Abstract
Background
Pemphigus vulgaris (PV) is a blistering disease in which TNF-α has a role in the pathogenesis.
Objectives
The objective was to evaluate the safety of infliximab (IFX) with prednisone compared to prednisone alone in the treatment of PV. In addition, treatment response was assessed and mechanistic studies were performed.
Methods
PV subjects who had ongoing disease activity while maintained on prednisone were randomized to receive either IFX or placebo in addition to prednisone. Response status and IgG anti-DSG1 and DSG3 antibodies were assessed at 18 and 26 weeks. .
Results
10 subjects were randomized to each group. There were no safety signals during the course of the study. At week 18, 1 subject in each group had responded. At week 26, 3 IFX treated subjects vs. none in the placebo group had responded (p =0 .21). At weeks 18 and 26, the median IgG anti-DSG1 and anti-DSG3 levels were lower in the IFX treated-patients (IgG anti DSG-1: week 18 p =0.035, week 26 p = 0.022; IgG anti-DSG3; week 18 p=0.035, week 26 p = 0.05)).
Limitations
This study is limited by the relative small sample size.
Conclusions
There was no significant difference between study arms in the proportion of subjects with treatment-related Adverse Events > Grade 3. IFX therapy was not shown to be effective for the treatment of patients with PV in this randomized, placebo-controlled trial, although IFX treatment may be associated with a decrease in anti-DSG1 and DSG3 antibodies.
Keywords: pemphigus vulgaris, infliximab, auto-antibodies, B cells
Introduction
Pemphigus vulgaris (PV) is an autoimmune blistering disease with a significant morbidity and mortality often requiring the addition of adjuvant therapy.1–3
The potential of TNF-α as a target for treatment of patients with PV has been suggested by case reports documenting patients with PV responding to anti-TNFα therapy. 4–7 Feliciani demonstrated that TNF-α is expressed in the skin lesions of patients with PV and serum levels of TNF-α appear to correlate with disease activity. 8;9 In vitro studies have also demonstrated that PV sera stimulate human keratinocytes to express TNF-α mRNA and undergo acantholysis, which was inhibited by anti-TNF-α antibodies. 10 These observations suggest that inhibition of TNF-α may a useful adjunctive therapy for patients with PV. To address this question we conducted a double-blind, placebo-controlled trial of infliximab (IFX) with prednisone versus prednisone alone to determine if blockade TNF-α would be a safe and effective treatment for patients with pemphigus vulgaris.
Methods
Subjects
20 patients with PV diagnosed by clinical presentation, histology and direct immunofluorescence findings were studied. Inclusion criteria required age greater than 18 years, ongoing disease activity (disease activity, mucosal and cutaneous, ≥ 2) (Table I), a stable dose of prednisone between 20 and 120 mg/day for two weeks prior to infusion and inability to reduce prednisone below 20 mg/day for 8 weeks. The disease activity score was modified from the methods previously utilized in studies of pemphigus vulgaris.11;12 Subjects were required to have discontinued other systemic immunosuppressive agents for at least 4 weeks before enrollment. Subjects were excluded if they had a positive PPD, history or presence of a severe or opportunistic infection, malignancy within 5 years, lymphoproliferative disorder, seizure or demyelinating disorder, or congestive heart failure.
Table 1.
Baseline Pemphigus Vulgaris Disease Activity Assessment
| Type of Disease | Extent of Disease Activity | Score |
|---|---|---|
| MUCOSAL | ||
| None | 0 | |
| 1–5 Lesions, small ulcers | 1 (mild) | |
| 6–10 Lesions, small ulcers | 2 (moderate) | |
| >10 Lesions or extensive erosions | 3 (severe) | |
| CUTANEOUS | ||
| No blisters or erosions | 0 | |
| < 20 blisters or < 1 to 3% body surface area (BSA) |
1(mild) | |
| 20 to 40 blisters or 3 – 10% BSA |
2 (moderate) | |
| > 40 blisters or > 10% BSA |
3 (severe) |
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of all the participating centers. Interim safety monitoring was provided by the Division of Allergy, Immunology and Transplantation of the National Institute of Allergy and Infectious Diseases with monthly reviews of adverse events (AEs), mortality and serious adverse events (SAEs). A data safety monitoring board evaluated accumulating data at approximately 6 month intervals.
Study Design
Subjects were randomized to infusions of IFX (5 mg/kg) or placebo at weeks 0, 2, 6 and 14 while receiving prednisone with follow-up at weeks 10, 18, 22 and 26. Corticosteroid dosage was allowed to be adjusted by the investigator using best medical judgment. If additional immunosuppressive medications were utilized during the trial the subject was deemed a treatment failure.
Subjects were evaluated for mucosal and cutaneous disease activity at each clinic visit and assessed using the Dermatology Quality of Life Index (DQLI) and Short Form 36 Health Survey (SF36) at weeks 0, 10, 18 and 26 13. Clinical disease activity was assessed in 16 of 20 subjects using the Pemphigus Disease Area Index (PDAI) at each study visit. 14
The primary safety endpoint was defined as the proportion of subjects who experienced AEs (> grade 3 National Cancer Institute –Common Terminology Criteria for Adverse Events, NCI-CTCAE, system version 3.0) occurring prior to 18 weeks. The secondary safety endpoints included severe infusion reactions > grade 3, severe infectious complications, AEs resulting in discontinuation of treatment and assessed as being at least possibly related to treatment, or an old or new disease activity score of 3 indicative of active disease.
