Abstract
The contribution of the nervous system to inflammation in general and inflammatory skin disease in particular has been underappreciated. It is now apparent that the conventional clinical manifestations of many inflammatory skin diseases require an intact neural component. We reviewed the literature and identified 23 cases of alterations in the appearance or distribution of skin disorders in patients with acquired central or peripheral neural damage or dysfunction. In 19 cases, near or complete resolution of pre-existing skin lesions occurred in areas directly or indirectly supplied by a subsequently injured nervous system. Exacerbation or new onset of skin lesions occurred in only 4 cases. The neural deficits described included damage within the peripheral or central nervous system resulting in pure sensory, pure motor, or combined sensory and motor deficits. These cases highlight the importance of neural innervation and neurogenic inflammation in the development of inflammatory skin disease and prompt further examination of the use of neural blockade as an adjunctive therapy in the treatment of inflammatory dermatoses.
Introduction
Peripheral nerve fibers are capable of releasing neuromediators including neuropeptides when activated. In 1901, Bayliss demonstrated that electrical stimulation of sensory dorsal roots induced vasodilation1. The term “neurogenic inflammation” was introduced to describe the vasodilation and protein extravasation caused by inflammatory neuropeptides. Recent demonstrations that sensory neurons respond directly to cytokines such as thymic stromal lymphopoietin, solidify the connections between the nervous and immune systems2. Neurogenic inflammation has been implicated in the pathogenesis of many skin disorders including psoriasis3-5, atopic dermatitis6,7 and rosacea8,9. Emotional stress may exacerbate these and other skin conditions. Moreover, some primary neurologic and psychiatric disorders are known to predispose to cutaneous diseases, presumably due to an alteration in neural activation10-12. To further investigate the relationship between nerves and skin disease, we systematically reviewed the literature to identify cases of alterations in the manifestations or distribution of skin disorders in patients with acquired central or peripheral neural damage or dysfunction.
Methods
A literature search using PubMed (1966 to present) and EMBASE (1974 to present) databases was performed to identify relevant studies and case reports. Additional cases were identified using Google as a search engine. We only included reports published in the English language. Search terms included combination of keywords related to nerve damage, neuropathy and neurapraxia. In addition to these neurocentric terms, keywords included: asymmetric, atopic dermatitis, acne, alopecia, Bell’s palsy, blister, bullous pemphigoid, carpal tunnel syndrome, cerebrovascular accident, contact dermatitis, dermatitis, dyshidrotic eczema, eczema, erythema, fixed drug eruption, healing, hemiparesis, hemiplegia, id reaction, lichen planus, multiple sclerosis, pemphigus vulgaris, pompholyx, psoriasis, rosacea, scleroderma, seborrheic dermatitis, skin lesion, spared, stroke, sunburn, sympathectomy, tan, transient ischemic attack, unilateral and urticaria.
Results
We identified a total of 23 cases described in 19 case reports, including 9 cases of eczema, 8 cases of psoriasis, 2 cases of scleroderma, and 1 case each of rosacea, contact dermatitis, bullous pemphigoid and a relapsing vesicular dermatitis. In 19 cases, near or complete resolution of pre-existing skin lesions was reported in the areas innervated by nerves that were injured or compromised (Table 1). Skin lesions cleared or diminished in all reports of patients with psoriasis and scleroderma and in 9 of 10 patients with eczematous dermatoses including allergic contact dermatitis. Recurrence of the skin lesions was observed following recovery from nerve injury in 4 of these cases (Figure 1). While nerve injury was associated with improvement in cutaneous involvement in the majority of cases, worsening or new onset of skin lesions occurred in areas affected by neurologic deficit in 4 cases (Table 1).
Table 1.
