Summary
Two hundred thirty-five consecutive patients presenting to a single center with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) after breast cancer treatment were compared with matched patients with de novo AML or MDS. There was no significant difference in median OS times between patients with therapy related AML and those with de novo AML (8.7 months vs. 10.2 months; P=.17). Patients with therapy related MDS had slightly lower median baseline platelet counts and a higher frequency of poor cytogenetics than those with de novo MDS, but the two groups had similar OS times (13.6 months vs. 18.9 months; P = .06). Multivariate analysis revealed that cytogenetic risk, baseline white blood cell count, age, and performance status were predictive for OS time in AML and that cytogenetic risk and performance status were predictive for OS time in MDS. Having therapy-related disease is not an independent risk factor in patients with myeloid neoplasms and with a history of breast cancer. Clinical trials should be designed to serve both populations.
Introduction
Although therapy-related myeloid neoplasms (t-MNs) are no longer subcategorized as “alkylating agent–related” and “topoisomerase II inhibitor–related,” they remain a distinct entity in the 2008 revision of the World Health Organization classification of myeloid neoplasms (MNs) and acute leukemias, with the recognition of myelodysplastic/myeloproliferative neoplasms that can occur after cytotoxic therapy.1 Roughly 10% of acute myeloid leukemia (AML) cases and about 20% of myelodysplastic syndrome (MDS) cases are therapy related (t-AML and t-MDS, respectively), and having t-MN (compared with de novo MN) is generally considered an independent adverse prognostic factor.1-5 However, results from multivariate analysis have been conflicting.6,7 Consequently, clinicians have disagreed on whether t-MN and de novo MN should be treated the same way for many years. There is cytogenetic evidence, however, that the diseases are similar, if not identical: 90% of t-MNs have cytogenetic abnormalities very similar to those observed in AML with myelodysplasia-related features and AML with recurrent cytogenetic abnormalities.1,8 Because the incidence of t-MN is increasing as more patients survive their primary cancers, a better understanding of the biologic and prognostic factors associated with this disease is needed.
Although several studies have characterized and reported outcomes of t-MN, these studies were limited by small sample size, lack of adequate controls, data that were exclusively from a few clinical trials with the potential for selection bias, lack of information about the primary cancers (such as disease status when the t-MN was diagnosed), inclusion of patients with a variety of primary cancers.2,6,9-11 To obtain further insight into t-MN and clarify whether t-AML/t-MDS is an independent poor prognostic factor, we evaluated 235 consecutive patients who presented to a single tertiary cancer center with AML or MDS and had the same primary cancer, breast cancer. The distribution and frequency of chromosome abnormalities, responses to therapy and outcomes of the t-MN were compared with those of matched patients with de novo MN.
Materials and methods
Study population and design
All patients who developed AML or MDS after breast cancer treatment, were diagnosed and received primary MN treatment at The University of Texas MD Anderson Cancer Center between 1983 and 2009 were identified. Of these 289 patients, 16 patients who had active primary cancer at the initial diagnosis of AML/MDS were excluded. In addition, 38 patients were excluded because there was inadequate info about their breast cancer treatment. The remaining 235 patients were included in this study and matched with patients who had de novo AML or MDS. Among those 235, 118 patients with AML (t-AML) and 75 patients with MDS (t-MDS) had received prior chemotherapy and/or radiation therapy for breast cancer; these patients were considered to have t-MN. The other 22 patients with AML and 20 patients with MD Shad received only surgery and/or hormonal therapy for their breast cancer; these patients were considered to have a second MN and analyzed separately.
For each of the 235 patients with t-MN or second MN and with a history of breast cancer, 2 (when possible) control female patients with de novo MN who were matched for age (±3 years) at initial diagnosis of MN, date of MN diagnosis (±5 years), French-American-British (FAB) classification (with M1, M2, and M0 grouped together), and race were randomly selected from the leukemia service database. Patients with de novo MN had not received any chemotherapy and/or radiation therapy and had no history of any antecedent hematologic disorder.
All patients with t-AML received anti–MNtherapy. Among them, 79 patients received a high-dose cytarabine (HDAC)–based induction regimen (≥1g/m2 per dose): HDAC only (n=4), HDAC plus idarubicin (n=33), HDAC plus fludarabine (n=25), HDAC plus daunorubicin (n=1), HDAC plus liposomal daunorubicin (n=5), or others (n=11). Thirty-two received non–HDAC-based or non–cytarabine–based; and 7 received biologic agents. Among 75 patients with t-MDS, 30 did not receive treatment. The treatments of patients with t-MDS varied according to risk status and age and consisted of chemotherapy (n=34) or biologic agents (n=11; 7 with decitabine, 3 with azacitidine, and 1 with lenalidomide). A complete response (CR) was defined based on the criteria established by the International Working Group.12,13 Cases of AML were grouped into three cytogenetic risk categories (favorable, intermediate and unfavorable) according to the European Leukemia Net criteria.14 All patients signed a consent form approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center for collection of samples and participation in the ongoing treatment.