The primary efficacy endpoint was response to treatment at week 18. Subjects were responders if they achieved a prednisone dosage ≤ 25% of the initial starting dose or ≤ 10 mg/day and had no new blisters within the previous 4 weeks. Secondary efficacy endpoints included achieving response status at weeks 22 or 26; modified response status, defined as achieving a prednisone dosage ≤ 25% of the initial starting dose or ≤ 10 mg/day at 18 weeks, regardless of status on new blister formation; time to cessation of new blister formation; time to 80 % healing of existing erosions/ulcerations at the time of enrollment; total prednisone dosage required to achieve cessation of new blisters and to achieve 80% healing of existing erosions at time of enrollment; and quality of life (Short Form-36, SF36) and dermatology related quality of life (DQLI) changes from baseline to week 26.
Mechanistic Studies
Serum IgG antibodies against desmogleins (DSG) 1 and 3 were assessed at each study visit using commercially available ELISAs (MBL Int Corp, Woburn, MA).
B cell phenotypes were analyzed in 10 subjects (5 placebo, 5 IFX) using FACS analysis as previously described.15
Statistical Methods
The goals of this study were to gather evidence of safety and possible efficacy of infliximab for treatment of pemphigus vulgaris. The sample size of 20 subjects reflected a balance between the study objectives and the desire to expose as few subjects as possible to infliximab in the absence of information on the safety in this population. It was anticipated that the planned sample size would not provide sufficient power to test hypotheses about study arm differences for endpoints unless the differences were very large. For example, if the probability of response was 5% in the placebo arm, the power would not reach 80% unless the probability of response was at least 60% in the infliximab arm.
Subjects were randomized in a 1:1 ratio to receive either infliximab or placebo. Since the study was small and equal group size was a priority, subjects were randomized without stratification by site in blocks of size 4 using an automated web-based system designed by Rho, Inc. The system sent randomized treatment assignments to unblinded site pharmacists who prepared the appropriate infusions for the subjects. All clinical personnel, who enrolled and interacted with the subjects, and immunology laboratory staff, who processed the coded specimens, were masked to the treatment assignments until the completion of the study. In addition, clinical staff members did not have access to any immunology laboratory results, and immunology laboratory staff did not have access to any clinical results until completion of the study. The treatment assignment for the one subject who died (placebo) was released prior to completion of the study.
For the primary endpoint analysis and secondary analyses on dichotomous endpoints, the proportion of responders (or modified responders) in each study arm was computed (with 90% confidence intervals), and study arms were compared using Fisher’s Exact test (two-sided). For the primary efficacy endpoint, the test for responder status at week 18 was evaluated in the ITT population at a Type 1 error rate of α=0.05. All secondary inferential analyses are considered supportive and are presented with p-values with no adjustment for multiple testing.
In general, for interval and ordinal scale variables, medians, minimums and maximums are presented, and groups are compared using Mann-Whitney tests. Time to cessation of new blisters and time to 80% healing are summarized with descriptive statistics by treatment group. Total accumulated prednisone dose from baseline to achievement of cessation of new blisters and to 80% healing are also summarized. To evaluate longitudinally how the number of days per week with new blisters changes over time, a random regression model was fit with fixed effects for new blisters days at baseline, and separate intercepts and slopes over time. Random subject effects for intercepts and slopes were assumed to have an unstructured covariance structure. The Kenward-Roger method for degrees of freedom was used for inference.
The overall incidence of treatment-related toxicities was computed for each study arm. For the primary and secondary safety endpoints, the proportion of subjects meeting endpoint and the estimated difference between arms (with 90% confidence intervals) were computed. For safety endpoints occurring in two or more subjects, Fisher’s exact test was performed to compare study arms.
The intent to treat (ITT) sample was defined as all randomized subjects. The safety sample included all subjects who received any dose of study therapy. All 20 subjects were included in both the ITT and safety samples. The per protocol sample was defined as all subjects who complied with the assigned treatment protocol and who completed all five follow up visits with no significant protocol deviations. A masked data review panel evaluated deviations from the protocol. Six IFX and 7 placebo subjects were included in the per protocol sample.
Results
Subject characteristics
20 subjects (10 each group) were randomized. No significant baseline differences in age, gender, race, disease activity, or IgG anti-DSG1 antibody levels between groups were noted. Subjects randomized to the placebo group had a somewhat higher level of IgG anti- DSG3 antibodies and a higher dose of prednisone at baseline compared to the IFX group, but these differences did not reach statistical significance (Table II).
Table 2.