Manifestations of skin diseases in patients with neurological deficits
| Case No. Age/Sex | Nerve damage* | Skin disease | Affected area(s) | Unaffected area(s) | Comments and Reference |
|---|---|---|---|---|---|
| CNS with sensory and motor deficit | |||||
| 1. M / 12 | Paraplegia caused by T12 cord section | Atopic dermatitis | Clearance of lesions | No change in lesions | 26 |
| 2. F / 94 | Left hemiparesis caused by cerebrovascular accident (CVA) | Nummular eczema | Clearance of lesions | Multiple lesions | 27 |
| 3. M / 64 | Right hemiparesis caused by CVA | Nummular eczema | Few lesions | Multiple lesions | 27 |
| 4. M / 62 | Right hemiparesis caused by multiple sclerosis | Eczema | Chronic stasis ulcers limited to skin surrounding right lateral malleolus | Lesions on left arm, trunk, thigh | 27 |
| 5. M / 71 | Left hemiparesis | Atopic eczema | Clearance of lesions | Lesions on right shoulder, mid trunk, upper arm, and thigh | Periodic disease since youth27 |
| 6. M / 61 | Right hemiparesis | Dyshidrotic eczema | Clearance of lesions | Multiple lesions | 27 |
| 7. F / 59 | Left hemiplegia caused by subarachnoid hemorrhage | Pustular Psoriasis and psoriatic arthritis | Clearance of lesions and sparing of left knee joint | Lesions on sole, knee, elbow and synovitis of the right knee | Decreased substance P immunoreactivity in synovial membrane of the left knee28 |
| 8. M / 62 | Left hemiparesis caused by CVA | Psoriasis | No lesions | Multiple lesions appearing within 6 months of the injury | New onset disease29 |
| 9. F / - | Left hemiplegia caused by lacunar stroke | Scleroderma | No lesions | Sclerodermatous skin changes and acroosteolysis of 2nd, 3rd, 4th, and 5th finger | 30 |
| 10. M / 78 | Left hemiplegia caused by CVA | Bullous pemphigoid | Bullous pemphigoid lesions | No lesions | IgG antibody at the dermo-epidermal junction on both sides of the body despite unilateral rash31 |
| CNS with motor but no sensory deficit | |||||
| 11. M / 42 | Right sided weakness due to progression of amyotrophic lateral sclerosis (ALS). Autonomic deficit also present. | Dyshidrotic eczema | Multiple pruritic vesicles and bullae | No Lesion | New onset disease32 |
| 12. F / 56 | Left hemiplegia caused by stroke | Scleroderma | No lesions | Sclerodermatous skin changes and telangiectasia | 33 |
| PNS with sensory and motor deficits | |||||
| 13. M / 49 | Traumatic brachial plexus neurapraxia | Plaque psoriasis | Clearance of lesions | Lesions present and unchanged | Lesions reappeared 4 months later with amelioration of nerve damage34 |
| 14. F / 66 | Carpal tunnel syndrome | Relapsing blisters | Blisters | No lesions | Blisters cleared after median nerve decompression surgery35 |
| PNS with sensory but no motor deficit | |||||
| 15. M / 16 | Peripheral nerve damage involving right axilla | Contact dermatitis | No lesions | Erythema and edema | Contact dermatitis caused by a deodorant36 |
| 16. M / 57 | Injury in left hand’s ulnar nerve distribution caused by occupational exposure to vibrating hand-held tools | Dyshidrotic eczema | Clearance of lesions | Erythema, scaling and vesicles | The number of PGP 9.5 immunoreactive sensory nerves was reduced in the biopsy of the lesion-free area37 |
