-specific, with the hippocampus being the most vulnerable area, thus correlating with the preponderance of hippocampally-induced cognitive deficits associated with Pb exposure [46]. MeHg exposure has been shown to trigger increased reactive oxygen species, specifically H2O2, which inhibited EAAT1/GLAST function as well as EAAT1/GLAST mRNA expression in primary cultures of astrocytes [47]. MeHg also contributes to the dysregulation of glutamate homeostasis by impairing GLAST and GLT-1 function in CHO cells transfected with these transporters [48]. Notably, the MeHg-induced glutamate dyshomeostasis and increased oxidative stress are attenuated by memantine, a N-methyl-D-aspartate (NMDA) receptor inhibitor, consistent with MeHg-induced dysregulation of glutamate homeostasis [49].
Mn toxicity associated with dysregulation of glutamate transporters has been a subject of recent studies by our group [50], and it is discussed in the following sections.
Mn neurotoxicity
Mn is an essential trace element that is required for numerous biochemical reactions. It is ubiquitously present in all human tissues and carries out a broad range of functions, including immune response, ATP generation, bone growth, digestion and reproduction [51]. In addition, Mn serves as a cofactor for multiple metalloenzymes, such as glutamine synthetase, mitochondrial superoxide dismutase, arginase and pyruvate decarboxylase [52-54]. However, accumulation of Mn in the brain following chronic exposure to excessive levels of this metal, from either environmental or occupational sources, leads to a neuropathologic sequalae, referred to as manganism. The clinical features of manganism are in large measure analogous to those seen in PD, characterized by cognitive and motor dysfunction [55, 56]. A variety of industries, including welding [57], mining [58], battery production [59], and Mn-alloy plants [60] represent occupational sources of Mn exposures. Environmental sources of Mn exposure include the usage of an Mn-containing gasoline anti-knock additive, methylcyclopentadienyl manganese tricarbonyl (MMT), and fungicidal pesticides, such as maneb [61]. In addition, consumption of soy-based formulas [62], total parenteral nutrition [63] and drinking water contaminated with high levels of Mn [64] represent other potential Mn sources. Mn is absorbed into the human body via inhalation or gastrointestinal absorption, followed by its transport into the brain by various transporters, such as the divalent metal transporter-1 (DMT-1) [65, 66], transferrin [67], and the divalent metal/bicarbonate ion symporters ZIP8 and ZIP14 [4], to name a few. Notably, Mn transport into the brain is enhanced by activation of NMDA receptors [66].
Mn and neurodegenerative disorders
In addition to manganism, which is characterized as a neurological disorder with shared features of PD, a growing body of evidence reveals that Mn overexposure is implicated in multiple neurodegenerative diseases. Chronic exposure to high content of Mn may precipitate PD progression by decreasing striatal dopamine turnover and promoting a-synuclein misfolding and aggregation [68, 69]. Mn overload has also been suggested to be closely related to ALS as a Mn smelter developed occupational manganism and bulbar ALS in Germany [70]. Moreover, High content of Mn has been reported in ALS cases in the spinal cord [71]. Mn overload has also been implicated in prion disease as Mn triggers misfolding and aggregation of prion protein [72] and elevated Mn levels have been observed in the CNS in human prion disease [73]. Mn overload may play a role in AD as a patient with elevated Mn levels showed dementia and typical pathological signs of AD in the brain such as numerous neuritic plaques and neurofibrillary tangles [74].
Mechanisms of Mn neurotoxicity
Given that Mn neurotoxicity is associated with multiple neurodegenerative diseases [4], numerous studies have been conducted in order to understand the cellular and molecular mechanisms involved in Mn effects in the brain. Oxidative stress, mitochondrial dysfunction, apoptosis, inflammation and excitotoxicity have been posited as the primary molecular and cellular mechanisms of Mn-induced neurotoxicity. Since Mn accumulates in dopamine-rich regions of the brain, such as the globus pallidus, substantia nigra and striatum, Mn-induced dopamine oxidation has also been suggested as a primary mechanism of its neurotoxicity [75, 76]. Moreover, Mn directly induces oxidative stress, as both in rats [77] and primates [78] it decreases glutathione (GSH) levels, and treatment with anti-oxidants (N-acetylcysteine) rescues the Mn-induced pathological phenotype [79].