Comparison of Treatment Groups at Baseline in the ITT population
| Baseline Characteristics | IFX (N=10) | Placebo (N= 10) | P-value [1] | |
|---|---|---|---|---|
| Age (Years) | median (min, max) | 52 (21, 63) | 54.5 (30, 71) | 0.7 |
| Gender | 6 male; 4 female | 6 male;4 female | 1.0 | |
| Race | 10 white | 7 white/3 black | 0.2 | |
| Initial Prednisone (mg/day) | median (min, max) | 22.5 (20, 100) | 45 (20, 65) | 0.1 |
| PV Disease Activity (0,mild-5,severe) | median (min, max) | 3.0 (1, 5) | 3.0 (0, 5) | 0.9 |
| IgG anti-DSG3 (u/ml) | median (min, max) | 248 (0.08, 1595) | 820 (77, 27788) | 0.09 |
| IgG anti-DSG1 (u/ml) | median (min, max) | 92 (0.73, 453) | 47 (6.0,669 ) | 0.9 |
P-values are derived from the 2-sided Mann-Whitney test for continuous variables or Fisher’s Exact for categorical variables.
Seven subjects (4 IFX, 3 placebo) were excluded from the per protocol sample. Four discontinued treatment early: three due to disease flares (2 IFX, day 15 and day 17; 1, placebo, day 44), and one (placebo) who died 17 days after randomization due to a pulmonary embolism. The remaining 3 exclusions (1 placebo, 2 IFX) were the result of the failure of subjects to meet entry criteria (PV Disease Activity score > 2) but who were nonetheless randomized into the trial resulting in major protocol deviations.
Treatment Response
The primary efficacy endpoint was achieved by 1 subject in the IFX group and 1 in the placebo group. Three subjects in the IFX group achieved responder status at 26 weeks compared to none of the subjects in the placebo group (p = 0.21). (Table III)
Table 3.
Primary and Secondary Efficacy Endpoint Analyses in the Intent to Treat Population
| Efficacy End-Point | IFX (N= 10) | Placebo (N= 10) | p-value | |
|---|---|---|---|---|
| Treatment Responder | N (%) | 1 (10%) | 1 (10%) | >0.99 |
| Week 18 (Primary Endpoint) | (90% CI) | (1%, 39%) | (1%, 39%) | |
| Treatment Responder | N (%) | 3 (30%) | 0% | 0.21 |
| Week 26 | (90% CI) | (9%, 61%) | (0%, 26%) | |
| Modified Treatment | N (%) | 5 (50%) | 3 (30%) | 0.65 |
| Responder Week 18 | (90% CI) | (22%, 78%) | (9%, 61%) | |
| Cessation of new blisters | ||||
| Subjects achieving event | N (%) | 5 (50%) | 3 (30%) | 0.65 |
| Days to achieve event | median (min,max) | 151 (103, 163) | 73 (66, 155) | |
| Total prednisone (mg) to achieve event | median (min,max) | 3380 (1830, 7305) | 6410 (2240, 10690) | |
| 80% Healing | ||||
| Subjects achieving event | N (%) | 5 (50%) | 4 (40%) | 0.99 |
| Days to achieve event | median (min,max) | 41 (15, 161) | 60 (14, 99) | |
| Total prednisone (mg) to achieve event | median (min,max) | 1490 (300, 7305) | 1902 (390, 7640) | |
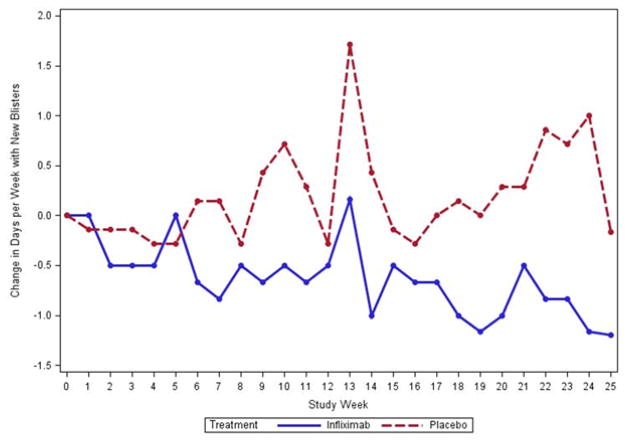
None of the secondary efficacy endpoints reached statistical significance (Table III). The number of days per week that subjects reported new blisters decreased on average by 0.68 days per week in the IFX group and increased on average by 0.76 days in the placebo group (p= 0.037) (Figure 1).
Figure 1.
Change in the number of days per week with new blisters in subjects treated with infliximab compared to placebo. Subjects in the per-protocol group treated with infliximab exhibited a decrease in the number of new blisters per week during the study whereas subjects treated with placebo showed an increase in the number of new blisters per week. (p= 0.037)
As the PDAI was validated after the initiation of the trial, it was completed for only 10 of 13 subjects in the per protocol population (5 IFX, 5 placebo). At week 0, the PDAI median total activity scores did not differ significantly by group (median score [min, max]: IFX 19.9 [3, 43]; Placebo 22.6 [7, 29.6]). For weeks 18, 22 and 26 reduced disease activity was noted in the IFX group (median scores [min,max]: week 18: IFX, 0 [0, 4], placebo 5 [2.3, 24.5], p =0.032; week 22: IFX 0 [0, 2], placebo 5 [3, 14.2], p =0.008; week 26: IFX 0 [0.3.3], placebo 9.2 [2.6, 31.3], p =0.032, Mann-Whitney). Four of 5 subjects in the IFX arm had a PDAI total disease activity score of 0 at week 26, compared to none in the placebo arm (p = 0.048). No significant differences in the DLQI or SF-36 between the treatment groups were noted (data not shown).