| 17. F / 53 | Intercostobrachialis nerve damage caused by right mastectomy | Plaque psoriasis | Clearance of lesions | No change in lesions | Lesions reappeared 18 months later with amelioration of nerve damage 38 |
| 18. M / 68 | Nerve injury caused by knee replacement surgery | Psoriasis | Clearance of lesions | No change in lesions | 39 |
| 19. M / 48 | Nerve injury caused by surgery for meniscal repair | Psoriasis | Clearance of lesions | No change in lesions | Lesions reappeared 2 years later with amelioration of nerve damage40 |
| PNS with motor but no sensory deficit | |||||
| 20. F / 80 | Bell’s palsy | Rosacea | Diffuse erythema, telangiectasia, and papulopustules | Mild malar erythema | Skin biopsy: superficial perivascular and periadnexa lymphoplas-macytic infiltrate, mild dermal edema and fibrosis, along dilated capillaries41 |
| Autonomic deficit | |||||
| 21. F / 32 | Right side sympathectomy | Palmar eczema & hyperhidrosis | Clearance of lesions | Lichenified, fissured eczema | 42 |
| CNS, S?, M?* | |||||
| 22. F / 68 | Unilateral lesions on the entire right side of the body after a bilateral craniotomy | Psoriasis | Clearance of lesions | Widespread plaques | The authors did not report a detailed motor or sensory exam. It was not clear from the report whether lesions appeared on the right side or cleared from the left side43 |
| 23. F / 85 | Confusional state | Psoriasis | Clearance of lesions | Clearance of lesions | No sensory or motor damage, lesions reappeared with resolution of confusion44 |
Central nervous system (CNS), Peripheral nervous system (PNS). Affected areas with near or complete resolution of skin lesions are highlighted by darker shading. Affected areas with new or worsening skin lesions are highlighted by lighter shading.
Figure 1.
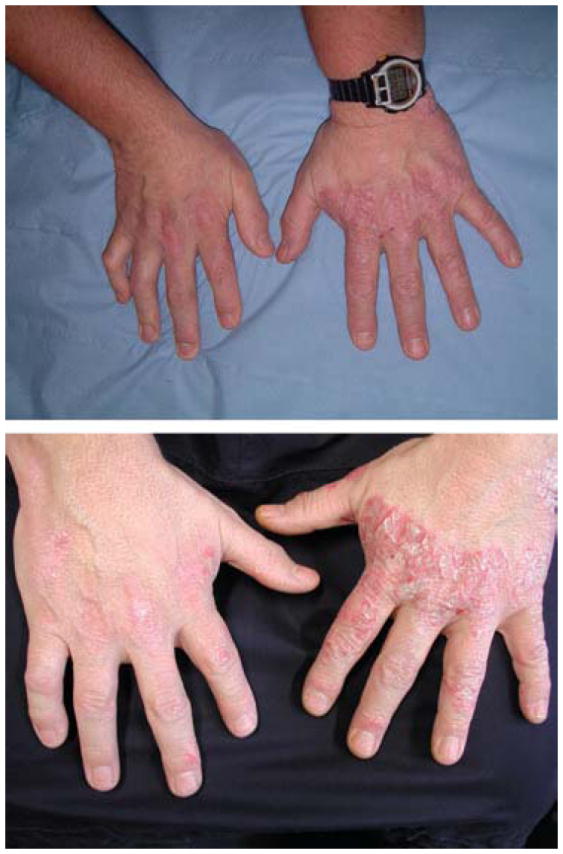
Altered manifestation of psoriasis following nerve injury. Top: Photograph showing remission of psoriasis in the right hand following posterior dislocation of the right shoulder that resulted in a brachial plexus neurapraxia in the distribution of the radial and median nerves. Psoriasis lesions remained intact on the left hand. The patient was not receiving any treatments for his skin lesions preceding or during this time. Bottom: Reappearance of psoriasis lesions in the right hand during early stages of recovery from peripheral nerve injury, approximately 4 months following his accident. Photogrpahs from reference #34.
Of the cases reviewed here, thirteen patients had central nervous system (CNS) deficits and 9 patients had peripheral nervous system (PNS) deficits. One patient (case 23) experienced a prolonged confusional state of unknown cause several months after experiencing a CVA, but there was no identifiable neurological deficit. Combined sensory and motor deficits were reported in 12 patients. Pure motor or pure sensory deficits were described as the primary finding in 3 and 5 patients respectively. In 2 reports, autonomic nervous system (ANS) dysfunction was observed, occurring in the setting of amyotrophic lateral sclerosis (ALS) in one case and following surgical sympathectomy in another (Table 2).