The preferential sequestration of Mn in mitochondria makes this energy producing organelle vulnerable to Mn toxicity. In mitochondria, Mn interferes with oxidative phosphorylation by inhibiting F1ATPase at low levels [80], and by inhibiting complex I of the electron transport chain at higher concentrations [81]. Mn-induced apoptosis and inflammation also play an important role in Mn neurotoxicity. During Mn-induced apoptotic cell death, both extrinsic and intrinsic apoptotic pathways are activated both in astrocytes and neurons [82, 83]. Moreover, Mn potentiates the release of several inflammatory molecules, including cytokines, such as TNF-α, IL-6 and IL-1β, prostaglandins, and nitric oxide from activated glial cells, leading to neuroinflammation [84-86]. Excitotoxic neuronal death also contributes to Mn neurotoxicity, as Mn reduces the expression and function of astrocytic glutamate transporters [87, 88]. Inhibition of NMDA receptors with MK 801, an NMDA antagonist, reverses Mn-induced lesions in rat striatum, affirming the involvement of excitotoxic neuronal injury in Mn-induced neurotoxicity [89].
Mn-induced astrocytic dysregulation
Astrocytes preferentially take up Mn, concentrating it up to 50-60 fold higher than in adjacent neurons [90]. Accordingly, astrocytes are considered as the primary site of early damage and dysfunction upon elevated cerebral Mn levels. In addition to manganism, another neuropathological condition that is associated with astrocyte-mediated Mn toxicity is hepatic encephalopathy (HE), which is characterized by brain edema secondary to astrocyte swelling [91]. HE is accompanied by increased levels of Mn in the blood and brain [92], and the development of Alzheimer's type II astrocytes by displaying enlarged pale nuclei and prominent nucleoli [93]. Oxidative/nitrosative stress and mitochondrial dysfunction are involved in Mn-induced astrocyte swelling since pretreatment of astrocytes with vitamin E (antioxidant), L-NAME (nitric oxide synthase inhibitor) and Cyclosporin A (mitochondrial permeability transition inhibitor) attenuate astrocyte swelling [91]. Mn-induced astrocyte swelling is mediated by enhanced membrane expression of water channel protein aquaporin-4, as knockdown of aquaporin-4 with siRNA significantly attenuates Mn-induced astrocyte swelling [94].
Reactive astrocyte-induced generation of pro-inflammatory molecules with a subsequent neuroinflammation represents a key feature of Mn-induced neurotoxicity. This Mn action in astrocytes might be important because neuroinflammation is also inherent to several neurodegenerative diseases, including ALS and PD [84, 95, 96]. Co-treatment of astrocytes with subtoxic levels of Mn and the inflammatory inducer, lipopolysaccharide, or interferon-γ potentiates production and expression of inflammatory mediators, such as prostaglandin E2 and COX-2 [97]. Notably, the production of inflammatory molecules, especially TNF-α and IL-1β is directly related to the impairment in GLT-1 function [98, 99].
A role for astrocytic nitric oxide synthase 2 (NOS2) has been implicated in Mn-induced neurotoxicity, as genetic deletion of NOS2 (in ducible NOS) attenuates its neurotoxicity [100], and neuronal injury coincides with increased reactive astrocytes expressing NOS2 in Mn-exposed mice [101]. Taken together, astrocytes appear to play a critical role in Mn-induced neurotoxicity by multiple mechanisms, such as astrocyte swelling, inflammation and glutamate transporter dysfunction. Among those, impaired glutamate transporter function might be the most important factor due to its propensity to trigger excitotoxic neuronal injury.
Mn-induced impairment of astrocytic glutamate transporters
Inflammation and oxidative stress are likely involved in Mn-induced dysregulation of astrocytic glutamate transporters since oxidative stress inhibits glutamate transporter function [102]. In astrocytes, oxidative stressors such as peroxynitrite and H2O2 inhibit glutamate uptake via EAAT1, EAAT2 and EAAT3, while treatment with reducing agents, such as dithithreotol restores glutamate uptake [103]. The role of Mn-induced neuroinflammation has been implicated in the impairment of glutamate transporter expression and function, as Mn treatment releases inflammatory cytokine TNF-α [104] which reduces expression and function of GLT-1 [99].