Safety
All subjects tolerated the infusions, and no safety signals were detected. 138 AEs were grade 1, 22 Grade 2, three Grade 3 and one each Grade 4 and Grade 5(Table IV). No infectious complications (>Grade 3) were seen in any subjects. There was no significant difference between study arms in the proportion of subjects with treatment-related AEs > Grade 3 on or before 18 weeks (IFX [0/10], placebo [1/10]).
TABLE 4.
Adverse Events by NCI –CTCAE Grade
| GRADE ADVERSE EVENT | INFLIXIMAB (N=10) | PLACEBO (N=10) | TOTAL (N=20) |
|---|---|---|---|
| 1 | 76 | 62 | 138 |
| 2 | 7 | 15 | 22 |
| 3 | 1 | 2 | 3 |
| 4 | 0 | 1 | 1 |
| 5 | 0 | 1 | 1 |
6 SAEs were reported (1 IFX, 5 placebo); 5 were of grade 3 or higher (1 INFX; 4 in 2 placebo subjects). One SAE in a placebo subject was deemed as possibly related to study drug while all other SAEs were deemed unlikely or unrelated to study drug.
Mechanistic Investigations
IgG anti DSG1 and anti DSG3 antibodies
For the longitudinal analysis of IgG anti-DSG1 and anti-DSG3 antibodies the per protocol subjects were used. This analysis allowed comparison across subjects who met all study criteria for entry and completed the entire study without additional therapy.
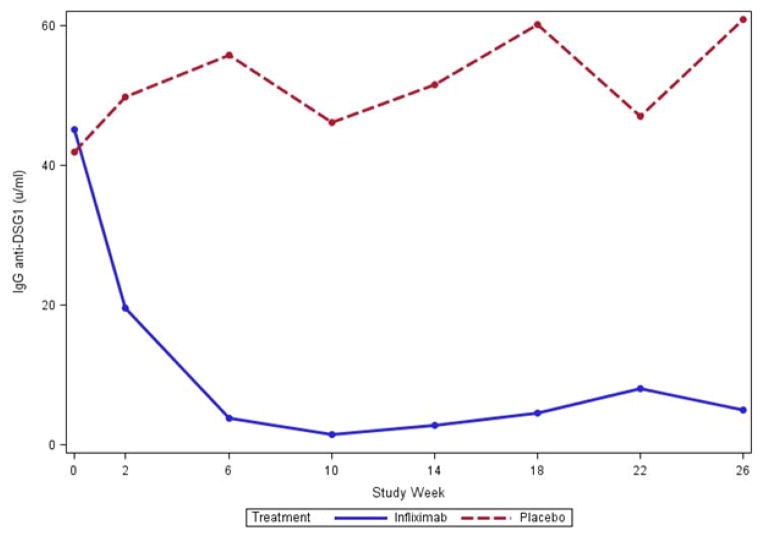
Median IgG anti-DSG1 antibody levels were similar at week 0 between per protocol treatment groups (median [min, max]: 45 [0.7, 368] IFX, 42 [8.7, 669] placebo). At weeks 18 and 26, median IgG anti-DSG1 level were significantly lower in the IFX treated than placebo treated subjects (Week 18: 4.6[(0, 47] IFX, vs. 60 [10, 553] placebo; p = 0.035; Week 26: 5.0 [0, 52] IFX vs. 61 [4.2, 832] placebo; p = 0.022) (Figure 2A).
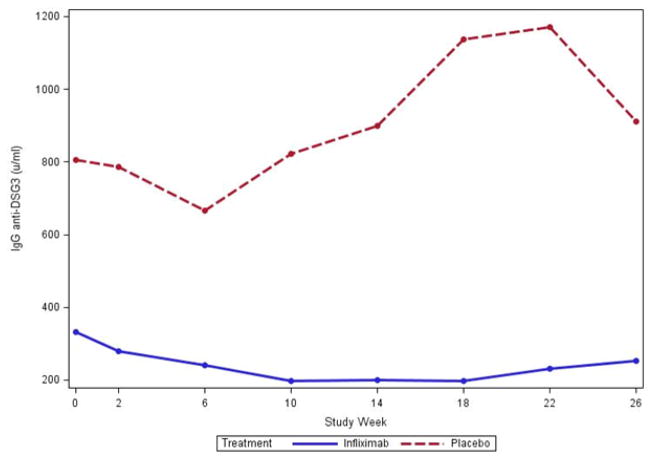
Figure 2.
IgG anti – desmoglein 1(A) and anti –desmoglein 3 (B) values. Median IgG anti- DSG1 (A) levels in the per-protocol population were lower in subjects treated with infliximab (N = 6) when compared to subjects treated with placebo (N=7) (week 0 = NS; week 26, p = 0.022). Median IgG anti DSG3 (B) levels were also lower at week 0 and week 26 in infliximab treated subjects (week 0 = NS; Week 26 p = 0.051).