Table 2.
Number of patients and associated sites and types of neurological deficits.
| Neurological Deficit | Combined sensory and motor | Pure motor | Pure sensory | ANS | Unidentified | Total |
|---|---|---|---|---|---|---|
| CNS | 10 | 2 | 0 | 1* | 1** | 13 |
| PNS | 2 | 1 | 5 | 1 | - | 9 |
| None specified | - | - | - | 1 | 1*** | |
| Total | 12 | 3 | 5 | 1 | 2 | 23 |
Case 11, an ALS patient (counted as pure motor CNS pathology), with ANS involvement.
Case 22 details of the neurological exam for defining the type of deficit were not reported.
Case 23.
Discussion
The interconnectivity of nerves, immune and epithelial cells, is remarkable in its complexity. Evidence from both the basic science and clinical arenas suggests intensive communication between and overlapping function of many of these cell types. For example, keratinocytes, initially considered to function solely in providing a protective barrier against water loss or other external damage, are now considered outposts of the immune and nervous systems. Neurogenic inflammation, driven by the effects of neural-derived transmitters or peptides on neighboring epithelial and endothelial cells, has been implicated in the pathogenesis of common disorders affecting the skin, gut and lung. It follows that alterations in peripheral or central nervous system function may influence immune responses and thereby affect manifestations of inflammatory skin disease. The compilation of cases reported here supports the concept that an intact nervous system is necessary for fully formed inflammatory skin lesions. In the absence of normal neural input, a forme fruste or absence of the disease is manifest.
Clinical observations suggesting the importance of neural contributions to the pathogenesis or maintenance of skin disease are consistent with our evolving understanding of neural-immune interactions based on preclinical and animal models of inflammatory skin disease. For example, cutaneous nerve density and neural expression of specific neuropeptides are increased in murine models of allergic, eczematous and psoriasiform skin diseases13,14. Langerhans and mast cells are anatomically associated with neuropeptidergic fibers and previous studies have demonstrated that neuropeptides and adrenergic transmitters including epinephrine and norepinephrine can influence the function of these immune cell types, modulating antigen presentation, mast cell degranulation, and cytokine release15-20. Consistent with some of the clinical reports cited here, loss of cutaneous innervation via traumatic nerve injury in a mouse model of psoriasis resulted in reduced acanthosis, decreased CD4+ and CD11+ cell infiltration, and decreased IL-23 protein expression21. Similar reports of the protective effects of spinal cord or peripheral nerve disruption or pharmacologic blockade of afferent C-fibers in animal models of arthritis have been described previously22,23. Thus, both in vitro and in vivo data underscore the importance of the nervous system in the generation or maintenance of inflammatory skin and joint disease.
Several possible explanations exist as to why inflammatory skin lesions are diminished or absent in the presence of altered innervation. One potential reason, supported by a growing number of basic science observations including those mentioned above, is that intact innervation is required for normal physiologic communication between different cell types in the skin, including crucial crosstalk between nerves, immunocytes and blood vessels. Neurogenic axon reflex flare size correlates with intraepidermal nerve fiber density in human skin and is often reduced in patients with small fiber polyneuropathies24. Another possibility is that sensory or motor responses were blunted or eliminated such that there was either no eliciting sensation or motor response to scratch or rub involved skin. This possibility is consistent with reports of the efficacy of occlusion therapy for conditions such as psoriasis and atopic dermatitis. However, while scratching can exacerbate involvement in these conditions, it is not required for the development of psoriatic or atopic skin lesions in humans or animal models, thus arguing against this explanation.
