Abstract
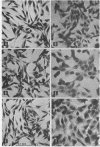
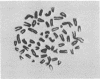
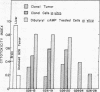
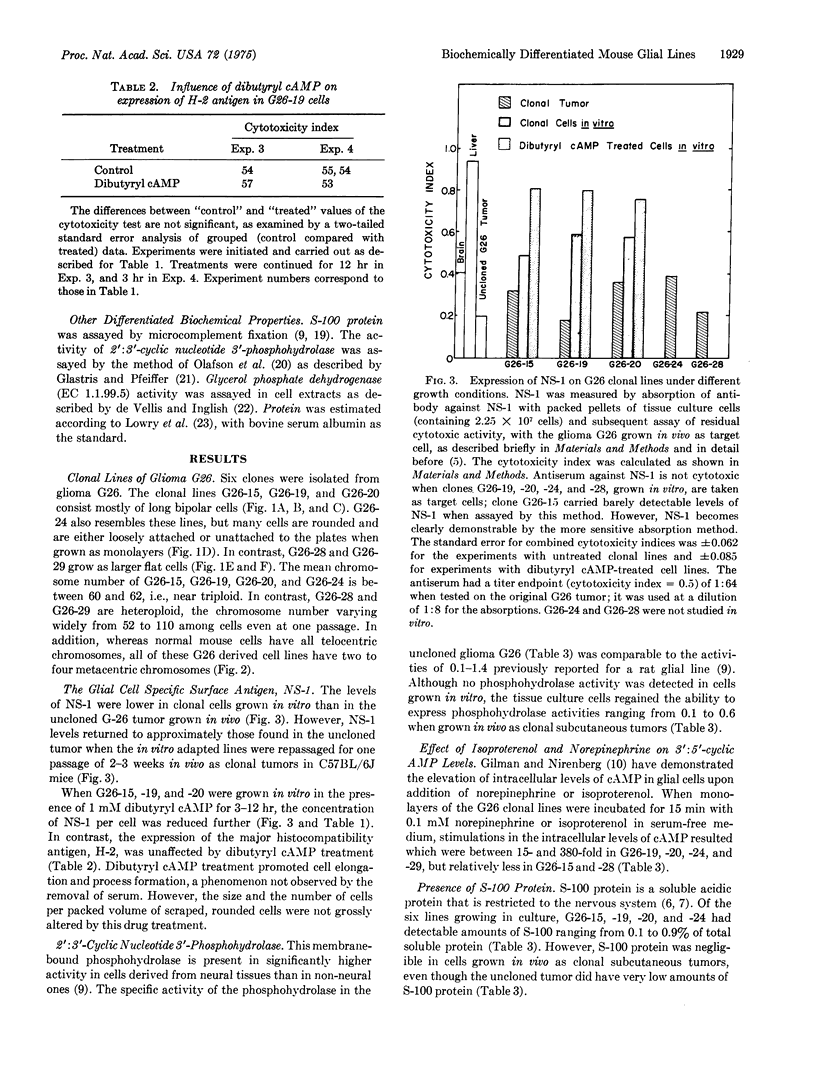
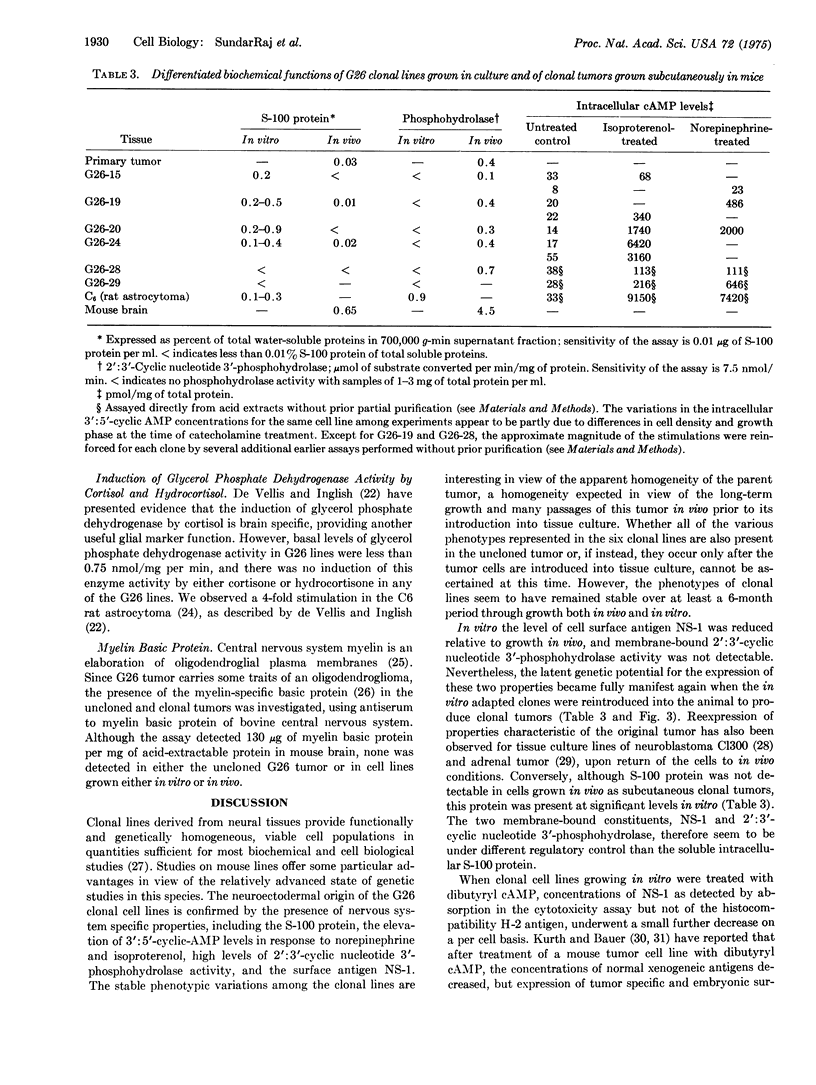
Six biochemically differentiated clonal lines have been established from a transplantable glioma (tg26) of the C57BL/6 inbred mouse strain. Antibodies have been previously raised against G26 tumor cells, which define a cell surface component(s), NS-1 (nervous system antigen-1), found exclusively in the nervous system. NS-1 concentrations approximate the levels of the original G26 tumor when the clonal lines are grown as clonal tumors in vivo, but are reduced when the cells are grown in vitro. NS-1 concentrations are further reduced in vitro upon incubation of the cells with 1 mM dibutyryl 3:5-cyclic AMP. H-2 histocompatibility antigen concentration, in contrast, is unaffected by dibutyryl cAMP. In addition to expressing NS-1, the neuroectodermal origin of these cell lines is further confirmed by their synthesis of the nervous system specific acidic protein S-100 and by the high specific activity of the enzyme 2:3-cyclic nucleotide 3-phosphohydrolase. In addition, they respond to catecholamines by the elevation of intracellular 3:5-cyclic AMP levels. Whereas expression of S-100 protein is high under in vitro conditions but negligible after one passage in vivo, 2:3-cyclic nucleotide 3-phosphohydrolase is not detectable in vitro but becomes detectable again in vivo. The two membrane-bound constituents, NS-1 and 2:3-cyclic nucleotide 3-phosphohydrolase, therefore seem to be subjected to different regulatory mechanisms from that of the soluble, intracellular S-100 protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Blecher M., Ro'Ane J. T., Flynn P. D. Metabolism of dibutyryl cyclic adenosine 3',5'-monophosphate during its regulation of lipolysis and glucose oxidation in isolated rat epididymal adipocytes. J Biol Chem. 1970 Apr 25;245(8):1867–1870. [PubMed] [Google Scholar]
- Browning E. T., Schwartz J. P., Breckenridge B. M. Norepinephrine-sensitive properties of C-6 astrocytoma cells. Mol Pharmacol. 1974 Jan;10(1):162–174. [PubMed] [Google Scholar]
- De Vellis J., Inglish D. Hormonal control of glycerolphosphate dehydrogenase in the rat brain. J Neurochem. 1968 Oct;15(10):1061–1070. doi: 10.1111/j.1471-4159.1968.tb06824.x. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heersche J. N., Fedak S. A., Aurbach G. D. The mode of action of dibutyryl adenosine 3',5'-monophosphate on bone tissue in vitro. J Biol Chem. 1971 Nov 25;246(22):6770–6775. [PubMed] [Google Scholar]