The IgG anti-DSG3 antibody levels were similar between the per protocol treatment groups at week 0 (331 [164, 1595] IFX vs. 806 [77, 27787] placebo). At weeks 18 and 26, IgG anti-DSG3 levels were lower in the IFX than the placebo group and these differences approached significance (week 18: 198 [63, 726], IFX vs. 1137 [4.4, 15289] placebo, p = 0.035; and week 26: 253 [31, 812] IFX vs. 912 [2.8, 32308] placebo, p = 0.051) (Figure 2B).
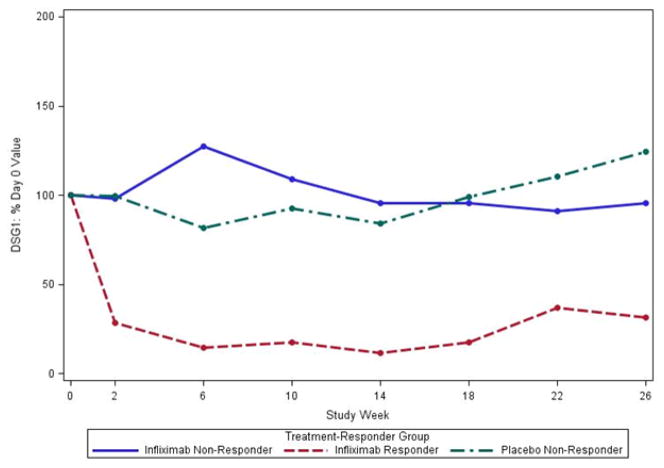
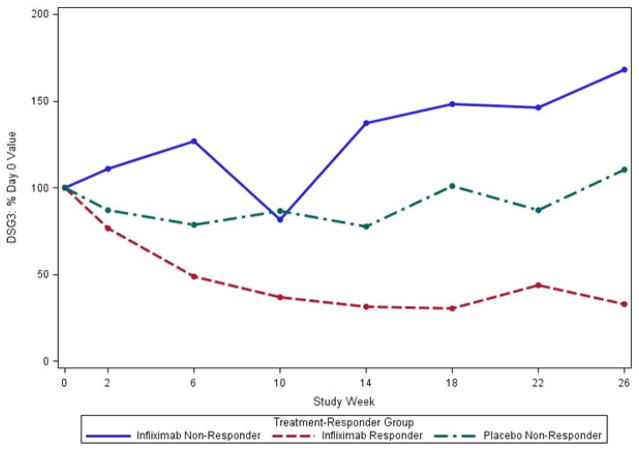
In separate analyses, we considered the % change in antibody levels relative to baseline (100%* level at week X/level at baseline). Median anti-DSG1 and anti DSG3 levels as a percent of week 0 decreased in the 3 responders in the IFX group, but not the non-responders regardless of treatment with IFX or placebo (Figure 3A, 3B).
Figure 3.
Median % change in IgG anti-DSG1 (A) and IgG anti –DSG3 (B) levels compared to baseline in the per-protocol population. IgG anti-DSG1 (A) levels at week 26 were decreased in the 3 subjects treated with infliximab that met responder criteria compared to the 3 non-responders treated with infliximab and to placebo treated subjects (N=7). IgG anti-DSG3 (B) levels also decreased in the 3 subjects treated with infliximab that met responder status but increased in infliximab treated non-responders (N=3) and showed little change in placebo treated subjects (N=7).
B cell subset studies
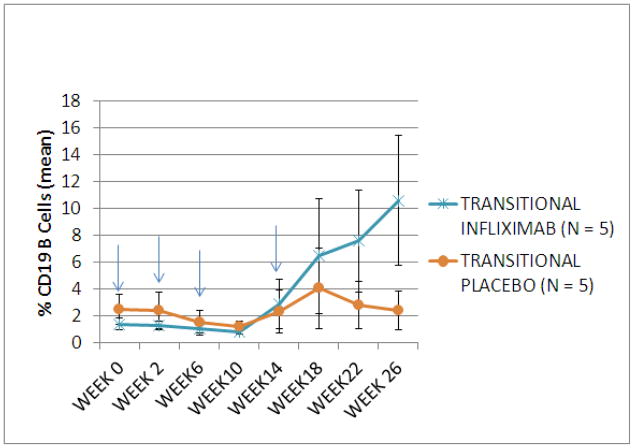
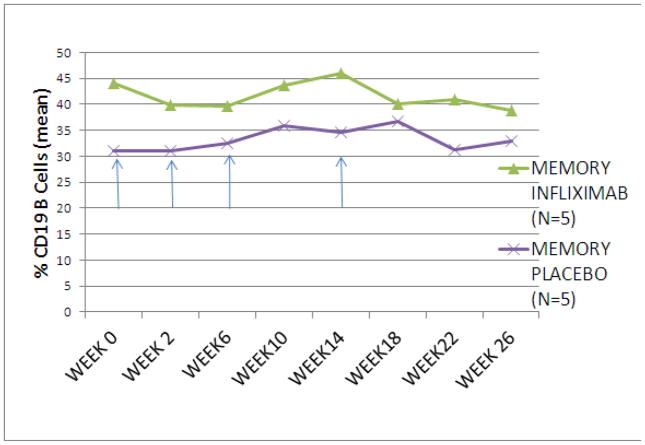
Analysis of the B cell phenotypes of 10 subjects (5 IFX; 5 placebo) at week 0 revealed no significant differences in proportion of B cells between the placebo or IFX study arms (Figure 4A, 4B). The proportion of transitional B cells (IgD+, CD38hi, CD27−) increased at week 18 through week 26 in subjects treated with IFX but remained stable in the subjects treated with placebo (week 26 mean [se]: 10.6 [4.8] IFX vs. 2.4 [1.5] placebo, p = 0.093,) (Figure 5A). There was no significant change in the proportion of memory B cells (IgD+/−, CD38+/−, CD27+) between the two treatment groups (Figure 5B).