One limitation of the cases presented here is that the specific neural populations that are compromised in individual cases are either not precisely characterized or more than one population was affected. This limitation makes it difficult to determine the relative importance of sensory versus motor neural contributions to skin disease. Sensory function was at least partially compromised with or without motor deficits in the majority of reports. In 2 cases (cases 11 and 21), disruption of autonomic innervation was the likely primary alteration. Somatosensory fibers, particularly peptidergic nerves that penetrate into the subcutaneous fat, dermis and epidermis or sudomotor fibers are the most likely relevant populations in modulating neuro-immune interactions. Worsening of cutaneous disease following nerve injury was described in 4 cases. In these cases, it is possible that the loss of a specific population of nerve fibers may have affected the balance of neural innervation to dermal or epidermal structures permitting pro-inflammatory conditions to prevail. We suggest that more specific descriptions of neural deficits be included in future reports. Such detail will aid in distinguishing the relative importance of various neural inputs into the skin.
The cases highlighted in this review essentially represent in people what conditional genetic mutations have demonstrated in animal models, providing powerful clinical evidence for the importance of neural-mediated signaling in inflammatory skin disease. These examples may be more robust and targeted than pharmacologic studies that employ topical neuropeptide blockers that may or may not inhibit nerves effectively or be the relevant molecular target for a given cutaneous disorder. We suggest that the clearance of inflammatory skin disease as a result of neural compromise begs further examination of the use of sensory neural blockade as a therapeutic option to treat common skin disorders. Antidepressants and neuromodulators are increasingly employed as adjunctive therapies in pruritic dermatoses. It may be that similar pharmacologic agents, as well as anesthetics or botulinum toxin, may also be helpful for controlling inflammatory dermatoses, whether or not itch is a primary symptom25.
What’s already known about this topic? The manifestation of inflammatory skin disease is altered, and typically diminished, following nerve damage.
What does this study add? Previous individual reports since 1966 were collected systematically and the clinical observations described therein placed within current concepts of neurogenic inflammation. We suggest that sensory neurons are necessary contributors to inflammatory skin disease and that neural blockade may serve as a primary or adjunctive approach to the treatment of certain inflammatory diseases.
Acknowledgments
Funding sources: This work was supported by a career development award to SBE from the Dermatology Foundation, an unrestricted research grant from LEO Pharma and NIH grant 1R01AR057744 to EAL.
Footnotes
The authors have no conflicts of interest or financial disclosures to declare.
References
- 1.Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. The Journal of physiology. 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson SR, The L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–95. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raychaudhuri SP, Raychaudhuri SK. Role of NGF and neurogenic inflammation in the pathogenesis of psoriasis. Progress in brain research. 2004;146:433–7. doi: 10.1016/S0079-6123(03)46027-5. [DOI] [PubMed] [Google Scholar]
- 4.Glinski W, Brodecka H, Glinska-Ferenz M, et al. Neuropeptides in psoriasis: possible role of beta-endorphin in the pathomechanism of the disease. International journal of dermatology. 1994;33:356–60. doi: 10.1111/j.1365-4362.1994.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 5.Farber EM, Raychaudhuri SP. Is psoriasis a neuroimmunologic disease? International journal of dermatology. 1999;38:12–5. doi: 10.1046/j.1365-4362.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 6.Misery L. Atopic dermatitis and the nervous system. Clinical reviews in allergy & immunology. 2011;41:259–66. doi: 10.1007/s12016-010-8225-z. [DOI] [PubMed] [Google Scholar]