- Henion W. F., Sutherland E. W., Posternak T. Effects of derivatives of adenosine 3',5'-phosphate on liver slices and intact animals. Biochim Biophys Acta. 1967 Oct 9;148(1):106–113. doi: 10.1016/0304-4165(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Morgan W. D., Pastan I. Regulation of cell motility by cyclic AMP. Nature. 1972 Jan 7;235(5332):54–56. doi: 10.1038/235054a0. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Kurihara T., Nussbaum J. L., Mandel P. 2',3'-cyclic nucleotide 3'-phosphohydrolase in purified myelin from brain of Jimpy and normal young mice. Life Sci II. 1971 Apr 22;10(8):421–429. doi: 10.1016/0024-3205(71)90303-1. [DOI] [PubMed] [Google Scholar]
- Kurth R., Bauer H. Influence of dibutyryl cyclicAMP and theophylline on cell surface antigens on oncornavirus transformed cells. Nat New Biol. 1973 Jun 20;243(129):243–245. doi: 10.1038/newbio243243a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Moore B. W. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965 Jun 9;19(6):739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., Drummond G. I., Lee J. F. Studies on 2',3'-cyclic nucleotide-3'-phosphohydrolase from brain. Can J Biochem. 1969 Oct;47(10):961–966. doi: 10.1139/o69-151. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., Eylar E. H. Allergic encephalomyelitis: preparation of the encephalitogenic basic protein from bovine brain. Arch Biochem Biophys. 1970 Jun;138(2):392–396. doi: 10.1016/0003-9861(70)90361-9. [DOI] [PubMed] [Google Scholar]
- PLESCIA O. J., BRAUN W., PALCZUK N. C. PRODUCTION OF ANTIBODIES TO DENATURED DEOXYRIBONUCLEIC ACID (DNA). Proc Natl Acad Sci U S A. 1964 Aug;52:279–285. doi: 10.1073/pnas.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S. E., Wechsler W. Biochemically differentiated neoplastic clone of Schwann cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2885–2889. doi: 10.1073/pnas.69.10.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. NS-1 (nervous system antigen-1), a glial-cell-specific antigenic component of the surface membrane. Proc Natl Acad Sci U S A. 1974 May;71(5):1795–1799. doi: 10.1073/pnas.71.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer B. P. Phenotypically variant adrenal tumor cell cultures with biochemical lesions in the ACTH-stimulated steroidogenic pathway. J Cell Physiol. 1969 Oct;74(2):115–122. doi: 10.1002/jcp.1040740203. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN H. M. The nature of gliomas as revealed by animal experimentation. Am J Pathol. 1955 Jan-Feb;31(1):1–29. [PMC free article] [PubMed] [Google Scholar]