Figure 4.
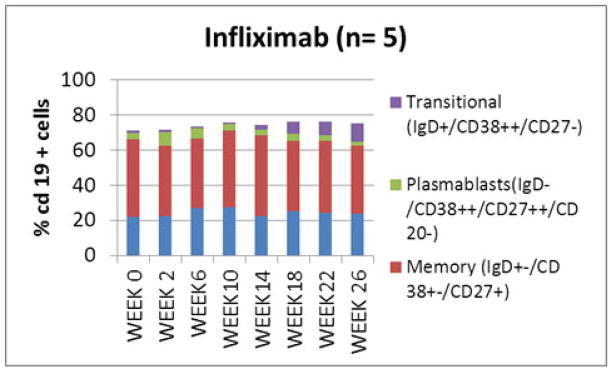
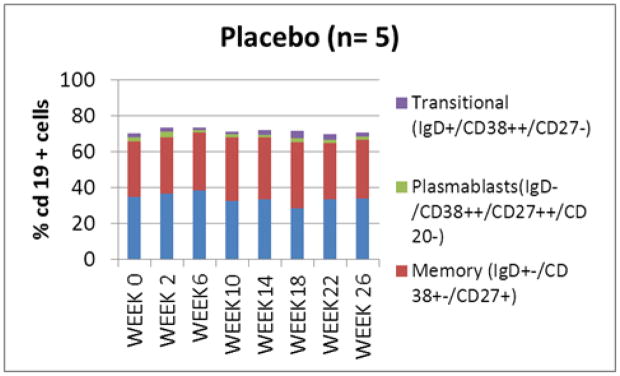
B (CD19+) cell subpopulations in subjects treated with infliximab(A) and placebo (B). No significant difference in B cell subpopulations between the study groups was seen at week 0. An increase in proportion of transitional (IgD+/CD38++/CD27−) cells was noted in the infliximab treated subjects at week 26.
Figure 5.
Proportion of transitional (IgD+/CD38++/CD27−) and memory (IgD+/CD38+-/CD27+) B cells in subjects treated with infliximab or placebo. Subjects treated with infliximab showed an increase in transitional B cells from week 18 thru week 26 compared to those treated with placebo (mean +/− SEM) (A). No change was seen in the mean proportion of memory B cells (B) during the study. Arrows signify infliximab or placebo infusion.
Discussion
In this study, IFX therapy was not associated with significant safety signals or SAEs. While these findings suggest that IFX treatment of patients with PV has an acceptable safety profile, the study number is small and it is possible that the AEs associated with IFX in the treatment of other diseases such as rheumatoid arthritis or psoriasis would occur with similar frequency and severity16–18;18;19 It is important to note that AEs greater than grade two and SAEs occurred more frequently in subjects treated with placebo and prednisone. These findings emphasize the morbidity and mortality associated with conventional treatment of moderate and severe pemphigus and the need for appropriate controls in studies of the treatment of patients with pemphigus.
The primary efficacy endpoint at 18 weeks was only achieved by one subject in each group. 3 subjects in the treatment group did achieve responder status at week 26 compared to no subjects in the placebo group (p = 0.21). The secondary efficacy endpoints, including the number of days till 80% healing, total prednisone dose till no new blisters, and total prednisone dose till 80% healing, all favored the IFX treated over the placebo treated subjects but none reached statistical significance (Table III). Adjusting for baseline differences between the study groups, the number of days per week when subjects observed new blisters decreased by an average of 0.68 days up to the end of the study in the IFX group compare to an increase of 0.76 days in the placebo group (p = 0.03) (Figure 1).
The PDAI was utilized for only 10 per protocol subjects (5 placebo and 5 IFX) as its validation was completed after the study began. IFX-treated subjects had a lower PDAI activity score than the placebo-treated subjects at weeks 18, 22 and 26 (p < 0.032). Although these results cannot be taken as evidence of the superiority of IFX with prednisone compared to prednisone alone, they are consistent with the interpretation that patients treated with IFX realized some clinical benefit.