- 7.Ostlere LS, Cowen T, Rustin MH. Neuropeptides in the skin of patients with atopic dermatitis. Clinical and experimental dermatology. 1995;20:462–7. doi: 10.1111/j.1365-2230.1995.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2011;15:53–62. doi: 10.1038/jidsymp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2011;15:33–9. doi: 10.1038/jidsymp.2011.8. [DOI] [PubMed] [Google Scholar]
- 10.Picardi A, Mazzotti E, Gaetano P, et al. Stress, social support, emotional regulation, and exacerbation of diffuse plaque psoriasis. Psychosomatics. 2005;46:556–64. doi: 10.1176/appi.psy.46.6.556. [DOI] [PubMed] [Google Scholar]
- 11.Kodama A, Horikawa T, Suzuki T, et al. Effect of stress on atopic dermatitis: investigation in patients after the great hanshin earthquake. The Journal of allergy and clinical immunology. 1999;104:173–6. doi: 10.1016/s0091-6749(99)70130-2. [DOI] [PubMed] [Google Scholar]
- 12.Arima M, Shimizu Y, Sowa J, et al. Psychosomatic analysis of atopic dermatitis using a psychological test. The Journal of dermatology. 2005;32:160–8. doi: 10.1111/j.1346-8138.2005.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 13.Wolfram JA, Diaconu D, Hatala DA, et al. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. The American journal of pathology. 2009;174:1443–58. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward NL, Hatala DA, Wolfram JA, et al. Cutaneous manipulation of vascular growth factors leads to alterations in immunocytes, blood vessels and nerves: Evidence for a cutaneous neurovascular unit. Journal of dermatological science. 2011;61:14–22. doi: 10.1016/j.jdermsci.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodali S, Friedman I, Ding W, et al. Pituitary adenylate cyclase-activating polypeptide inhibits cutaneous immune function. European journal of immunology. 2003;33:3070–9. doi: 10.1002/eji.200324085. [DOI] [PubMed] [Google Scholar]
- 16.Kodali S, Ding W, Huang J, et al. Vasoactive intestinal peptide modulates Langerhans cell immune function. Journal of immunology. 2004;173:6082–8. doi: 10.4049/jimmunol.173.10.6082. [DOI] [PubMed] [Google Scholar]
- 17.Forsythe P, Bienenstock J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chemical immunology and allergy. 2012;98:196–221. doi: 10.1159/000336523. [DOI] [PubMed] [Google Scholar]
- 18.Seiffert K, Hosoi J, Torii H, et al. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans cells. Journal of immunology. 2002;168:6128–35. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 19.Hosoi J, Murphy GF, Egan CL, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–63. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 20.Ding W, Manni M, Stohl LL, et al. Pituitary adenylate cyclase-activating peptide and vasoactive intestinal polypeptide bias Langerhans cell Ag presentation toward Th17 cells. European journal of immunology. 2012;42:901–11. doi: 10.1002/eji.201141958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski SM, Belkadi A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. The Journal of investigative dermatology. 2011;131:1530–8. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iarussi D, Bassmaji J, Vitale P, et al. Arrhythmia during the postoperative course in patients after heart surgery. Bollettino della Societa italiana di cardiologia. 1976;21:209–15. [PubMed] [Google Scholar]
- 23.Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. British journal of pharmacology and chemotherapy. 1967;31:138–51. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickel A, Heyer G, Senger C, et al. C-fiber axon reflex flare size correlates with epidermal nerve fiber density in human skin biopsies. Journal of the peripheral nervous system : JPNS. 2009;14:294–9. doi: 10.1111/j.1529-8027.2009.00241.x. [DOI] [PubMed] [Google Scholar]
