Abstract
Rationale
Buprenorphine (BPN) has been shown to rapidly improve mood in treatment-resistant depressed patients in small clinical studies. However, BPN’s effects in preclinical tests for mood and antidepressant efficacy are largely unexplored.
Objective
The current study examined the effects of BPN in the forced swim test (FST) and novelty-induced hypophagia (NIH) test as measures of antidepressant and anxiolytic-like effects in C57BL/6J mice. Microdialysis was used to measure whether BPN engaged KORs in the nucleus accumbens shell (NAcSh) at a behaviorally active dose (0.25 mg/kg).
Methods
BPN was tested in the FST at both 30 min and 24 h post administration. Also measured in the FST at 24 h post administration were the KOR antagonist norbinaltorphimine (nor-BNI), the MOR agonist morphine and the reference antidepressant desipramine. The anxiolytic effects of BPN were examined in the NIH test 24 h after treatment. The effects of acute injection of BPN and the KOR agonist U50,488 were measured on extracellular DA levels in the NAcSh.
Results
BPN produced significant reductions in FST immobility without changing locomotor activity and reduced approach latencies in the novel environment of the NIH test when tested 24 h after treatment. Repeated daily BPN injections for 6 d did not produce tolerance to these behavioral effects. nor-BNI produced a similar antidepressant-like response in the FST 24 h postinjection but morphine and desipramine were ineffective. BPN (0.25 mg/kg) did not alter DA levels when given alone but prevented the KOR agonist U50,488 from reducing DA levels.
Conclusions
Acute and subchronic treatment with BPN produced antidepressant and anxiolytic-like responses in mice at doses that engage KORs. These studies support the clinical evidence that BPN may be a novel rapid-acting antidepressant medication and provides rodent models for investigating associated neurochemical mechanisms.
Keywords: Buprenorphine, Kappa-opioid receptor, Depression, Anxiety, Antidepressant, microdialysis, dopamine
Introduction
Major Depressive Disorder (MDD) is one of the most prevalent and debilitating psychiatric disorders, with a lifetime prevalence of 17% in the United States (Kessler et al. 2005). The long-term economic impact of depression exceeds that of most other medical illnesses, in terms of work absenteeism and family burden (Crown et al. 2002). Even though several types of antidepressant medications are currently available, these are not effective in approximately 40% of patients (Cipriani et al. 2009). In fact, approximately 30% of patients suffer from treatment-resistant depression (TRD), where they do not respond to at least two types of antidepressant medications (Al-Harbi 2012). TRD has a highly significant economic impact, estimated over $80 billion in the US alone (Fostick et al. 2010). Moreover, most antidepressants require 4 to 6 weeks to produce their effects when they do work, which can place persistently resistant patients suffering from suicidal ideations at prolonged risk. Hence, there is currently an unmet need for the development of novel, more efficacious and faster-acting antidepressant medications.
The endogenous opioid system, especially the kappa-opioid receptor (KOR) and its endogenous ligand dynorphin, has been implicated in the regulation of mood disorders and in mediating the aversive properties of stress (Lutz and Kieffer 2013; McLaughlin et al. 2003; McLaughlin et al. 2006). KOR agonists have been shown to induce prodepressive behaviors and exposure to stress has been known to increase levels of dynorphins in limbic regions (Shirayama et al. 2004; McLaughlin et al. 2006; Carr et al. 2010). Furthermore, several animal studies have shown that KOR antagonists produce anxiolytic and antidepressant-like responses (Mague et al. 2003; Knoll et al. 2007; Carr et al. 2010; Carr and Lucki 2010; Knoll et al. 2011). These studies have been largely performed in rats and have used long-lasting selective KOR antagonists such as nor-BNI and JDTic, but the duration of their effects lasting for weeks post-treatment have raised concerns of risk to patients if they were developed for human use.
Buprenorphine (BPN) is an opiate drug with mixed pharmacology, with its most potent actions a high-affinity partial agonist at mu opioid receptors (MOR) and antagonist at KORs (Cowan 2007). BPN is a currently approved medication by the Food and Drug Administration for the treatment of opiate dependence or chronic pain. Due to its partial agonism at MORs, BPN is considered safer than other opiates because it produces fewer side effects, has a ceiling effect on respiratory depression and is not immunosuppressive compared to other potent MOR agonists like morphine or heroin (Davis 2012). Moreover, opioid-dependent patients show milder withdrawal symptoms, less drug dependence, and fewer or no cognitive deficits when treated with BPN (Shmygalev et al. 2011; Davis 2012). In a limited number of clinical studies, BPN has also been shown to produce antidepressant effects in non-opioid dependent treatment-resistant patients (Bodkin et al. 1995; Nyhuis et al. 2008; Karp et al 2014). These studies, along with recent trial results for ALKS5461 presented by Alkermes (Ehrich et al. 2012; ClinicalTrials.gov identifier NCT01381107), showed significant improvements in mood within one week of BPN treatment. However, only two preclinical studies, one using rats and another using mice, have examined BPN in anxiety-like behaviors and none for antidepressant effects. BPN was found to produce anxiolytic-like effects in a rat model of fear-potentiated startle due to MOR activation (Glover and Davis 2008) but induced an anxiogenic-like effect in mice using the light-dark box test (Lelong-Boulouard et al. 2006). These discrepancies in anxiety-like behavior could be attributed to the different tests performed in different rodent species and are not unusual for a field with less than a handful of studies.