The protein kinase C (PKC) pathway might play a critical role in mediating Mn-induced dysfunction of astrocytic glutamate transporters [105, 106]. Mn increases the phosphorylation of both the PKC-α and -δ isoforms in astrocytes [107], whereas inhibition of PKC reverses the Mn-induced decrease in glutamate uptake, as well as GLT-1 and GLAST protein levels [106]. Mn-induced activation of the apoptotic pathway also plays a role in the reduction of astrocytic glutamate transporter expression, since a pan caspase inhibitor, Z-VAD-FMK, efficiently attenuates Mn-induced reduction in glutamate uptake, as well as GLT-1 and GLAST protein levels [108].
Mechanisms of Mn-induced reduction of GLT-1/GLAST at the transcriptional level
Given that the reduction in expression and function of glutamate transporters is inherent to a myriad of neurodegenerative diseases, identifying the molecular target of Mn-induced reduction in astrocytic glutamate transporter expression at the transcription level would be highly desirable. It may facilitate the development of novel therapeutics to restore impairment in glutamate transporter function. Several studies have shown that NF-κB mediates the stimulatory effects of various positive regulators of GLT-1, including neuronal secreting factors [109], epidermal growth factor, dibutyryl cAMP [110], ceftriaxone [111], as well as estrogenic compounds such as estrogen, tamoxifen and raloxifene [112, 113].
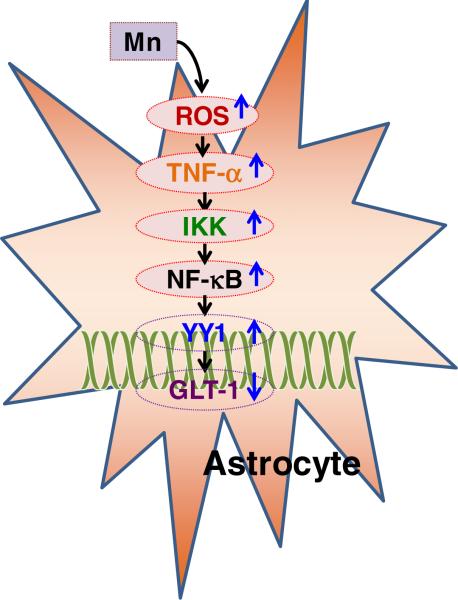
However, the negative regulatory mechanism of GLT-1 at the transcriptional level remains largely unexplored. Recently, findings from our group revealed that the transcription factor yin yang 1 (YY1) is a critical repressor of GLT-1 and also mediates Mn-induced down-regulation of GLT-1 in astrocytes as shown in Figure 1 [104].
Figure 1.
Mn produces ROS and TNF-α which activate YY1 via the IKK/NF-κB pathway. YY1 is a repressor of the GLT-1 promoter and decreases GLT-1 expression. IKK, IκB kinase; TNF-α: tumor necrosis factor-α; NF-κB, nuclear factor κB.
YY1 is a critical mediator for Mn-induced reduction of GLT-1 expression and function
YY1 is a multifunctional transcription factor that can initiate, activate or repress gene transcription depending on its interaction with other available cellular factors [114]. It is composed of 414 amino acids with four zinc fingers at the C-terminus in human YY1 [115]. Although the role of YY1 in the physiological as well as pathological conditions has been studied extensively in developmental and cancer biology [116, 117], YY1 has also been recognized to play a critical role in the nervous system such as neural development [118] and developmental myelination [119]. In astrocytes, YY1 is involved in regulation of glutamate homeostatis by modulating glutamate transporters, GLAST and GLT-1. It has been reported that excess glutamate increased YY1 binding to GLAST promoter and decreased glutamate uptake in chick Bergman glia cells [120]. Moreover, astrocyte elevated gene-1 (AEG-1) recruited YY1 to repress GLT-1 promoter activity and glutamate uptake in primary fetal human astrocytes [121]. Recently, our findings established that Mn enhanced YY1 promoter activity, YY1 mRNA and protein levels via NF-κB activation. Mn-induced TNF-α release from astrocytes may mediate the effects of Mn on GLT-1 repression via YY1 as TNF-α increased YY1 promoter activity, mRNA and protein levels, whereas it represses GLT-1 mRNA levels as shown in Fig. 1 [99].