Statistical analysis
Descriptive statistics were calculated for all groups of patients. Overall survival (OS) time for each patient was measured from the date of entry at MD anderson due to diagnosis of the MN until the date of death and censored on the date of the last follow-up if alive; Kaplan-Meier survival curves were used to estimate unadjusted survival times for groups. Differences in continuous variables between groups were assessed by the Wilcoxon rank sum test. Differences in categorical variables between groups were assessed by chi-square or Fisher exact tests. The Cox proportional hazards model was used to evaluate the ability of variables to predict OS time. Variables with potentially significant effect (P<0.05) in the univariate analysis were included in the multivariate mode. All computations were carried out in SAS version 9.3 and TIBCO Spotfire S+ version 8.2.
Results
Clinical, cytogenetic, and molecular features in t-MN vs de novo MN
The median ages at diagnosis of t-AML and matched de novo AML were 63 years and 64 years, respectively (ranges, 30–81 years and 26–86 years, respectively; Table 1). Patients with t-AML had significantly lower median baseline white blood cell counts (P=.03), platelet counts (P =.01), and bone marrow blast percentages (P=.01) and significantly higher hemoglobin levels (P=.03) than patients with de novo AML. The two groups did not differ by the percentage of patients receiving HDAC-based regimens.
Table 1.
Comparison of presenting features, initial treatment type, and outcomes between patients with t-AML and de novo AML
| Characteristic | t-AML (n=118) | De novo AML (n=237) |
P value |
|---|---|---|---|
| Age, years | |||
| Median (range) | 63.3 (29.5, 81) | 63.6 (25.6, 86.1) | .79 |
| WBC, × 109/L | |||
| Median (range) | 5 (0.4, 205) | 9.5 (0.4, 341.5) | .03 |
| Platelet, × 109/L | |||
| Median (range) | 43.5 (3, 378) | 55 (3, 658) | .01 |
| HGB, g/dL | |||
| Median (range) | 8.8 (3.8, 12.1) | 8.2 (2.8, 14.1) | .03 |
| BM blasts (%) | |||
| Median (range) | 42 (0, 96) | 52 (0, 98) | .01 |
| Race, white | 98(83.1%) | 206(86.9%) | .33 |
| FAB classification# | |||
| M0, M1, M2 | 41(41.4%) | 110(55.3%) | .03 |
| M3 | 8(8.1%) | 11(5.5%) | |
| M4 | 18(18.2%) | 37(18.6%) | |
| M5, M5a, M5b | 10(10.1%) | 22(11.1%) | |
| RAEB-T | 22(22.2%) | 19(9.5%) | |
| Unknown | 19 | 38 | |
| Performance status* | .53 | ||
| 0–1 | 85(72%) | 163(68.8%) | |
| >1 | 33(28%) | 74(31.2%) | |
| Cytogenetic risk | <.01 | ||
| Favorable | 13(11.4%) | 21(9.6%) | |
| Intermediate | 40(35.1%) | 137(62.8%) | |
| Poor | 61(53.5%) | 60(27.5%) | |
| Unknown | 4 | 19 | |
| Abnormal karyotype | 88(77.2%) | 109(50.0%) | <.01 |
| Del(5q)/-5, del(7q)/-7, and/or complex karyotype | 30(25.4%) | 21(8.9%) | <.01 |
| 11q23 abnormalities | 20 | 8 | <.01 |
| 11q23 rearrangements | 9 | 2 | .01 |
| Initial treatment regimens | .58 | ||
| HDAC | 79(66.9%) | 149(62.9%) | |
| Non-HDAC | 32(27.1%) | 67(28.3%) | |
| Non-Ara-c | 7(5.9%) | 21(8.9%) | |
| Complete remission | 62 (52.5%) | 144 (60.8%) | .14 |
| Median OS (rane), months | 8.7 (6.7, 13.4) | 10.2 (7.9, 12.9) | .17 |
| 5-year OS rate (range) | 15% (10%, 23%) | 20% (16%, 26%) | |
| Median RFS (range), months | 12.4 (8.7, 56.7) | 14.4 (11.5, 20.4) | .89 |
| 5-year RFS rate(range) | 30% (20%, 50%) | 30% (23%, 39%) |
AML, acute myeloid leukemia; WBC white blood cell; HGB, hemoglobin; BM, bone marrow.; FAB, French-American-British Classification; OS, overall survival; RFS, relapse free survival; HDAC, high dose a ra-C based regimen; RAEB-T, refractory anemia with excess blasts in transformation.
Eastern Cooperative Oncology Group;
The median ages at diagnosis of t-MDS and de novo MDS were 64 years for both groups (ranges, 46-87 years and 43-89 years, respectively; Table 2). T-MDS was associated with slightly lower median baseline platelet counts (P=.006) than de novo MDS, but the two groups had similar median baseline white blood cell counts, hemoglobin levels, and bone marrow blast percentages.