The present study characterized and established an antidepressant and anxiolyticlike response of BPN in C57BL/6J mice using the FST and NIH test. These particular tests demonstrate strong predictive validity for the antidepressant and anxiolytic effects of drug treatments in mice (Dalvi and Lucki 1999; Dulawa and Hen 2005). The results of this study showed that a single injection of BPN was effective in producing significant reductions of immobility in the FST and in latency to approach a palatable food in a novel environment in the NIH test 24 h post-administration. Furthermore, subchronic administration of BPN produced similar responses in the FST and NIH. The selective KOR antagonist nor-BNI, but not the MOR agonist morphine, produced a comparable response in the FST. Additionally, microdialysis studies showed that a behaviorally active dose of BPN prevented the KOR agonist U50,488 from reducing extracellular dopamine (DA) levels in the nucleus accumbens shell (AcbSh), indicating pharmacological engagement of KORs. These studies suggest a role for KORs actions in in mediating the antidepressant-like responses of BPN.
Materials and methods
Animals
Male C57BL/6J mice, 8 weeks old on arrival, were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in groups of five per cage for the FST studies and in pairs for the NIH experiments in polycarbonate cages and maintained in a 12 h light-dark cycle (lights on at 0700) in a temperature (22°C)- and humidity-controlled environment. Food and water were available ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Drugs
Buprenorphine hydrochloride (Sigma, St. Louis, MO), norbinaltorphimine (nor-BNI; Tocris Bioscience, Ellisville, MO), desipramine hydrochloride (DMI; Sigma), and U50,488 (Sigma) were dissolved in distilled water and injected intraperitoneally (i.p.). Morphine (Spectrum Chemical, New Brunswick, NJ) was diluted in 0.9% saline and injected i.p. Mice in the control groups were injected with 0.9% saline. All doses were calculated according to the base weight of the drug and administered in a volume of 10 ml/kg.
Forced swim test (FST)
Swim sessions were conducted either 30 min or 24 h after treatment and were performed as described previously (Crowley et al. 2004). Briefly, mice were placed in individual polycarbonate cylinders (25.3 cm tall×22.2 cm in diameter) filled with water (25 ± 1°C) to a depth of 15 cm. Each session lasted 6 min and water was changed between each animal. Sessions were recorded with a video camera from an overhead view to allow for manual scoring of immobile behavior. Immobility was defined as the absence of movement, except that necessary to maintain the head above the water. Passive floating, where the animal drifts across the cylinder without initiation of swimming, was also included as immobility.
Mice were initially tested in the FST 30 min after injection of DMI or BPN, as is commonly done when testing the acute effects of antidepressant drugs in mice (Lucki et al. 2001; Crowley et al. 2004). Because BPN increased locomotor activity at this point, mice were subsequently tested 24 h after the injection of all drugs.
Novelty induced hypophagia (NIH)
Performed as previously described (Balu et al. 2009), subjects were pair housed and trained for 12 d (15 min sessions) to eat a palatable food (3 peanut butter chips presented in a small, clear petri dish) in a home cage environment. Opaque, black, plastic dividers were placed inside each cage to separate the mice during training and home test sessions. Mice were allowed to habituate to the dividers for 1 h before the start of the training session. For the 24 h BPN study, mice were injected with saline or 0.25 mg/kg BPN immediately after their last session and tested 24 h later in the novel environment. For the daily BPN study, mice were injected for 6 d following their last training session. On the last 3 d of treatment, mice were re-exposed to the training procedure. Following drug treatment, mice were tested in a novel and home environment. For novel cage testing, mice were placed in an empty, clear polycarbonate cage (25.5×46×20 cm) with bright lighting (60W light bulb). There was no food deprivation or habituation period prior to the novel test. The novel test session was videotaped and the latency to approach during the 15-min test session was measured. The approach latency was defined as the time it took the mouse to approach the dish in the center of the arena and to start eating the food. The home cage test was performed the day after the novel cage test.
Locomotor activity
Activity was measured 30 min or 24 h after drug treatment during a 1 h session. Mice were monitored in a home cage environment (28.9×17.8×12 cm) placed inside an infrared-sensitive motion detection system (30×24×8 cm) with an 8-beam photocell array strip (Med Associates Inc, St. Albans, VT). Total ambulatory activity was measured by counting the number of beam breaks.
Microdialysis
Microdialysis probes were custom-made and surgically implanted in mice as described previously (Knobelman et al. 2001). Under isofluorane anesthesia, the probe was implanted in the nucleus accumbens shell (AcbSh) at the following coordinates: AP +1.2, ML ± 0.5, and DV −4.5 mm from bregma (Franklin and Paxinos, 1997) using a stereotaxic instrument. Following surgery, the mice were placed into a 21.5 cm high, clear polycarbonate cylindrical in vivo microdialysis apparatus with a counterbalance arm holding a liquid swivel (Instech Laboratories, Plymouth Meeting, PA) and allowed to recover overnight.
Microdialysis experiments started 17–20 h after surgery. Dialysate samples were collected into polypropylene microcentrifuge vials at 20-min intervals. Four fractions were collected to measure baseline values before systemic administration of drugs and samples were collected for three additional hours after systemic drug challenge. The effects of BPN (0.25 mg/kg) on extracellular DA levels were compared with U50,488 (5 mg/kg) given alone and simultaneously with BPN. Samples were stored at −80°C until analyzed for DA as described previously (Andrews and Lucki, 2001) using a Shimadzu Prominence HPLC system (includes a LC-20AD pump, Sil-20AC refrigerated microsampler) using a Unijet microbore column (3 uM ODS/ 100×1mm) coupled with an Antec Decade II electrochemical detector. DA levels were identified by comparing their elution times with those of reference standards and quantified from their respective peak heights using a linear regression analysis of the peak heights obtained from a series of reference standards. At the completion of the experiment, brains were removed, placed in cold isopentane and frozen at −80°C. The brains were then sectioned (35 µm) with a refrigerated cryostat and the tissue examined for the location of the dialysis probe. No animals were excluded for improper probe placement.