We focused on the per protocol group to determine the potential effects of IFX treatment on the autoantibody responses since subjects withdrawn from the protocol did not receive a full course of therapy or received other therapy which may have impacted antibody levels. Median IgG anti-DSG1 antibody levels were significantly lower in the IFX treated subjects at weeks 18 and 26. Median IgG anti-DSG3 levels were significantly lower in the IFX subjects at weeks 18 and nearly significant at week 26 (without adjustment for multiplicity). Comparison of the changes in anti-DSG1 and anti-DSG3 levels between responders and non-responders expressed as a percentage of the baseline value showed that IgG anti-DSG1 and anti-DSG3 antibodies decreased in responders, but remained relatively stable in non-responders regardless of treatment (Fig. 3A, 3B). Mouquet and co-workers evaluated patients with pemphigus with complete remission after rituximab therapy, observing a decrease in both IgG anti DSG1 and anti-DSG3 antibodies at 6 months which correlated with B cell depletion. 20 Our observations document a similar decrease in auto-antibodies in responders treated with IFX but without B cell depletion, suggesting that the neutralization of TNF-α is another mechanism by which autoantibody levels may be affected in PV. 20;21
Previous studies have demonstrated that treatment with IFX results in a decrease in memory B cells with an increase in the immature transitional B cells. 22 We demonstrated a similar increase in the proportion of transitional B cells (CD38++/IgD+/CD27−) at weeks 18 and 26 in subjects treated with IFX, with no significant change in the proportion of memory B cells at any of the time points. This increase in transitional B cells was first observed at week 18, and persisted without additional IFX infusions, demonstrating a biological effect of the IFX infusion in our subjects.
The main limitation of this study is the small numbers of subjects. The objective of the study was to evaluate safety in 20 patients with PV and the study was underpowered to evaluate efficacy endpoints except with very large differences between treatment and placebo arms (see statistical methods). This limitation was exacerbated by the need to exclude three subjects due to protocol violations. Efficacy and mechanistic endpoints were examined to explore trends and generate hypotheses for future research. Given that some patients in our trial continued to improve during the 8 week follow up period after only 4 treatments with IFX additional improvement may have been observed if the patients had been treated for a longer period of time. The use of a threshold prednisone dose and the absence of new blister formation as the clinical endpoints may have represented too high a bar for treatment success given that the PDAI was significantly improved in IFX treated subjects.
In summary, IFX therapy was not shown in this randomized, placebo-controlled trial to be effective for the treatment of patients with PV, although the trends suggested some patient may derive benefit from this treatment approach. The use of IFX did not produce any unexpected serious toxicity in patients with PV despite the concomitant treatment with prednisone. IFX therapy was associated with a decrease in anti-DSG1 and DSG3 antibody levels, suggesting that inhibition of TNF-α may down-regulate these autoantibody responses. Further studies of TNF-α blockade will be necessary to confirm these preliminary findings and determine if this treatment approach can be utilized for the benefit of this patient population.
What is known about this topic?
TNF-α has been shown to play a role in the pathogenesis of pemphigus vulgaris
Case reports have shown infliximab to be beneficial in the treatment of pemphigus vulgaris.
What does this study add?
Treatment with infliximab and prednisone was not significantly better than prednisone alone in treating patients with active pemphigus vulgaris
Infliximab therapy is associated with decreased autoantibodies and change in peripheral B cell phenotypes.
Acknowledgments
Supported By Grants from:
Autoimmunity Centers of Excellence, NIAID (Grant #5U 19AI056363-7) (Participated in study design, data analysis through DSMB, manuscript review. No participation in data collection, publication decision)
Janssen Biologics (Participation in design, data collection, data analysis, manuscript preparation, publication decision: None)
Registered on ClinicalTrials.gov: Use of Infliximab for the treatment of Pemphigus Vulgaris, NCT00283712ID:DAIT APV01
Abbreviations
- PV
Pemphigus vulgaris
- IFX
Infliximab
- TNF-α
Tumor necrosis factor alpha
- DQLI
Dermatology Quality of Life Index
- PDAI
Pemphigus Disease Area Index
- DSG
Desmoglein
- ITT
Intent to Treat
- AEs
Adverse Events
- SAEs
Serious Adverse Events
Footnotes
Presented in abstract form: Annual meeting Society for Investigative Dermatology, May 9–12, 2012.