Table 2.
Comparison of presenting features, initial treatment type, and outcomes between patients with t-MDS and de novo MDS
| Characteristic | t-MDS (n=75) | De novo MDS (n=134) |
P value |
|---|---|---|---|
| Age, years | |||
| Median (range) | 64 (45.6, 87.3) | 63.5 (43, 89) | .48 |
| WBC, × 109/L | |||
| Median (range) | 2.9 (0.3, 57.6) | 3.4 (0.4, 72.9) | .43 |
| Platelet, × 109/L | |||
| Median (range) | 57 (6, 628) | 84 (3, 660) | .006 |
| HGB, g/dL | |||
| Median (range) | 9.9 (6.2, 13.1) | 9.7 (3.2, 13.5) | .38 |
| BM blasts (%) | |||
| Median (range) | 6 (0, 19) | 7 (0, 19) | .26 |
| Race, white | 74(98.7%) | 132(98.5%) | 1.0 |
| WHO classification | .79 | ||
| 5q- | 0(0%) | 3(2.2%) | |
| RA | 14(18.7%) | 24(17.9%) | |
| RARS | 5(6.7%) | 8 (6.0%)) | |
| RAEB | 48 (64.0%) | 90 (67.2) | |
| CMML | 4(5.3%) | 6(4.5%) | |
| MDS-U | 2(2.7%) | 1(0.7%) | |
| RCMD | 2(2.7%) | 2(1.5%) | |
| Performance status* | .78 | ||
| 0–1 | 67(89.3%) | 118(88.1%) | |
| >1 | 8(10.7%) | 16(11.9%) | |
| IPSS | .15 | ||
| Low-risk (0) | 7(9.7%) | 25(18.7%) | |
| Inter1 (0.5–1) | 15(20.8%) | 37(27.6%) | |
| Inter2 (1.5–2) | 39(54.2%) | 58(43.3%) | |
| High-risk (2.5–3) | 11(15.3%) | 14(10.4%) | |
| Unknown | 3 | 0 | |
| Abnormal karyotype | 57(85.1% out of 67) | 66(51.6% out of 128) | <.01 |
| Del(5q)/-5, del(7q)/-7, and/or complex karyotype | 32(42.7%) | 22(16.4%) | <.01 |
| Initial treatment regimen | .09 | ||
| Chemotherapy | 34 (45.3%) | 50 (37.3%) | |
| Biologic agents | 11 (14.7%) | 11 (8.2%) | |
| No treatment | 30 (40%) | 73 (54.5%) | |
| Complete remission | 10 (21.7% out of 46) | 15 (22.1% out of 68) | .97 |
| Median OS time (range), months | 13.6 (9.38, 20.6) | 18.9 (15.48, 27.1) | .06 |
| 5-year OS rate (range) | 8% (3%, 19%) | 19% (13%, 28%) |
t-MDS, therapy-related myelodysplastic syndrome; WBC white blood cell; HGB, hemoglobin; BM, bone marrow; WHO, World Health Organization.; OS, overall survival; IPSS, International prognostic score system; RA, refractory anemia; RARS, refractory anemia with ringed sideroblsts, RAEB, refractory anemia with excess blasts; CMML, chronic myelomonocytis leukaemia; MDS-U, myelodysplastic syndrome-unclassified; RCMD. Refractory cytopenia with multilineage dysplasia.
Eastern Cooperative Oncology Group
T-AML was associated with a higher frequency of abnormal karyotypes than de novo AML (77.2% vs 50.0%; P<.01; Table 1). A similar trend was seen fort-MDS and de novo MDS (85.1% vs 51.6%; P<.01; Table 2). T-AML and t-MDS exhibited significantly higher frequencies of del(5q)/-5, del(7q)/-7, and/or complex cytogenetic abnormalities than de novo AML (25.4% vs 8.9%, respectively; P<.01; Table 1) and de novo MDS (42.7% vs 16.4%, respectively; P<.01; Table 2).
Fourteen patients with t-AML were tested for NPM1 mutations and all were negative, whereas 45 patients with de novo AML were tested for NPM1 mutations and 14 were positive (0% vs 31%; P=.03). Of the 10 patients with MDS (7 patients with t-MDS) tested for NPM1 mutations, none had such mutations. There were no significant differences in frequencies of Ras, FLT3-ITD, or FLT3-D835 mutations between t-AML and de novo AML (P = .3, P = .05 and P = 1.0, respectively) or between t-MDS and de novo MDS (data not shown).