Data analysis
One-way and two-way ANOVA were performed to examine the significance of differences between treatments. Significant overall main effects were followed by Dunnett’s multiple comparison or Bonferroni post-hoc tests. Unpaired two-tailed Student’s t-tests were applied where appropriate. Microdialysis data were expressed as a percentage of baseline values determined by the mean of 4 samples collected immediately before drug injections. Drug effects on extracellular DA levels were analyzed by a two-way ANOVA, with repeated measures over time following injections, and compared at individual time points using Tukey’s test. For all tests, p < 0.05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
Effects of BPN in the FST and locomotor activity 30 min post-administration
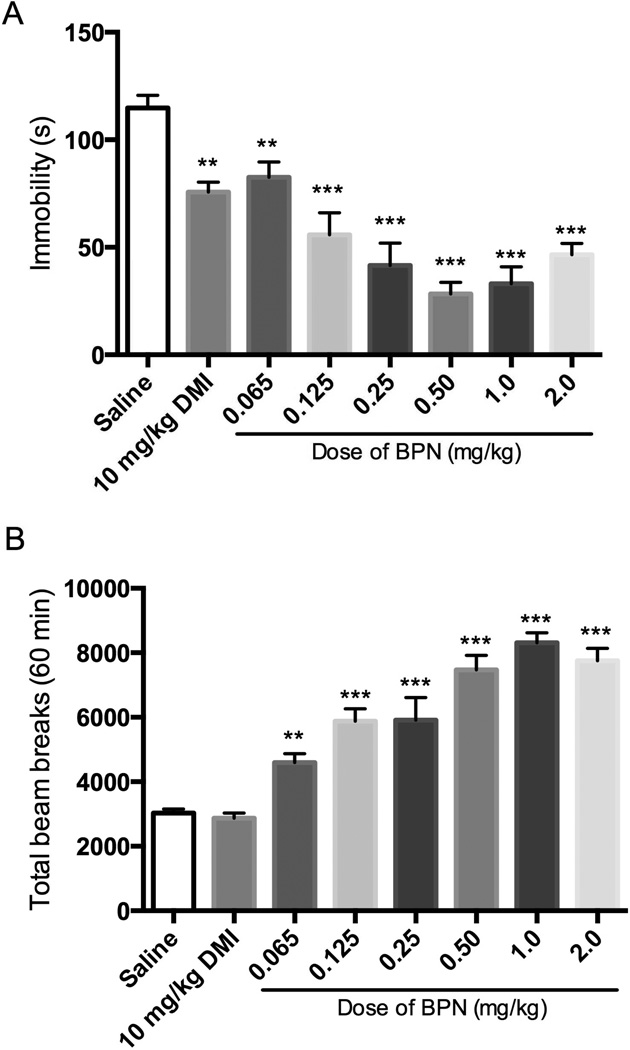
Figure 1 shows the behavioral effects produced by BPN administration 30 min before testing. BPN significantly reduced immobility across all doses tested (Fig 1A). One-way ANOVA revealed a significant effect of treatment on immobility [F(7, 88) = 21.09, p < 0.0001]. Immobility following each BPN dose response was reduced significantly compared with saline-treated mice. Desipramine (10 mg/kg i.p.), used as a reference antidepressant, also reduced immobility significantly when compared to saline. However, all doses of BPN produced significant increases in locomotor activity (Fig 1B), F(7, 88) = 44.28, p < 0.0001. Each BPN dose produced a significantly higher locomotor response when compared to saline. Desipramine treatment did not produce an increase in locomotor activity 30 min post-administration.
Figure 1. Effects of BPN in the FST and locomotor activity when tested 30 min postadministration.
A) Effects of BPN and DMI on immobility, n = 28 for saline group; n = 9–10 per BPN and DMI group. B) Effects of BPN and DMI on locomotor activity, n = 29 for saline group; n = 9–10 per BPN and DMI group. Data are depicted as mean ± SEM (* p < 0.05, ** p < 0.01, *** p < 0.001).
Effects of BPN in the FST and locomotor activity 24 h post-administration
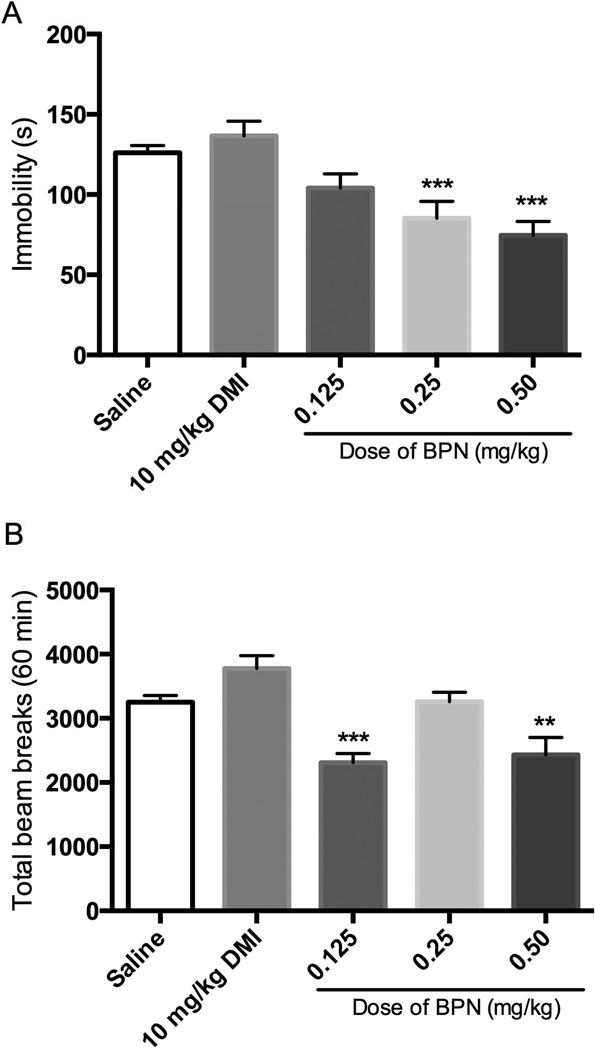
When a separate group of mice were tested 24 h after treatment, BPN produced a significant reduction in immobility without inducing hyperactivity (Fig 2). One-way ANOVA revealed a significant effect of treatment on immobility [F(4, 65) = 11.08, p < 0.0001]. Only the 0.25 and 0.5 mg/kg doses of BPN produced significant decreases in immobility when compared to saline, whereas 0.125 mg/kg BPN and 10 mg/kg DMI had no effect (Fig 2A). Although there were overall differences between groups in locomotor activity [F(4,64) = 11.50, p < 0.0001], the differences were caused by reductions of locomotor activity after 0.125 and 0.5 mg/kg BPN. BPN did not produce hyperactivity at any dose tested (Fig 2B).