Role of Histone deacetylases (HDACs) in Mn-activated YY modulation on GLT-1
Epigenetically, HDACs classes I and II serve as critical co-repressors of YY1 for GLT-1 promoter activity, and Mn enhances the interaction between YY1 and HDAC1 [104]. Interestingly, YY1 is a dominant factor by overriding the positive action of NF-κB on GLT-1 promoter activity when the two are simultaneously activated. Mn enhances the interaction of YY1 with NF-κB [104], suggesting that Mn activates NF-κB for GLT-1 activity, but decreases GLT-1 expression. HDAC inhibitors increase GLT-1 promoter activity and reverse the Mn- induced repression of GLT-1 promoter activity [104]. These results demonstrate that YY1, using HDACs as co-repressors, is a critical negative transcriptional regulator of GLT-1 and mediates Mn-induced GLT-1 repression. In addition, our findings provide molecular insight into Mn neurotoxicity, as well as neurological disorders associated with impairment of GLT-1 expression.
Summary
Astrocytes play a pivotal role in normal physiological functions of the brain including neuronal synaptic activities through various mechanisms. In addition, as predominant sources of anti-oxidants and numerous growth factors, astrocytes play a critical role in neuroprotection against various forms of toxic stimuli and brain injuries. More importantly, maintaining optimal glutamate levels in the synaptic cleft by removing excess glutamate into astrocytes via glutamate transporters represents one of the most important functions of these cells. Given that various neurological disorders, including manganism, are associated with dysfunction of astrocytic glutamate transporters, elucidation of molecular mechanisms by which Mn decreases expression of these transporters is imperative for identifying molecular targets and the development of potential therapeutics against neurodegenerative diseases. Our findings that YY1 plays a critical role in Mn-induced repression of GLT-1 gene transcription offers new insights for targeting such signaling pathways to treat neurological disorders associated with GLT-1 dysfunction and increased concetrations of synaptic glutamate levels.
Acknowledgements
The present study was supported in part by NIH grants, NIGMS SC1089630 (EL) andR01ES10563 and R01ES10563S1 (MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kimelberg HK. Functions of mature mammalian astrocytes: a current view. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- 2.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochemistry international. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman AB, Kwakye GF, Herrero Hernandez E, Aschner M. Role of manganese in neurodegenerative diseases. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS) 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Y, Xu Z, Xu B, Xu D, Tian Y, Feng W. The protective effects of riluzole on manganese-induced disruption of glutamate transporters and glutamine synthetase in the cultured astrocytes. Biological trace element research. 2012;148:242–249. doi: 10.1007/s12011-012-9365-1. [DOI] [PubMed] [Google Scholar]
- 6.Erikson KM, Suber RL, Aschner M. Glutamate/aspartate transporter (GLAST), taurine transporter and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology. 2002;23:281–288. doi: 10.1016/s0161-813x(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 7.Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochemical research. 1997;22:1443–1447. doi: 10.1023/a:1021994126329. [DOI] [PubMed] [Google Scholar]
- 8.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science (New York, NY) 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 9.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 10.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. Journal of the neurological sciences. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. European journal of biochemistry / FEBS. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindenau J, Noack H, Possel H, Asayama K, Wolf G. Cellular distribution of superoxide dismutases in the rat CNS. Glia. 2000;29:25–34. doi: 10.1002/(sici)1098-1136(20000101)29:1<25::aid-glia3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. The Journal of comparative neurology. 1995;355:479–489. doi: 10.1002/cne.903550402. [DOI] [PubMed] [Google Scholar]
- 14.Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 15.White RE, Rao M, Gensel JC, McTigue DM, Kaspar BK, Jakeman LB. Transforming growth factor alpha transforms astrocytes to a growth-supportive phenotype after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15173–15187. doi: 10.1523/JNEUROSCI.3441-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaheer A, Zhong W, Uc EY, Moser DR, Lim R. Expression of mRNAs of multiple growth factors and receptors by astrocytes and glioma cells: detection with reverse transcription-polymerase chain reaction. Cellular and molecular neurobiology. 1995;15:221–237. doi: 10.1007/BF02073330. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 18.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 19.Slotboom DJ, Konings WN, Lolkema JS. Structural features of the glutamate transporter family. Microbiology and molecular biology reviews : MMBR. 1999;63:293–307. doi: 10.1128/mmbr.63.2.293-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunch L, Erichsen MN, Jensen AA. Excitatory amino acid transporters as potential drug targets. Expert opinion on therapeutic targets. 2009;13:719–731. doi: 10.1517/14728220902926127. [DOI] [PubMed] [Google Scholar]