Clinical and cytogenetic features in second MN vs de novo MN
The 42 patients who had a history of breast cancer treated with surgery and/or hormonal therapy and developed second MN (Table 3) did not differ significantly from patients with de novo MN in median baseline white blood cell counts, platelet counts, hemoglobin levels, and bone marrow blast percentages. The cytogenetic profiles in the two groups showed no significantly difference in frequencies of abnormal karyotypes (P=.14) or the cytogenetic abnormalities del(5q)/-5, del(7q)/-7, and/or complex karyotype (P=.37).
Table 3.
Comparison of cytogenetic abnormalities and outcomes between patients with second MN after breast cancer (surgery/hormonal therapy alone) and matched de novo MN
| Characteristic | Second MN (n=42) | De novo MN (n=82) | P value |
|---|---|---|---|
| Age, years | |||
| Median (range) | 70.4 (39.5, 82.7) | 69.8 (39, 81.5) | .78 |
| WBC, × 109/L | |||
| Median (range) | 4.9 (1.2, 161.0) | 6.0 (0.5, 233.0) | .43 |
| Platelet, × 109/L | |||
| Median (range) | 60 (8, 398) | 60 (4, 606) | .41 |
| HGB, g/dL | |||
| Median (range) | 9.1 (5.0, 13.3) | 8.9 (4.0, 15.4) | .82 |
| BM blasts (%) | |||
| Median (range) | 10 (0, 93) | 17 (0, 97) | .66 |
| Performance status* | |||
| 0–1 | 31(73.8%) | 62(75.6%) | .83 |
| >1 | 11(26.2%) | 20(24.4%) | |
| Abnormal karyotype | 21(55.3% out of 38) | 31(40.8% out of 76) | .14 |
| Del(5q)/-5, del(7q)/-7, and/or complex karyotype | 7(16.7%) | 9(11%) | .37 |
| Complete remission | 15(35.7%) | 27(32.9%) | .76 |
| Median OS time (range), months | 15.6 (10.0, 32.5) | 14.7 (9.1, 17.3) | .71 |
| 5-year OS rate (range) | 18% (9%, 35%) | 18% (11%, 30%) |
MN, myeloid neoplasm; WBC, white blood cell; HGB, hemoglobin; BM, bone marrow.; OS, overall survival.
Eastern Cooperative Oncology Group
Of note, patients with t-MN and second MN differed significantly in age (P=.002), baseline white blood cell counts (P=.02) and abnormal karyotypes (P=.003;).
Outcomes
CR rates were similar in patients with t-AML and those with de novo AML (52.5% vs 60.8%; P=.14; Table 1) and in patients with t-MDS and those with de novo MDS (21.7% vs 22.1%; P=.97; Table 2).
The median follow-up times for surviving patients with t-AML and de novo AML were 82 months and 105 months, respectively. There was no significant difference in median OS times between patients with t-AML and those with de novo AML (8.7 months and 10.2 months, respectively, P=.17; Figure 1A; Table 1). The median follow-up times for surviving patients with t-MDS and de novo MDS were 79 months and 78 months, respectively. The t-MDS and de novo MDS groups also had similar median OS times (13.6 months and 18.9 months, respectively; P=.06) (Figures 1C–D; Table 2). The median OS time in patients with second MN was similar to that in patients with de novo MN (P=.71; Table 3). Furthermore, there were no significant differences in OS times between the t-AML and de novo AML groups when patients treated with HDAC (P = .21) and non-HDAC regimens (P = .43) were considered separately (data not shown). In addition, the t-AML and de novo AML groups did not differ significantly in OS times when patients with adverse, intermediate, or favorable cytogenetic risks were considered separately (P=.37, .79, and .91, respectively; data not shown); 5-year OS rates were 5% versus 3% for the adverse cytogenetic risk group, 15% versus 24% for the intermediate cytogenetic risk group, and 69% versus 56% for the favorable cytogenetic risk group (data not shown). Of note, the cytogenetic risk groups were predictive of OS time (P<.001; Figure 2A) in patients with t-AML. International Prognostic Scoring System (IPSS) scores were predictive of OS time in patients with t-MDS (P<.001; Figure 2B).
Figure 1.