Figure 2. Effects of BPN in the FST and locomotor activity when tested 24 h postadministration.
A) Effects of BPN and DMI on immobility in the FST, n = 29 for saline group and n = 9–10 per BPN and DMI groups. B) Effects of BPN and DMI on locomotor activity, n = 29 for saline group; n = 9–10 per BPN and DMI group. Data are depicted as mean ± SEM (* p < 0.05).
Effects of BPN in the NIH test 24 h post-administration
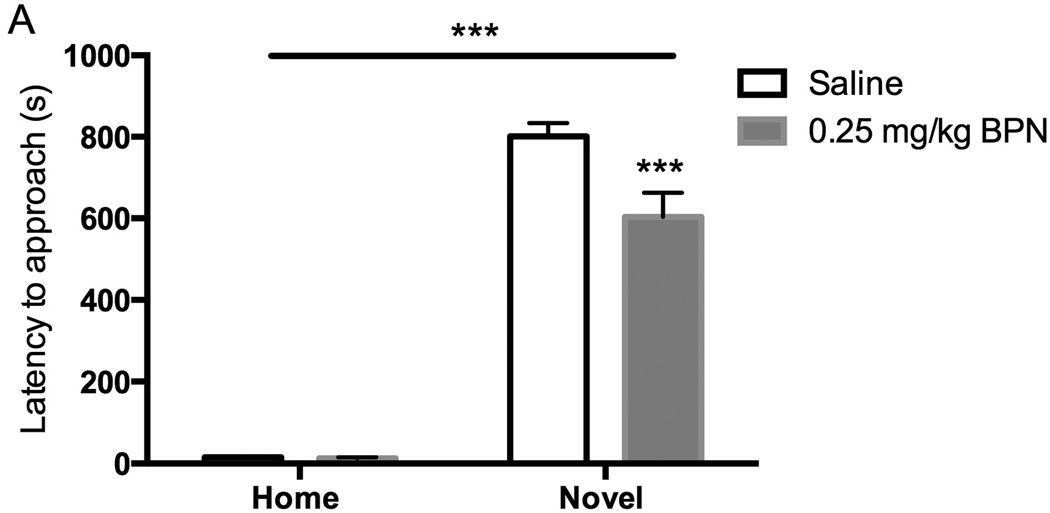
BPN (0.25 mg/kg i.p.) treatment produced a significant reduction in the latency to approach and ingest a palatable food in the novel arena (p < 0.001; Fig 3). There were significant main effects of drug [F(1,33) = 7.73, p < 0.01] and environment [F(1,33) = 366.28, p < 0.001] as well as an interaction [F(1,33) = 7.39, p < 0.05]. There were no significant effects observed in the home cage test (saline: 15.11 ± 2.36 s; BPN: 12.89 ± 2.62 s)
Figure 3.
Effects of BPN and saline on the latency to approach and ingest food in the novel arena in the NIH test 24 h post-administration, n = 9–10 per group. Data are depicted as mean ± SEM (*** p < 0.001).