- 21.Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owe SG, Marcaggi P, Attwell D. The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. The Journal of physiology. 2006;577:591–599. doi: 10.1113/jphysiol.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 24.Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, Schmalzing G, Hidalgo P, Fahlke C. A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. The Journal of biological chemistry. 2004;279:39505–39512. doi: 10.1074/jbc.M408038200. [DOI] [PubMed] [Google Scholar]
- 25.Grewer C, Balani P, Weidenfeller C, Bartusel T, Tao Z, Rauen T. Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry. 2005;44:11913–11923. doi: 10.1021/bi050987n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdon G, Boudker O. Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog. Nature structural & molecular biology. 2012;19:355–357. doi: 10.1038/nsmb.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science (New York, NY) 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 28.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. The European journal of neuroscience. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. Journal of neurochemistry. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 30.Schallier A, Smolders I, Van Dam D, Loyens E, De Deyn PP, Michotte A, Michotte Y, Massie A. Region- and age-specific changes in glutamate transport in the AbetaPP23 mouse model for Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2011;24:287–300. doi: 10.3233/JAD-2011-101005. [DOI] [PubMed] [Google Scholar]
- 31.Scimemi A, Meabon JS, Woltjer RL, Sullivan JM, Diamond JS, Cook DG. Amyloid-beta1-42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5312–5318. doi: 10.1523/JNEUROSCI.5274-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. The New England journal of medicine. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 34.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of neurology. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 35.Mhatre M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer's disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. Journal of Alzheimer's disease : JAD. 2004;6:147–157. doi: 10.3233/jad-2004-6206. [DOI] [PubMed] [Google Scholar]
- 36.Ueda Y, Doi T, Tokumaru J, Yokoyama H, Nakajima A, Mitsuyama Y, Ohya-Nishiguchi H, Kamada H, Willmore LJ. Collapse of extracellular glutamate regulation during epileptogenesis: down-regulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. Journal of neurochemistry. 2001;76:892–900. doi: 10.1046/j.1471-4159.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 37.Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Annals of neurology. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 38.Markiewicz I, Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta neurobiologiae experimentalis. 2006;66:343–358. doi: 10.55782/ane-2006-1623. [DOI] [PubMed] [Google Scholar]
- 39.Chung EK, Chen LW, Chan YS, Yung KK. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. The Journal of comparative neurology. 2008;511:421–437. doi: 10.1002/cne.21852. [DOI] [PubMed] [Google Scholar]
- 40.Hazell AS, Itzhak Y, Liu H, Norenberg MD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) decreases glutamate uptake in cultured astrocytes. Journal of neurochemistry. 1997;68:2216–2219. doi: 10.1046/j.1471-4159.1997.68052216.x. [DOI] [PubMed] [Google Scholar]
- 41.Yang YL, Meng CH, Ding JH, He HR, Ellsworth K, Wu J, Hu G. Iptakalim hydrochloride protects cells against neurotoxin-induced glutamate transporter dysfunction in in vitro and in vivo models. Brain research. 2005;1049:80–88. doi: 10.1016/j.brainres.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarese C, Zoia C, Pecora N, Piolti R, Frigo M, Bianchi G, Sala G, Begni B, Riva R, Frattola L. Reduced platelet glutamate uptake in Parkinson's disease. Journal of neural transmission (Vienna, Austria : 1996) 1999;106:685–692. doi: 10.1007/s007020050189. [DOI] [PubMed] [Google Scholar]
- 43.Lievens JC, Salin P, Nieoullon A, Kerkerian-Le Goff L. Nigrostriatal denervation does not affect glutamate transporter mRNA expression but subsequent levodopa treatment selectively increases GLT1 mRNA and protein expression in the rat striatum. Journal of neurochemistry. 2001;79:893–902. doi: 10.1046/j.1471-4159.2001.00644.x. [DOI] [PubMed] [Google Scholar]