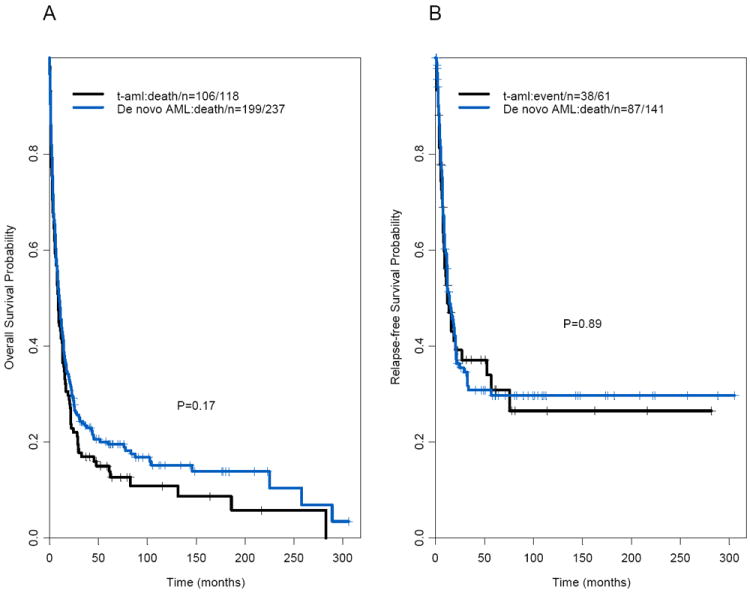
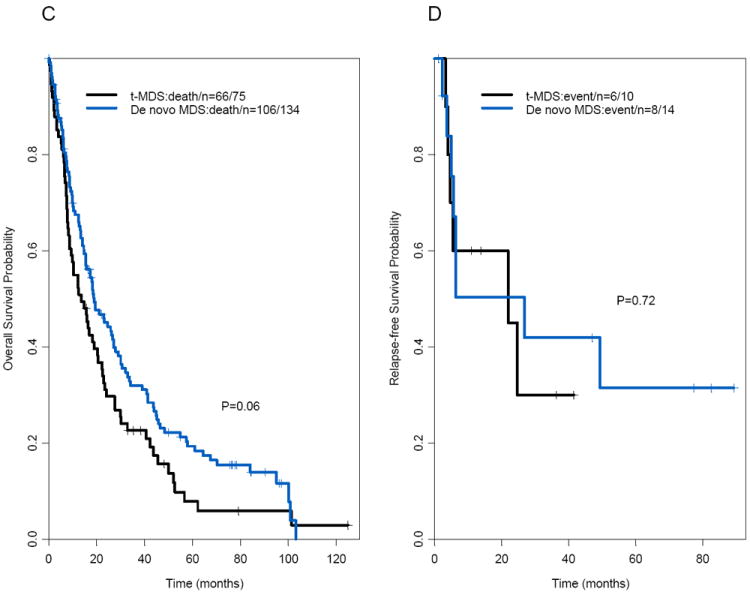
(A) Overall survival and (B) relapse-free survival in patients with therapy-related acute myeloid leukemia and matched patients with de novo acute myeloid leukemia. (C) Overall survival and (D) relapse-free survival in patients with therapy-related-myelodysplastic syndrome and matched patients with de novo myelodysplastic syndrome.
Figure 2.
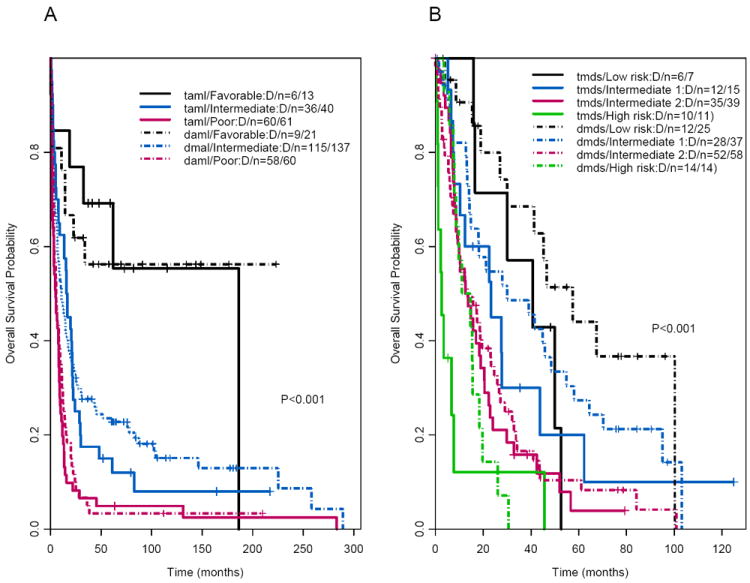
(A) Overall survival in patients with therapy-related and matched de novo acute myeloid leukemia by cytogenetic risk group. (B) Overall survival in patients with therapy-related and matched de novo myelodysplastic syndrome by cytogenetic risk group.
Multivariate analysis
To identify potential prognostic factors for OS times in AML and MDS, we used Cox proportional hazards models that included age, baseline white blood cell count, race, platelet count, hemoglobin level, bone marrow blast percentage, MN type (de novo vs. t-MN), treatment type for the MN, performance status, cytogenetic (diploid Vs. other), cytogenetic risk (for AML), and IPSS score (for MDS). Variables with potentially significant effect (P<0.05) in the univariate analysis were included in the final multivariate mode. Age, white blood cell count, performance status, cytogenetic risk, and treatment type were independent prognostic factors for OS in patients with AML, and performance status and abnormal cytogenetic karyotype were independent prognostic factors for OS in patients with MDS (Table 4).
Table 4.