Effects of subchronic BPN treatment in the FST, NIH test and locomotor activity
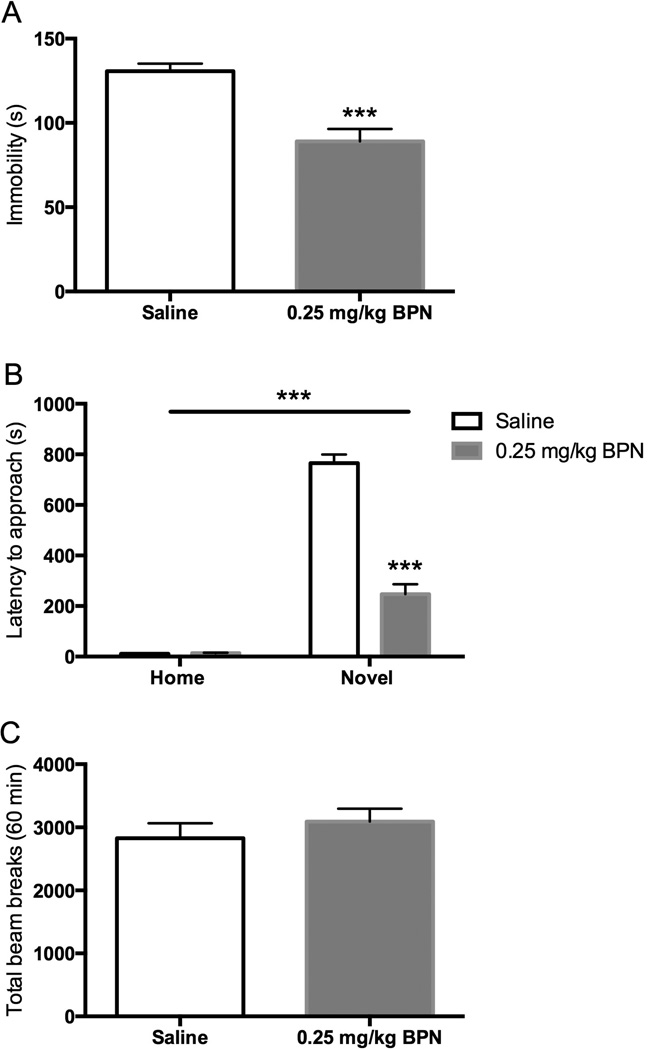
Daily BPN (0.25 mg/kg i.p.) treatment given for 6 d produced a significant reduction of immobility in the FST when tested 24 h after the last injection (Fig 4A; t = 4.917, p < 0.001). Furthermore, subchronic BPN treatment produced an even more pronounced reduction in the latency to approach and ingest food in the novel arena of the NIH test (Fig 4B). There were significant main effects of drug [F(1,35) = 103.59, p < 0.0001] and environment [F(1,35) = 380.34, p < 0.0001] as well as an interaction [F(1,35) = 105.53, p < 0.0001]. Bonferroni post hoc analysis indicated that BPN significantly reduced approach latency in the novel arena when compared to saline-treated subjects (p < 0.001). No significant differences were observed in the home cage test (saline: 11.10 ± 1.15 s; BPN: 13.5 ± 1.63 s). Moreover, no differences in locomotor activity were observed between BPN-treated and saline-treated mice (Fig 4C).
Figure 4. Effects of treatment with BPN for 6 days.
A) BPN reduced immobility in the FST, n = 9–10 per group. B) BPN reduced the latency to approach and ingest food in the novel arena, n = 9–10 per group. C) BPN did not change locomotor activity, n = 10 per group. Data are depicted as mean ± SEM (*** p < 0.001).
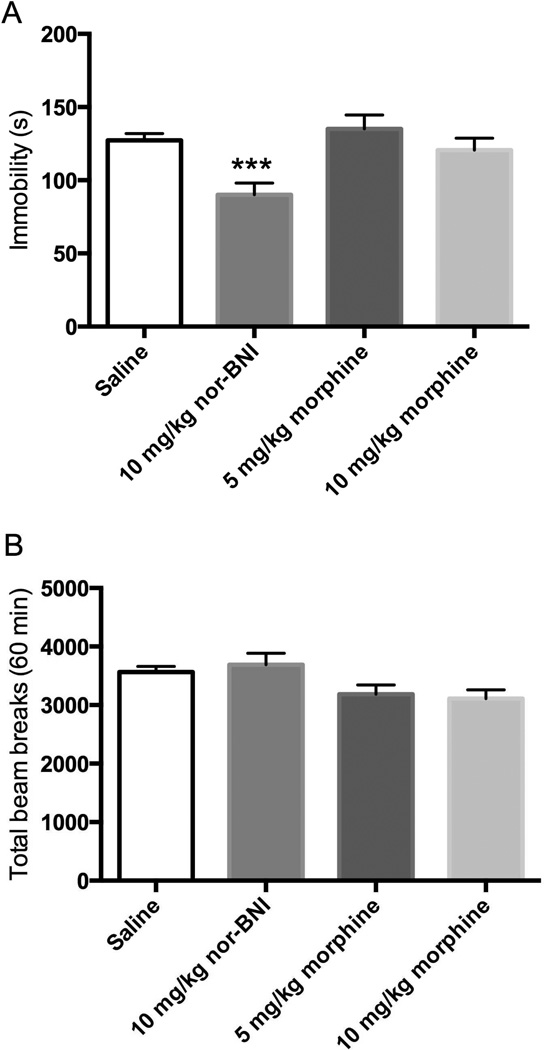
Effects of nor-BNI and morphine in the FST and locomotor activity 24 h post-administration
In a separate group of mice, nor-BNI (10 mg/kg i.p.) treatment significantly reduced immobility, while treatment with morphine (5 and 10 mg/kg i.p.) had no effect in the FST 24 h post-administration (Fig 5A). One-way ANOVA revealed a significant effect of treatment on immobility [F(3, 54) = 6.058, p < 0.01]. No differences in locomotor activity were observed between saline-treated mice and nor-BNI or morphine-treated mice (Fig 5B).
Figure 5. Effects of opioid compounds in the FST and locomotor activity tested 24 h post-administration.
A) Treatment with nor-BNI (n = 9) and morphine (n = 10) on immobility in the FST compared with saline control (n = 19). B) Treatment with nor-BNI (n = 10) or morphine (n = 10) did not change locomotor activity compared with saline control (n = 30). Data are depicted as mean ± SEM (*** p < 0.001).