- 44.Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L. Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson's disease. The European journal of neuroscience. 2004;20:1255–1266. doi: 10.1111/j.1460-9568.2004.03591.x. [DOI] [PubMed] [Google Scholar]
- 45.Struzynska L, Chalimoniuk M, Sulkowski G. Changes in expression of neuronal and glial glutamate transporters in lead-exposed adult rat brain. Neurochemistry international. 2005;47:326–333. doi: 10.1016/j.neuint.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert ME, Mack CM, Lasley SM. The influence of developmental period of lead exposure on long-term potentiation in the adult rat dentate gyrus in vivo. Neurotoxicology. 1999;20:57–69. [PubMed] [Google Scholar]
- 47.Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain research. 2001;902:92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- 48.Mutkus L, Aschner JL, Syversen T, Aschner M. Methylmercury alters the in vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells. Biological trace element research. 2005;107:231–245. doi: 10.1385/BTER:107:3:231. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Xu Z, Deng Y, Xu B, Wei Y, Yang T. Protective effects of memantine against methylmercury-induced glutamate dyshomeostasis and oxidative stress in rat cerebral cortex. Neurotoxicity research. 2013;24:320–337. doi: 10.1007/s12640-013-9386-3. [DOI] [PubMed] [Google Scholar]
- 50.Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 51.Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Metal ions in life sciences. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bentle LA, Lardy HA. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. The Journal of biological chemistry. 1976;251:2916–2921. [PubMed] [Google Scholar]
- 53.Stallings WC, Metzger AL, Pattridge KA, Fee JA, Ludwig ML. Structure-function relationships in iron and manganese superoxide dismutases. Free radical research communications. 1991;12-13(Pt 1):259–268. doi: 10.3109/10715769109145794. [DOI] [PubMed] [Google Scholar]
- 54.Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Current topics in cellular regulation. 1984;24:153–169. doi: 10.1016/b978-0-12-152824-9.50021-6. [DOI] [PubMed] [Google Scholar]
- 55.Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharmacol. 1837;1:41–42. [Google Scholar]
- 56.Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB. Chronic manganese intoxication. Archives of neurology. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- 57.Flynn MR, Susi P. Neurological risks associated with manganese exposure from welding operations--a literature review. International journal of hygiene and environmental health. 2009;212:459–469. doi: 10.1016/j.ijheh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, Rosas I, Sabido Pedraza E, Miranda J, Siebe C, Texcalac JL, Santos-Burgoa C. Motor alterations associated with exposure to manganese in the environment in Mexico. The Science of the total environment. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 59.Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. International archives of occupational and environmental health. 1999;72:521–527. doi: 10.1007/s004200050410. [DOI] [PubMed] [Google Scholar]
- 60.Bast-Pettersen R, Ellingsen DG, Hetland SM, Thomassen Y. Neuropsychological function in manganese alloy plant workers. International archives of occupational and environmental health. 2004;77:277–287. doi: 10.1007/s00420-003-0491-0. [DOI] [PubMed] [Google Scholar]
- 61.ATSDR Toxicological Profile for Manganese. 2000. [PubMed]
- 62.Crinella FM. Does soy-based infant formula cause ADHD? Update and public policy considerations. Expert review of neurotherapeutics. 2012;12:395–407. doi: 10.1586/ern.12.2. [DOI] [PubMed] [Google Scholar]
- 63.Alves G, Thiebot J, Tracqui A, Delangre T, Guedon C, Lerebours E. Neurologic disorders due to brain manganese deposition in a jaundiced patient receiving long-term parenteral nutrition. JPEN Journal of parenteral and enteral nutrition. 1997;21:41–45. doi: 10.1177/014860719702100141. [DOI] [PubMed] [Google Scholar]
- 64.Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Archives of environmental health. 1989;44:175–178. doi: 10.1080/00039896.1989.9935883. [DOI] [PubMed] [Google Scholar]
- 65.Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. The Journal of physiology. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Itoh K, Sakata M, Watanabe M, Aikawa Y, Fujii H. The entry of manganese ions into the brain is accelerated by the activation of N-methyl-D-aspartate receptors. Neuroscience. 2008;154:732–740. doi: 10.1016/j.neuroscience.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 67.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain research bulletin. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 68.Andre C, Truong TT, Robert JF, Guillaume YC. Effect of metals on herbicides-alpha-synuclein association: a possible factor in neurodegenerative disease studied by capillary electrophoresis. Electrophoresis. 2005;26:3256–3264. doi: 10.1002/elps.200500169. [DOI] [PubMed] [Google Scholar]