Multivariate analysis for overall survival time
| OS time
|
||||
|---|---|---|---|---|
| MN | Variable | HR | 95% CI | P value |
| AML | De novo AML vs t-AML | 0.85 | 0.64–1.12 | .25 |
| Age | 1.12 | 1.01–1.03 | .003 | |
| Log WBC | 1.11 | 1.01–1.22 | .03 | |
| BM blast percentage | 1.25 | 0.68–2.27 | .47 | |
| PS (>1 vs ≤1) | 1.40 | 1.04–1.87 | .02 | |
| Cytogenetics (favorable vs poor) | 0.34 | 0.18–0.65 | .001 | |
| Cytogenetics (intermediate vs poor) | 0.64 | 0.49–0.85 | .002 | |
| HDAC vs non-HDAC | 1.53 | 1.10–2.14 | .01 | |
| Biologic agents vs non-HDAC | 1.18 | 0.71–1.99 | .52 | |
|
| ||||
| MDS | Hemoglobin | 0.94 | 0.84–1.05 | .28 |
| Log platelet | 0.92 | 0.79–1.07 | .29 | |
| BM blast percentage | 1.04 | 0.99–1.08 | .10 | |
| PS*(>1 vs ≤1) | 2.08 | 1.26–3.44 | <.01 | |
| Cytogenetics (diploid vs others) | 2.01 | 1.29–3.14 | <.01 | |
| IPSS (inter1 vs low) | 1.27 | 0.71–2.29 | .42 | |
| IPSS (inter2 vs low) | 1.76 | 0.88–3.52 | .11 | |
| IPSS (inter3 vs low | 1.98 | 0.72–5.43 | .18 | |
t-AML, therapy-related acute myeloid leukemia; CI, confidence interval; HR, hazard ratio; WBC, white blood cell; BM, bone marrow; OS, overall survival; IPSS, International prognostic score system; and inter, intermediate.; HDAC, high-dose ara-C based regimens.
Eastern Cooperative Oncology Group
Discussion
In the present study, we compared the presenting characteristics and outcomes of patients with t-AML (n=118) or t-MDS (n=75) with those of matched de novo AML or MDS cases. We clearly demonstrated that CR rates and median OS times are similar inpatients with t-MN and patients with de novo MN. To the best of our knowledge, this is the first time to date to prove that t-AML/t-MDS is not an independent poor prognostic factor by comparing a large series of patients with t-MN after the same primary breast cancer with those of matched patients with de novo MN.
The median OS time of 8.7 months that we found in t-AML patients is in accordance with a University of Chicago study group’s finding of an OS time of 8.0 months in patients with t-AML and different primary cancers and the OS time of 10.2 months that we found inpatients with de novo AML is consistent with the OS time of <1 year in elderly patients with AML who received standard treatment.15
Our finding that t-MN is not independent poor prognostic factor agrees with observations by Ostgard et al,6 who reported that in a study of a population-based cohort of 157 consecutive patients with secondary AML (including 37 patients with t-AML); and with a more recent study by Nardi et al16 of 181 patients with t-AML; both studies found that the presence of secondary AML lost prognostic significance after correction for age, cytogenetic abnormalities, and performance status. Those authors concluded that the impact of secondary AML on survival is very limited.6 Our finding is also in agreement with a report of 121 patients with t-AML with different primary cancers versus 1,511 patents with de novo AML by the German AML Cooperative Group. They found that unfavorable cytogenetics were more frequent in t-AML (46.2% vs 20.4%) and that median OS time was shorter in t-AML (10 vs 15 months; P<.001).7 In that report, the median survival times of patients with t-AML ranged from 26.7 months for favorable karyotypes to 5.6 months for unfavorable karyotypes. The authors concluded that the presence of t-AML does not independently confer a poorer prognosis than that of de novo AML; the apparently poorer prognosis is simply due to t-AML’s commonly unfavorable karyotype. In fact, they observed no statistically significant differences in OS times for t-AML versus de novo AML within the unfavorable risk group (P=.06) and within the intermediate risk group (P=.31), although they had initially thought that t-AML is a poor prognostic factor for OS.2 Others have reported that therapy-related acute promyelocytic leukemia before and after the introduction of all-trans retinoic acid and t-AML with inv(16) or t(8;21) have biologic and clinical outcomes similar to those of their de novo counterparts.17-19 Likewise, our study found that OS times were similar fort-AML and de novo AML patients within the favorable, intermediate, and adverse cytogenetic risk groups and when the analyses were stratified by treatment type (HDAC Vs. non-HDAC). Our results contrast those of a report from the Medical Research Council trials19,20 in which it was observed that patients with t-AML had worse outcomes than patients with de novo AML (P = .04); however, that study had younger patients than ours did. Of note, t-AML is significantly associated with higher frequency of abnormal karyotypes or del(5q)/-5, del(7q)/-7 and/or complex cytogenetic abnormalities, but has the same OS time compared to de novo AML, this could be due to t-AML is associated with lower lever of baseline WBC which is predictive for OS time.