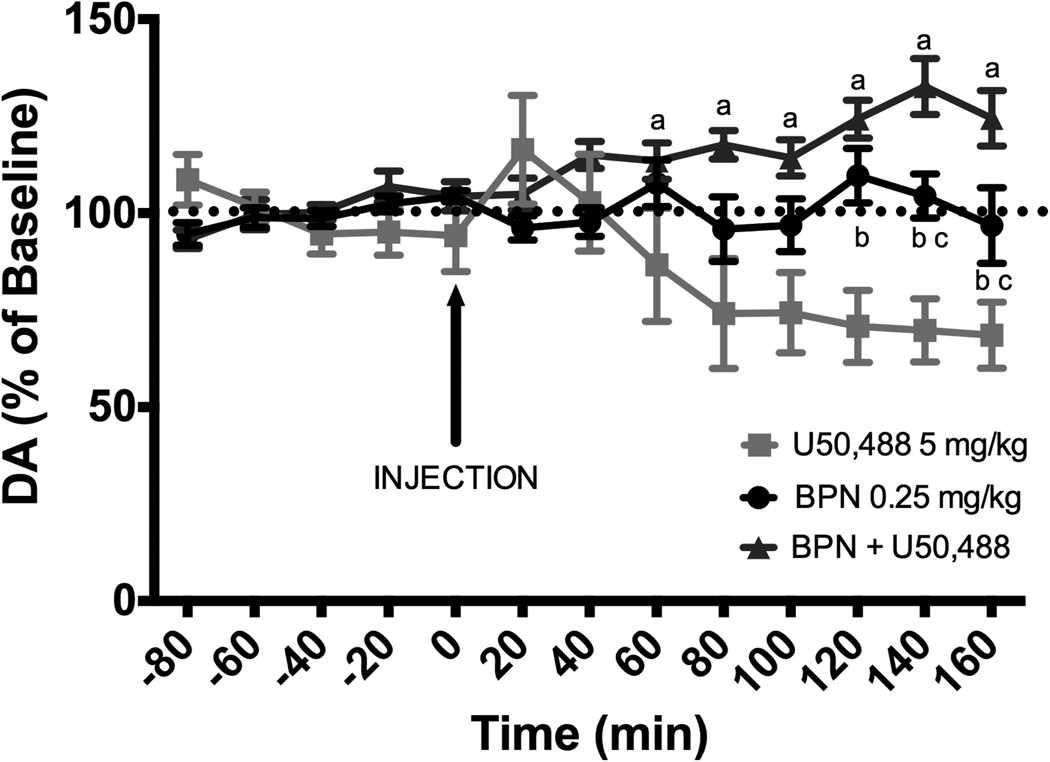
Effects of BPN and U50,488 on DA levels in the AcbSh
Systemic administration of U50,488 (5 mg/kg) produced a significant decrease of extracellular DA levels in the AcbSh. Administration of BPN (0.25 mg/kg) immediately prior to U50, 488 blocked the reduction of DA levels. When administered alone, BPN did not alter DA levels (Fig 6). A repeated-measures two-way ANOVA revealed a significant effect of treatment [F(2, 16) = 8.927, p < 0.01], time [F(7, 112) = 2.216, p < 0.05] and an interaction [F(14, 112) = 7.293, p < 0.001]. Injection of U50,488 produced a 40% decrease from baseline in dialysate concentrations of DA at 80 – 160 min post-injection. In contrast, injection of BPN prior to U50,488 produced a 25% increase from baseline in dialysate concentrations of DA at 120–160 min post-injection. Injection of BPN did not produce significant changes in DA dialysate concentrations over time.
Figure 6.
DA levels in the NAchSh in response to systemic administration of U50,488 (n = 5), BPN (n = 6) or in combination (n = 8). Data are expressed as percent change from individual baseline and are presented as mean ± SEM. Significant differences between groups are indicated by the following symbols: a (U50,488 vs. BPN + U50,488, p < 0.001), b (BPN vs. U50,488, p < 0.05), and c (BPN vs BPN + U50,488, p < 0.05).
Discussion
The aim of the current study was to determine whether BPN produced antidepressant and anxiolytic like responses on behavioral tests in mice. The major result of this study is that BPN produced antidepressant-like and anxiolytic responses 24 h after treatment in the FST and NIH paradigms, respectively. BPN produced a stimulant-like increase in locomotor activity 30 min after treatment, hence producing false-positive effects in the FST. Moreover, subchronic administration of BPN continued to produce significant reductions in immobility and latency to ingest a palatable food in a novel arena. In addition, the KOR antagonist nor-BNI produced a reduction of immobility in the FST that was comparable to the effects of BPN.
Antidepressant drugs are commonly tested in the mouse FST 30 min to 1 hr after administration in order to measure the acute effects of drug treatments (Lucki et al. 2001; Crowley et al. 2004). At the 30 min timepoint, BPN produced a MOR-dependent hyperactivity (Marquez et al. 2007) that likely resulted in reducing immobility in the FST as observed in Figure 1. Drug-induced hyperactivity make the results obtained in the FST ambiguous regarding antidepressant activity since increased activity can account for the reduction of immobility. Based on BPN’s slow receptor kinetics/receptor dissociation rates and on previous studies with selective KOR antagonists like nor-BNI where behavioral testing in the FST in rats was performed 24 h after administration, BPN was subsequently tested in the mouse FST 24 h post-administration (Cowan and Lewis 1995; Carr et al. 2010; Paronis and Bergman 2011). Interestingly, a single injection of BPN produced protracted antidepressant-like and anxiolytic effects lasting at least 48 h (data not shown). This pattern of sustained behavioral effects is also seen with ketamine (Autry et al. 2011), a novel fast-acting antidepressant (Murrough et al. 2013), and could be an emerging hallmark for rapid acting antidepressants in animal studies. In the limited clinical studies, most of the TRD patients responded rapidly within 1 week to BPN treatment (Bodkin et al. 1995; Nyhuis et al. 2008). Future studies are needed to elaborate on the duration and possible underlying mechanisms of these protracted effects of BPN.