- 25.Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Archives of dermatology. 1972;105:128–9. doi: 10.1001/archderm.1972.01620040088028. [DOI] [PubMed] [Google Scholar]
- 26.Amon U, Wolff HH. Healing of chronic atopic dermatitis lesions in skin areas of paraplegia after trauma. The Journal of dermatology. 1994;21:982–3. doi: 10.1111/j.1346-8138.1994.tb03325.x. [DOI] [PubMed] [Google Scholar]
- 27.Troilius A, Moller H. Unilateral eruption of endogenous eczema after hemiparesis. Acta dermato-venereologica. 1989;69:256–8. [PubMed] [Google Scholar]
- 28.Veale D, Farrell M, Fitzgerald O. Mechanism of joint sparing in a patient with unilateral psoriatic arthritis and a longstanding hemiplegia. British journal of rheumatology. 1993;32:413–6. doi: 10.1093/rheumatology/32.5.413. [DOI] [PubMed] [Google Scholar]
- 29.Sowell JK, Pippenger MA, Crowe MJ. Psoriasis contralateral to hemiparesis following cerebrovascular accident. International journal of dermatology. 1993;32:598–9. doi: 10.1111/j.1365-4362.1993.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 30.Azevedo VF, Mueller C, Aragao SC. Asymmetric scleroderma in a CVA patient. Clinical rheumatology. 2008;27:1321–3. doi: 10.1007/s10067-008-0915-z. [DOI] [PubMed] [Google Scholar]
- 31.Long CC, Lever LR, Marks R. Unilateral bullous pemphigoid in a hemiplegic patient. The British journal of dermatology. 1992;126:614–6. doi: 10.1111/j.1365-2133.1992.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakai K, Yoneda K, Moriue T, et al. Unilateral palm pompholyx in a patient with amyotrophic lateral sclerosis. European journal of dermatology : EJD. 2011;21:445–6. doi: 10.1684/ejd.2011.1349. [DOI] [PubMed] [Google Scholar]
- 33.Sethi S, Sequeira W. Sparing effect of hemiplegia on scleroderma. Annals of the rheumatic diseases. 1990;49:999–1000. doi: 10.1136/ard.49.12.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph T, Kurian J, Warwick DJ, et al. Unilateral remission of psoriasis following traumatic nerve palsy. The British journal of dermatology. 2005;152:185–6. doi: 10.1111/j.1365-2133.2005.06330.x. [DOI] [PubMed] [Google Scholar]
- 35.Baroni A, Piccolo V, Russo T, et al. Recurrent blistering of the fingertips as a sign of carpal tunnel syndrome: an effect of nerve compression. Archives of dermatology. 2012;148:545–6. doi: 10.1001/archdermatol.2011.3199. [DOI] [PubMed] [Google Scholar]
- 36.Rebhun J. Effect of innervation on contact dermatitis. Annals of allergy. 1980;45:33. [PubMed] [Google Scholar]
- 37.Wallengren J, Tegner E, Sundler F. Cutaneous sensory nerve fibers are decreased in number after peripheral and central nerve damage. Journal of the American Academy of Dermatology. 2002;46:215–7. doi: 10.1067/mjd.2002.118540. [DOI] [PubMed] [Google Scholar]
- 38.Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. International journal of dermatology. 1990;29:418–20. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 39.Raychaudhuri SP, Farber EM. Are sensory nerves essential for the development of psoriatic lesions? Journal of the American Academy of Dermatology. 1993;28:488–9. doi: 10.1016/s0190-9622(08)81760-4. [DOI] [PubMed] [Google Scholar]
- 40.Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Archives of dermatology. 1971;104:220–1. [PubMed] [Google Scholar]
- 41.Cabete J, Serrao V, Lestre S. Unilateral rosacea in a patient with Bell’s palsy. The Journal of dermatology. 2013;40:403–4. doi: 10.1111/1346-8138.12100. [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury MM, Hedges R, Lanigan SW. Unilateral resolution of palmar eczema and hyperhidrosis complicated by Horner’s syndrome following ipsilateral endoscopic cervical sympathectomy. The British journal of dermatology. 2000;143:653–4. doi: 10.1111/j.1365-2133.2000.03733.x. [DOI] [PubMed] [Google Scholar]
- 43.Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Archives of dermatology. 2004;140:1531–6. doi: 10.1001/archderm.140.12.1531-e. [DOI] [PubMed] [Google Scholar]
- 44.Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. Journal of the American Academy of Dermatology. 1998;38:768–70. doi: 10.1016/s0190-9622(98)70210-5. [DOI] [PubMed] [Google Scholar]