- 69.Peneder TM, Scholze P, Berger ML, Reither H, Heinze G, Bertl J, Bauer J, Richfield EK, Hornykiewicz O, Pifl C. Chronic exposure to manganese decreases striatal dopamine turnover in human alpha-synuclein transgenic mice. Neuroscience. 2011;180:280–292. doi: 10.1016/j.neuroscience.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 70.H V. Progressive bulbar paralysis and amyotrophic lateral sclerosis from chronic manganese poisoning. Arch Gewerbepathol Gewerbehyg. 1939;9:464–476. [Google Scholar]
- 71.Miyata S, Nakamura S, Nagata H, Kameyama M. Increased manganese level in spinal cords of amyotrophic lateral sclerosis determined by radiochemical neutron activation analysis. Journal of the neurological sciences. 1983;61:283–293. doi: 10.1016/0022-510x(83)90012-6. [DOI] [PubMed] [Google Scholar]
- 72.Levin J, Bertsch U, Kretzschmar H, Giese A. Single particle analysis of manganese-induced prion protein aggregates. Biochemical and biophysical research communications. 2005;329:1200–1207. doi: 10.1016/j.bbrc.2005.02.094. [DOI] [PubMed] [Google Scholar]
- 73.Hesketh S, Sassoon J, Knight R, Brown DR. Elevated manganese levels in blood and CNS in human prion disease. Molecular and cellular neurosciences. 2008;37:590–598. doi: 10.1016/j.mcn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Banta RG, Markesbery WR. Elevated manganese levels associated with dementia and extrapyramidal signs. Neurology. 1977;27:213–216. doi: 10.1212/wnl.27.3.213. [DOI] [PubMed] [Google Scholar]
- 75.Donaldson J, LaBella FS, Gesser D. Enhanced autoxidation of dopamine as a possible basis of manganese neurotoxicity. Neurotoxicology. 1981;2:53–64. [PubMed] [Google Scholar]
- 76.Oikawa S, Hirosawa I, Tada-Oikawa S, Furukawa A, Nishiura K, Kawanishi S. Mechanism for manganese enhancement of dopamine-induced oxidative DNA damage and neuronal cell death. Free radical biology & medicine. 2006;41:748–756. doi: 10.1016/j.freeradbiomed.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 77.Dobson AW, Weber S, Dorman DC, Lash LK, Erikson KM, Aschner M. Oxidative stress is induced in the rat brain following repeated inhalation exposure to manganese sulfate. Biological trace element research. 2003;93:113–126. doi: 10.1385/BTER:93:1-3:113. [DOI] [PubMed] [Google Scholar]
- 78.Erikson KM, Dorman DC, Lash LH, Aschner M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2007;97:459–466. doi: 10.1093/toxsci/kfm044. [DOI] [PubMed] [Google Scholar]
- 79.Hazell AS, Normandin L, Norenberg MD, Kennedy G, Yi JH. Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment. Neuroscience letters. 2006;396:167–171. doi: 10.1016/j.neulet.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 80.Gavin CE, Gunter KK, Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicology and applied pharmacology. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- 81.Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): special reference to mitochondrial [Fe-S] containing enzymes. Toxicology and applied pharmacology. 2001;175:160–168. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alaimo A, Gorojod RM, Kotler ML. The extrinsic and intrinsic apoptotic pathways are involved in manganese toxicity in rat astrocytoma C6 cells. Neurochemistry international. 2011;59:297–308. doi: 10.1016/j.neuint.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez LE, Juknat AA, Venosa AJ, Verrengia N, Kotler ML. Manganese activates the mitochondrial apoptotic pathway in rat astrocytes by modulating the expression of proteins of the Bcl-2 family. Neurochemistry international. 2008;53:408–415. doi: 10.1016/j.neuint.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Filipov NM, Dodd CA. Role of glial cells in manganese neurotoxicity. Journal of applied toxicology : JAT. 2012;32:310–317. doi: 10.1002/jat.1762. [DOI] [PubMed] [Google Scholar]
- 85.Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicological sciences : an official journal of the Society of Toxicology. 2005;84:139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- 86.Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotoxicity research. 2009;16:42–49. doi: 10.1007/s12640-009-9045-x. [DOI] [PubMed] [Google Scholar]
- 87.Aschner M, Shanker G, Erikson K, Yang J, Mutkus LA. The uptake of manganese in brain endothelial cultures. Neurotoxicology. 2002;23:165–168. doi: 10.1016/s0161-813x(02)00056-6. [DOI] [PubMed] [Google Scholar]
- 88.Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. Journal of neurochemistry. 2009;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Experimental neurology. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- 90.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, Forte G, Martorana A, Bernardi G, Sancesario G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Rama Rao KV, Reddy PV, Hazell AS, Norenberg MD. Manganese induces cell swelling in cultured astrocytes. Neurotoxicology. 2007;28:807–812. doi: 10.1016/j.neuro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995;346:270–274. doi: 10.1016/s0140-6736(95)92164-8. [DOI] [PubMed] [Google Scholar]