The NIH test is an anxiety-like behavior test in rodents that is sensitive to the anxiolytic effects of antidepressants emerging following chronic treatment for weeks, but not following subchronic or acute treatments (Dulawa et al. 2004; Dulawa and Hen 2005; Bechtholt et al., 2008; Balu et al. 2009). Interestingly, a single injection of BPN produced anxiolytic effects in the NIH test only 24 h after injection. This result is similar to that observed with the KOR antagonist DIPPA in the rat NIH test, suggesting a role for KOR blockade in mediating an anxiolytic response that would ordinarily require chronic treatment with other antidepressants (Carr and Lucki 2010). Previous studies in rats have shown anxiolytic effects for the KOR antagonists nor-BNI and JDTic on other rodent tests of anxiety behavior, such as the elevated plus maze (Knoll et al. 2007; Knoll et al. 2011), although this behavioral test does not predict the anxiolytic effects of chronic antidepressant treatment (Borsini et al. 2002). BPN was shown to potently block fearpotentiated startle in rats, an effect attributed to MOR agonist effects (Glover and Davis 2008). Similarly, BPN was found to effectively reduce emotional fear recognition in normal healthy volunteers (Ipser et al. 2013). Only one preclinical study has demonstrated an anxiogenic role for BPN in mice, using the light-dark test (Lelong-Boulouard et al. 2006). The discrepancy between that study and the present one can be attributed to differences in mouse strain (NMRI vs C57BL/6J), the time of testing (24 h post-administration), and the different paradigms used. The light-dark test is based on approach-avoidance behavior, whereas the NIH test is considered a conflict-based anxiety test where the anxiety-inducing novel environment suppresses approach behavior and the ingestion of a highly palatable food, possibly suggesting an anxiolytic role for KOR antagonists in more stress-related tests (Cryan and Sweeney 2011). Furthermore, current antidepressants, like selective serotonin reuptake inhibitors (SSRIs), produce anxiogenic effects during the first days of treatment in rodents and humans (Borsini et al. 2002; Cryan and Sweeney 2011), whereas the anxiolytic effects of BPN were evident as early as 24 h after acute treatment. This suggests that BPN may have clear benefits for patients dealing with anxiety during the early phase of treatment when compared to SSRIs.
In the present study, BPN continued to produce significant reductions of immobility in the FST and approach latency in the NIH test after subchronic (6 d) treatment. No evidence for behavioral tolerance to the antidepressant-like and anxiolytic responses was found; in fact the anxiolytic response was even larger following 6 d of BPN. In contrast, tolerance to some behavioral effects of BPN has been shown to develop following repeated administration (Glover and Davis 2008; Gringauz et al 2001; Paronis and Bergman 2011). Several studies have shown that chronic treatments with conventional antidepressants also retain their antidepressant-like effect in both the mouse and rat FST (Detke et al 1997; Cryan et al 2005; Jiao et al 2011). The sustained effects in the FST and NIH tests suggest that BPN could remain clinically effective for mood and anxiety disorders after chronic administration. Indeed, BPN maintenance programs for opioid-dependent patients have shown some psychotherapeutic benefits of the drug independent of its drug abuse mitigating effects (Kosten et al 1990; Tenore 2008). Future animal studies will determine the efficacy of BPN treatment in measures of antidepressant and anxiolytic behaviors after longer treatment periods, which would be critical for demonstrating its likely sustained therapeutic activity for mood disorders in humans.
The convergent antidepressant-like and anxiolytic response of BPN in the FST and NIH test agree with previous evidence on the role of the dynorphin/KOR system in mediating the behavioral responses to stress. Because the most potent effects of BPN involve mixed effects as a partial MOR agonist and KOR antagonist (Lufty and Cowan 2004), more extensive efforts are necessary to identify the mechanisms underlying its effects on emotional behaviors. Studies in rodents have identified the dynorphin/KOR system as necessary for the expression of a number of stress-induced behaviors (McLaughlin et al. 2003; McLaughlin et al. 2009; Van’t Veer and Carlezon 2013). In the present study, nor-BNI produced a significant reduction of immobility in the mouse FST, comparable to that of BPN 24 h post-administration. In contrast, morphine, a full MOR agonist which was reported to produce antidepressant-like effects in the mouse FST when tested 30 min post-administration (Zomkowski et al. 2005), was ineffective in the FST when tested 24 h after treatment. Existing literature has identified a role for each of the opioid receptors in the regulation of mood (Lutz and Kieffer, 2013). Even though this study suggests that BPN could be producing its effect through its actions at KORs, it does not rule out a possible role for other receptors like the delta opioid receptor (DOR) and the nociceptin receptor (NOP), since BPN and its active metabolites bind to multiple opioid receptors. BPN’s action as a low efficacy partial agonist at NOP receptors, as well as norbuprenorphine’s potent agonist activity at DOR, could also contribute to its antidepressant effects (Huang et al 2001; Brown et al 2011). Although additional pharmacological studies are needed to completely define the mechanism of BPN’s effects on emotional behavior, the present results suggest that BPN’s antidepressant-like response is more closely associated with its actions as a KOR antagonist than a MOR agonist.
In the present study, brain microdialysis was used to demonstrate an interaction of BPN with KORs in the AcbSh. Extracellular DA levels in the mouse AcbSh shell were reduced by 40% following systemic administration of the KOR agonist U50,488. This result agrees with previously published studies conducted in rats (Di Chiara and Imperato 1988; Maisonneuve et al 1994). Co-treatment with BPN (0.25 mg/kg), the lowest dose that was active behaviorally in the FST and NIH tests, prevented U50,488 from reducing DA levels supporting other reports that BPN is a potent antagonist of KORs (Richards and Sadee 1985; Leander 1987; Paronis and Bergman 2011). Moreover, BPN alone was ineffective at altering DA levels. This suggests that increases in DA transmission associated with the rewarding effects of opioid compounds and higher doses of BPN may not be necessary for BPN to produce its antidepressant-like behavioral effects in opioidnaïve animals. The association between the rewarding effects of BPN and its effects on mood-related behaviors should be specifically investigated.