Conflicts of interest: None declared
Reference List
- 1.Risser J, Lewis K, Weinstock MA. Mortality of bullous skin disorders from 1979 through 2002 in the United States. Archives Dermatology. 2009;145(9):1005–1008. doi: 10.1001/archdermatol.2009.205. [DOI] [PubMed] [Google Scholar]
- 2.Langan SM, Smeeth L, Hubbard R, Fleming K, Smith C, West J. Incidence and mortality of bullous pemphigoid and pemphigus vulgaris in the UK. Br Med J. 2008;337:a180. doi: 10.1136/bmj.a180. doi:10:1136bmja180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin LK, Werth VP, Villaneuva EV, et al. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. JAAD. 2011;64:903–8. doi: 10.1016/j.jaad.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardo J, Mercader P, Mahiques L, et al. Infliximab in the management of severe pemphigus vulgaris. BJD. 2005;153:222–3. doi: 10.1111/j.1365-2133.2005.06672.x. [DOI] [PubMed] [Google Scholar]
- 5.Jacobi A, Schuler G, Hertl M. Rapid control of therapy-refractory pemphigus vulgaris by treatment with the tumour necrosis factor-alpha inhibitor infliximab. BJD. 2005;153:448–9. doi: 10.1111/j.1365-2133.2005.06744.x. [DOI] [PubMed] [Google Scholar]
- 6.Berookhim B, Fischer HD, Weinberg JM. Treatment of recalcitrant pemphigus vulgaris with the tumor necrosis factor alpha antagonist etanercept. Cutis. 2004;74:245–247. [PubMed] [Google Scholar]
- 7.Lin M. Successful treatment of recalcitrant pemphigus vulgaris and pemphigus vegetans with etanercept and carbon dioxide laser. Archives. 2005;141:680–2. doi: 10.1001/archderm.141.6.680. [DOI] [PubMed] [Google Scholar]
- 8.Feliciani C, Toto P, Amerio P, et al. In vitro and in vivo expression of interleukin-1alpha and tumor necrosis factor-alpha mRNA in pemphigus vulgaris: interleukin-1alpha and tumor necrosis factor-alpha are involved in acantholysis. Journal of Investigative Dermatology. 2000;114:7l–7. doi: 10.1046/j.1523-1747.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 9.Ameglio F, D’Auria L, Cordiali-Fei P, et al. Anti-intercellular substance antibody log titres are correlated with serum concentrations of interleukin-6, interleukin-15 and tumor necrosis factor-alpha in patients with Pemphigus vulgaris relationships with peripheral blood neutrophil counts, disease severity and duration and patients’ age. Journal of Biological. Regulators & Homeostatic Agents. 1999;13:220–4. [PubMed] [Google Scholar]
- 10.Feliciani C, Toto P, Wang B, et al. Urokinase plasminogen activator mRNA is induced by IL-1+α and TNF-+α in in vitro acantholysis. Experimental Dermatology. 2003;12:466–71. doi: 10.1034/j.1600-0625.2002.120415.x. [DOI] [PubMed] [Google Scholar]
- 11.Amagai M, Ikeda S, Shimizu H, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. JAAD. 2009;60:595–603. doi: 10.1016/j.jaad.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Beissert S, Mimouni D, Kanwar AJ, et al. Treating Pemphigus Vulgaris with Prednisone and Mycophenolate Mofetil: A Multicenter, Randomized, Placebo-Controlled Trial. J Invest Dermatol. 2010;130:2041–8. doi: 10.1038/jid.2010.91. [DOI] [PubMed] [Google Scholar]
- 13.Basra MKA, Fenech R, Gatt RM, et al. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. BJD. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbach M, Murrell D, Bystryn JC, Delay S, Dick S, Fakharzadeh S, Hall R, Korman N, Lin J, Okawa J, Pandya AG, Payne AS, Rose M, Rubenstein David S, Woodley D, Vitoria JC, Werth BB, Williams EA, Taylor L, Troxel A, Werth VP. Comparison of reliability and validity between two outcome instruments for pemphigus. J Invest Dermatol. 2009;129(10):2404–2410. doi: 10.1038/jid.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levesque MC, Moody MA, Hwang KK, et al. Polyclonal B Cell Differentiation and Loss of Gastrointestinal Tract Germinal Centers in the Earliest Stages of HIV-1 Infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmon-Ceron D, Tubach F, Lortholary O, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70:616–23. doi: 10.1136/ard.2010.137422. [DOI] [PubMed] [Google Scholar]
- 17.Atteno M, Peluso R, Costa L, et al. Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin Rheumatol. 2010;29:399–403. doi: 10.1007/s10067-009-1340-7. [DOI] [PubMed] [Google Scholar]
- 18.Delabaye I, De Keyser F the REMITRACT study group. 74-week follow-up of safety of infliximab in patients with refractory rheumatoid arthritis. Arthritis Research & Therapy. 2010;12:R121. doi: 10.1186/ar3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinisch W, Sandborn WJ, Rutgeerts P, et al. Long-term infliximab maintenance therapy for ulcerative colitis: The ACT-1 and -2 extension studies. Inflamm Bowel Dis. 2012;18:201–11. doi: 10.1002/ibd.21697. [DOI] [PubMed] [Google Scholar]
- 20.Mouquet H, Musette P, Gougeon ML, et al. B-cell depletion immunotherapy in pemphigus: effects on Cellular and Humoral immune responses. J Invest Dermatol. 2008;128:2859–69. doi: 10.1038/jid.2008.178. [DOI] [PubMed] [Google Scholar]
- 21.Joly P, Mouquet H, Roujeau JC, et al. A Single Cycle of Rituximab for the Treatment of Severe Pemphigus. N Engl J Med. 2007;357:545–52. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 22.Anolik JH, Ravikumar R, Barnard J, et al. Cutting Edge: Anti-Tumor Necrosis Factor Therapy in Rheumatoid Arthritis Inhibits Memory B Lymphocytes via Effects on Lymphoid Germinal Centers and Follicular Dendritic Cell Networks. J Immunol. 2008;180:688–92. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]