As other studies have reported, we confirmed a significantly higher incidence of del(5q)/-5, del(7q)/-7, and/or complex karyotypes in patients with t-MN than in those with de novo MN.9,10,21-23 In our study, we clearly demonstrated that cytogenetic profile in patients with second MN who had a history of breast cancer treated with surgery and/or hormonal therapy had cytogenetic profiles similar to those of patients with de novo MN, suggesting that patients with a history of breast cancer without receiving chemotherapy and/or radiation therapy may not have a higher risk of genetic susceptibility to developing a second cancer than healthy people do. Our findings are somewhat in line with those of a study of 34 patients with MN as second cancer10 and with a recent study of 77 patients with MN as second cancer, which found no significant difference in the incidence of high-risk cytogenetics between the patients with AML as a second cancer and the patients with de novo AML (P=.06).24 Our results disagree with those of a study of 38 patients with second MN who had received surgery only for a prior primary cancer; their cytogenetic profiles were similar to those of patients with t-MN who had received chemotherapy and/or radiation therapy for prior primary cancer.11
It has been long suspected that some of t-MN may be part of the nature history of the primary cancer, treated or untreated, which has been unmasked by early diagnosis and the development of more effective cancer treatment and prolonged survival. Some evidence suggests that genetic factors contribute to t-MN risk after primary cancer treatment. Specifically, variants in drug-metabolizing genes, DNA repair genes, and genes that regulate hematopoietic development are associated with increased t-MN susceptibility.25-27 Cytotoxic therapy is a potent surrogate for the environmental exposures that drive sporadically occurring cancers. Our findings of a similar incidence of adverse-risk cytogenetics in second MN and de novo MN (Table 3) indicate that a history of previous breast cancer in itself does not necessarily carry a cytogenetic risk of subsequent MN, and a higher incidence of adverse-risk cytogenetics in t-MN (but not in second MN) than in de novo MN (Table 1 and 2) indicate that chemotherapy and/or radiation therapy play a major role in the development of t-MN,1,28-30 although recent report that that radiation did not increase risk of subsequent MDS.31 These findings are supported by a recent population-based study that found stage III breast cancer is associated with a significantly higher risk of subsequent diagnosis of AML than stage I breast cancer, suggesting that AML may be a sequela of treatment.32
Our data confirmed others’ reports that, as in de novo MN, cytogenetic risk is an important prognostic factor for OS among patients with t-AML (P<.001; Figure 2A).2,7 Similarly, IPSS scores are significant prognostic factors in patients with t-MDS (P<.001; Figure 2B). A recent study reported that patients with t-MN after radiation therapy alone had a statistically significant survival benefit and a lower incidence of high-risk cytogenetics than patients who had received chemotherapy or radiation plus chemotherapy.16 In contrast, our study found no significant difference in OS times (P =.43) and frequencies of high-risk cytogenetics (68.4% vs 50.5%; P = .36) between patients with t-MN arising after radiation therapy (n=19) and patients with t-MN arising after chemotherapy or radiation plus chemotherapy (n=118; data not shown); these results confirmed an earlier finding that t-MN secondary to radiation therapy has similar cytogenetic characteristics and clinical outcomes to those of t-MN arising after chemotherapy or radiation plus chemotherapy.9,10
In summary, although differences in some characteristics between t-MN and de novo MN suggest differences in etiology, t-MN and de novo MN have similarity when they are matched for cytogenetics and age. Because t-MN and de novo MN are biologically similar, the study of t-MN may provide further insight into the pathogenesis of de novo disease and why some cancer patients develop leukemia whereas most patients treated with the same agents do not. MN as a second cancer in patients with a history of breast cancer and de novo MN has very similar clinical features, cytogenetics, and clinical outcomes
Acknowledgments
Financial Support: This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Conception and design: Yiming Chen, Zeev Estrov and Miloslav Beran
Provision of study materials or patients: Gautam Borthakur, Farhad Ravandi, Tapan Kadia, Stefan Faderl, Susan O’Brien, Elias Jabbour, Guillermo Garcia-Manero, Jorge Cortes, Zeev Estrov and Miloslav Beran
Collection and assembly of data: Yiming Chen, MarkBrandt, Sherry Pierce and Miloslav Beran
Data analysis and interpretation: Yiming Chen, Zeev Estrov Sherry Pierce, Wei Qiao, Gautam Borthakur, Farhad Ravandi, Tapan Kadia, Stefan Faderl, Susan O’Brien, Elias Jabbour, Guillermo Garcia-Manero, Jorge Cortes and Miloslav Beran
Manuscript writing: Yiming Chen, Zeev Estrov and Miloslav Beran
Final approval of manuscript: All authors
Conflict-of-interest-disclosure:
The authors declare no competing financial interests.