To our knowledge, the present study is the first to demonstrate an antidepressant response of BPN in mice in a test of antidepressant efficacy with strong predictive validity. The results of this study provide a method for further investigation into the mechanisms underlying these antidepressant and anxiolytic behaviors. The pattern of these results also support further clinical research into the use of BPN in treatmentresistant depressed patients, who suffer from co-morbid anxiety and so greatly need novel and more effective therapeutics. Although BPN might be developed for treating depression and anxiety in non opioid-dependent populations, concerns remain to be met about mitigating its abuse potential.
Acknowledgement
This research was supported by USPHS grants R01 MH92412 and T32 MH14654.
Footnotes
Conflict of Interest
IL was a consultant for Alkermes. EF, KM, and SAR have no conflict to disclose.
References
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Pref and Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews CM, Lucki I. Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology. 2001;155:221–229. doi: 10.1007/s002130100704. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharm. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtolt AJ, Valentino RJ, Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology. 2008;33:2117–2130. doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J of Clin Psychopharm. 1995;15(1):49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology. 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Brown SM, Holtzman M, Kim T, Kharasch ED. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115:1251–1260. doi: 10.1097/ALN.0b013e318238fea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I. Comparison of the kappa opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology. 2010;210:295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Cowan A. Buprenorphine: the basic pharmacology revisited. J Addict Med. 2007;1:68–72. doi: 10.1097/ADM.0b013e31806c9202. [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, editors. Buprenorphine: combatting drug abuse with a unique opioid. New York: Wiley-Liss; 1995. [Google Scholar]
- Crowley JJ, Jones MD, O’Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM. The impact of treatment-resistant depression on health care utilization and costs. Clin Psychiatry. 2002;63(11):963–971. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology. 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi A, Lucki I. Murine models of depression. Psychopharmacology. 1999;147:14–16. doi: 10.1007/s002130051131. [DOI] [PubMed] [Google Scholar]
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209–219. doi: 10.1016/j.suponc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Sellers E, Jones R, Fava M. Early clinical development of the opioid modulator ALKS 5461 in the treatment of depression. NCDEU; 2012. [Google Scholar]
- Fostick L, Silberman A, Beckman M, Spivak B, Amital D. The economic impact of depression: Resistance or severity? Eur Neuropsychopharm. 2010;20:671–675. doi: 10.1016/j.euroneuro.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2004. [Google Scholar]
- Glover EM, Davis M. Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology. 2008;198:167–180. doi: 10.1007/s00213-008-1112-0. [DOI] [PubMed] [Google Scholar]
- Gringauz M, Rabinowitz R, Stav A, Korczyn AD. Tolerance to the analgesic effect of buprenorphine, butorphanol, nalbuphine, and cyclorphan, and cross-tolerance to morphine. J Anesth. 2001;15:204–209. doi: 10.1007/s005400170004. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Paksepp J, Malcolm-Smith S, Thomas K, Stein DJ, van Honk J. Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology. 2013;38:166–170. doi: 10.1016/j.psyneuen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Jiao J, Nitzke AM, Doukas DG, Seiglie MP, Dulawa SC. Antidepressant response to chronic citalopram treatment in eight inbred mouse strains. Psychopharmacology. 2011;213:509–520. doi: 10.1007/s00213-010-2140-0. [DOI] [PubMed] [Google Scholar]
- Karp JF, Butters MA, Begley A, Miller MD, Lenze EJ, Blumberger D, Mulsant B, Reynolds CF., III Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in mid-life and older adults. J Clin Psychiatry. 2014 doi: 10.4088/JCP.13m08725. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demier O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Lucki I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine1A and 5-hydroxytryptamine1B autoreceptors in different brain regions of the mouse. J Pharmacol Exp Ther. 2001;298:1083–1091. [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA. Anxiolytic-like effects of kappa opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. J Subst Abuse Treat. 1990;7:51–54. doi: 10.1016/0740-5472(90)90035-o. [DOI] [PubMed] [Google Scholar]
- Leander DJ. Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology. 1987;26:1445–1447. doi: 10.1016/0028-3908(87)90112-2. [DOI] [PubMed] [Google Scholar]
- Lelong-Boulouard V, Quentin T, Moreaux F, Debruyne D, Boulouard M, Coquerel A. Interactions of buprenorphine and dipotassium clorazepate on anxiety and memory functions in the mouse. Drug Alcohol Depen. 2006;85:103–113. doi: 10.1016/j.drugalcdep.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Lufty K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36(3):195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Marquez P, Ballram R, Kieffer BL, Lufty K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology. 2007;52:1336–1341. doi: 10.1016/j.neuropharm.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharm. 2008;28(5):593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawl. J Pharmacol Exp Ther. 2011;336:488–495. doi: 10.1124/jpet.110.173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards ML, Sadee W. Buprenorphine is an antagonist at the kappa opioid receptor. Pharmacol Res. 1985;2:178–181. doi: 10.1023/A:1016340106299. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Shmygalev S, Damm M, Weckbecker K, Berghaus G, Petzke F, Sabatowski R. The impact of long-term maintenance treatment with buprenorphine on complex psychomotor and cognitive function. Drug Alcohol Depend. 2011;117:190–197. doi: 10.1016/j.drugalcdep.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. 2008;27:49–65. doi: 10.1080/10550880802122646. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomkowski ADE, Santos ARS, Rodrigues ALS. Evidence for the involvement of the opioid system in the agmatine antidepressant-like effect in the forced swimming test. Neurosci Lett. 2005;381:279–283. doi: 10.1016/j.neulet.2005.02.026. [DOI] [PubMed] [Google Scholar]