- 93.Norenberg MD. The role of astrocytes in hepatic encephalopathy. Neurochemical pathology. 1987;6:13–33. doi: 10.1007/BF02833599. [DOI] [PubMed] [Google Scholar]
- 94.Rao KV, Jayakumar AR, Reddy PV, Tong X, Curtis KM, Norenberg MD. Aquaporin-4 in manganese-treated cultured astrocytes. Glia. 2010;58:1490–1499. doi: 10.1002/glia.21023. [DOI] [PubMed] [Google Scholar]
- 95.McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 96.Almer G, Vukosavic S, Romero N, Przedborski S. Inducible nitric oxide synthase up-regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. Journal of neurochemistry. 1999;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- 97.Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochemistry international. 2007;50:905–915. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 98.Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. Journal of neurochemistry. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. The EMBO journal. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Streifel KM, Moreno JA, Hanneman WH, Legare ME, Tjalkens RB. Gene deletion of nos2 protects against manganese-induced neurological dysfunction in juvenile mice. Toxicological sciences : an official journal of the Society of Toxicology. 2012;126:183–192. doi: 10.1093/toxsci/kfr335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 102.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends in pharmacological sciences. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 103.Miralles VJ, Martinez-Lopez I, Zaragoza R, Borras E, Garcia C, Pallardo FV, Vina JR. Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain research. 2001;922:21–29. doi: 10.1016/s0006-8993(01)03124-9. [DOI] [PubMed] [Google Scholar]
- 104.Karki P, Webb A, Smith K, Johnson J, Jr., Lee K, Son DS, Aschner M, Lee E. Yin Yang 1 Is a Repressor of Glutamate Transporter EAAT2, and It Mediates Manganese-Induced Decrease of EAAT2 Expression in Astrocytes. Molecular and cellular biology. 2014;34:1280–1289. doi: 10.1128/MCB.01176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sidoryk-Wegrzynowicz M, Aschner M. Role of astrocytes in manganese mediated neurotoxicity. BMC pharmacology & toxicology. 2013;14:23. doi: 10.1186/2050-6511-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee E, Sidoryk-Wegrzynowicz M, Yin Z, Webb A, Son DS, Aschner M. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia. 2012;60:1024–1036. doi: 10.1002/glia.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia. 2011;59:1732–1743. doi: 10.1002/glia.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sidoryk-Wegrzynowicz M, Lee E, Aschner M. Mechanism of Mn(II)-mediated dysregulation of glutamine-glutamate cycle: focus on glutamate turnover. Journal of neurochemistry. 2012;122:856–867. doi: 10.1111/j.1471-4159.2012.07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear factor-kappaB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochemical research. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- 111.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. The Journal of biological chemistry. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karki P, Webb A, Smith K, Lee K, Son DS, Aschner M, Lee E. cAMP response element-binding protein (CREB) and nuclear factor kappaB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. The Journal of biological chemistry. 2013;288:28975–28986. doi: 10.1074/jbc.M113.483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia. 2014;62:1270–1283. doi: 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng Z, Cao P, Wan MM, Sui G. Yin Yang 1: a multifaceted protein beyond a transcription factor. Transcription. 2010;1:81–84. doi: 10.4161/trns.1.2.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hyde-DeRuyscher RP, Jennings E, Shenk T. DNA binding sites for the transcriptional activator/repressor YY1. Nucleic acids research. 1995;23:4457–4465. doi: 10.1093/nar/23.21.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 117.Lee TC, Zhang Y, Schwartz RJ. Bifunctional transcriptional properties of YY1 in regulating muscle actin and c-myc gene expression during myogenesis. Oncogene. 1994;9:1047–1052. [PubMed] [Google Scholar]
- 118.Donohoe ME, Zhang X, McGinnis L, Biggers J, Li E, Shi Y. Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Molecular and cellular biology. 1999;19:7237–7244. doi: 10.1128/mcb.19.10.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosas S, Vargas MA, Lopez-Bayghen E, Ortega A. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: a role for YY1. Journal of neurochemistry. 2007;101:1134–1144. doi: 10.1111/j.1471-4159.2007.04517.x. [DOI] [PubMed] [Google Scholar]
- 121.Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, Emdad L, Howlett EL, Jeon HY, Su ZZ, Yoo BK, Sarkar D, Kim SH, Kang DC, Fisher PB. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer research. 2011;71:6514–6523. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]