References
- 1.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 3.Josting A, Wiedenmann S, Franklin J, et al. Secondary myeloid leukemia and myelodysplastic syndromes in patients treated for Hodgkin’s disease: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21(18):3440–3446. doi: 10.1200/JCO.2003.07.160. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 5.Feldman EJ. Does therapy-related AML have a poor prognosis, independent of the cytogenetic/molecular determinants? Best Practice & Research Clinical Haematology. 2011;24(4):523–526. doi: 10.1016/j.beha.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Ostgard LS, Kjeldsen E, Holm MS, et al. Reasons for treating secondary AML as de novo AML. Eur J Haematol. 2010;85(3):217–226. doi: 10.1111/j.1600-0609.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- 7.Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22(12):2510–2511. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22(2):240–248. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Keating MJ, Walters RS, et al. Therapy-related leukemia and myelodysplastic syndrome: clinical, cytogenetic, and prognostic features. J Clin Oncol. 1986;4(12):1748–1757. doi: 10.1200/JCO.1986.4.12.1748. [DOI] [PubMed] [Google Scholar]
- 11.Abdelhameed A, Pond GR, Mitsakakis N, et al. Outcome of patients who develop acute leukemia or myelodysplasia as a second malignancy after solid tumors treated surgically or with strategies that include chemotherapy and/or radiation. Cancer. 2008;112(7):1513–1521. doi: 10.1002/cncr.23325. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 14.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 15.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25(14):1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 16.Nardi V, Winkfield KM, Ok CY, et al. Acute myeloid leukemia and myelodysplastic syndromes after radiation therapy are similar to de novo disease and differ from other therapy-related myeloid neoplasms. J Clin Oncol. 2012;30(19):2340–2347. doi: 10.1200/JCO.2011.38.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21(11):2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 18.Quesnel B, Kantarjian H, Bjergaard JP, et al. Therapy-related acute myeloid leukemia with t(8;21), inv(16), and t(8;16): a report on 25 cases and review of the literature. J Clin Oncol. 1993;11(12):2370–2379. doi: 10.1200/JCO.1993.11.12.2370. [DOI] [PubMed] [Google Scholar]
- 19.Detourmignies L, Castaigne S, Stoppa AM, et al. Therapy-related acute promyelocytic leukemia: a report on 16 cases. J Clin Oncol. 1992;10(9):1430–1435. doi: 10.1200/JCO.1992.10.9.1430. [DOI] [PubMed] [Google Scholar]
- 20.Acute myeloid leukemia 17 trial webpage. http://aml17.cardiff.ac.uk/files/files.htm.
- 21.Le Beau MM, Albain KS, Larson RA, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol. 1986;4(3):325–345. doi: 10.1200/JCO.1986.4.3.325. [DOI] [PubMed] [Google Scholar]
- 22.Mauritzson N, Albin M, Rylander L, et al. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976-1993 and on 5098 unselected cases reported in the literature 1974-2001. Leukemia. 2002;16(12):2366–2378. doi: 10.1038/sj.leu.2402713. [DOI] [PubMed] [Google Scholar]
- 23.Montesinos P, Gonzalez JD, Gonzalez J, et al. Therapy-related myeloid neoplasms in patients with acute promyelocytic leukemia treated with all-trans-retinoic Acid and anthracycline-based chemotherapy. J Clin Oncol. 2010;28(24):3872–3879. doi: 10.1200/JCO.2010.29.2268. [DOI] [PubMed] [Google Scholar]
- 24.Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 25.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5(12):943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 26.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;137(6):513–529. doi: 10.1111/j.1365-2141.2007.06613.x. [DOI] [PubMed] [Google Scholar]
- 27.Knight JA, Skol AD, Shinde A, et al. Genome-wide association study to identify novel loci associated with therapy-related myeloid leukemia susceptibility. Blood. 2009;113(22):5575–5582. doi: 10.1182/blood-2008-10-183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone G, Fianchi L, Voso MT. Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011;23(6):672–680. doi: 10.1097/CCO.0b013e32834bcc2a. [DOI] [PubMed] [Google Scholar]
- 29.Larson RA. Therapy-related myeloid neoplasms. Haematologica. 2009;94(4):454–459. doi: 10.3324/haematol.2008.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czader M, Orazi A. Therapy-related myeloid neoplasms. Am J Clin Pathol. 2009;132(3):410–425. doi: 10.1309/AJCPD85MCOHHCOMQ. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee S, Reddy CA, Ciezki JP, et al. Risk for developing myelodysplastic syndromes in prostate cancer patients definitively treated with radiation. J Natl Cancer Inst. 2014;106(3):djt462. doi: 10.1093/jnci/djt462. [DOI] [PubMed] [Google Scholar]
- 32.Martin MG, Welch JS, Luo J, Ellis MJ, Graubert TA, Walter MJ. Therapy related acute myeloid leukemia in breast cancer survivors, a population-based study. Breast Cancer Res Treat. 2009;118(3):593–598. doi: 10.1007/s10549-009-0376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


