Abstract
Ample genetic and physiological evidence establishes that renal salt handling is a critical regulator of blood pressure. Studies also establish a role for the immune system, T-cell infiltration and immune cytokines in hypertension. This study aimed to connect immune cytokines, specifically IFN-γ and IL-17A, to sodium transporter regulation in the kidney during angiotensin II (AngII) hypertension. C57BL/6J (wild type, WT) mice, responded to AngII infusion (490 ng/kg/min, 2 weeks) with a rise in blood pressure (to 170 mmHg) and a significant decrease in the rate of excretion of a saline challenge. In comparison, mice that lacked the ability to produce either IFN-γ (IFN-γ−/−) or IL-17A (IL-17A−/−) exhibited a blunted rise in blood pressure (to <150 mmHg), and both genotypes maintained baseline diuretic and natriuretic responses to a saline challenge. Along the distal nephron, AngII infusion increased abundance of the phosphorylated forms of the Na-K-2Cl cotransporter, Na-Cl cotransporter and Ste20/SPS-1 related proline-alanine rich kinase, in both the WT and IL-17A−/− but not in IFN-γ−/− mice; epithelial Na channel abundance increased similarly in all three genotypes. In the proximal nephron, AngII infusion significantly decreased abundance of Na/H-exchanger isoform 3 and the motor myosin VI in IL-17A−/− and IFN-γ−/− , but not WT; the Na-phosphate cotransporter decreased in all three genotypes. Our results suggest that during AngII hypertension both IFN-γ and IL-17A production interfere with the pressure natriuretic decrease in proximal tubule sodium transport and that IFN-γ production is necessary to activate distal sodium reabsorption.
Keywords: angiotensin II, cytokines, pressure natriuresis, NHE3, NCC, NKCC2, SPAK, ENaC
Introduction
Blood vessels, kidney, and central nervous system are all implicated in the genesis of experimental hypertension, and T-cells may provide a key link. Animal models of chronic hypertension exhibit increased immune infiltration into the vascular adventitia and kidney.1-3 Mice and rats lacking T-lymphocytes exhibit blunted hypertensive responses to experimental hypertension, restored by adoptive transfer of T-cells.4-6 Trott et al recently provided evidence that a population of CD8+ T-cells, not CD4+ T-cells, play a critical role in the development Ang II infusion hypertension.6 Recent evidence also indicates that isoketal-modified proteins are formed in dendritic cells, and that these are recognized as non-self and promote T cell activation.7 In the kidney, cytokines released from these cells activate local production of angiotensin II (AngII), even when systemic levels of AngII are low.8, 9 Local AngII is antinatriuretic, produces ROS locally, and activates Na+ transporters along the nephron.10 These responses can attract more immune cells, creating a positive feedback loop manifest as chronic inflammation and hypertension. 3, 11
There is evidence for involvement of cytokines produced by T-cells in the genesis and maintenance of hypertension. TNF-α and IFN-γ are produced by CD4+ Th1 cells, IL-17 produced by CD4+ Th17 cells, and IFN-γ and TNF-α are produced by CD8+ cells.12 TNF-α KO mice do not exhibit hypertension in response to AngII infusion.13 Madhur et al 14 reported that the rise in systolic pressure was blunted in IL-17A −/− versus wild type (WT) mice after 4 wk AngII infusion, and Nguyen et al 15 demonstrated that 1 wk infusion of IL-17 raised systolic blood pressure indicating direct actions of this cytokine. IFN-γ is elevated in kidneys during experimental AngII hypertension16 and while Marko et al found AngII infusion elevated blood pressure similarly in IFN-γ receptor knockout and wild type mice, there was less renal tubulointerstitial injury and T- cell infiltration.17
Physically, blood pressure (BP) can be elevated by vasoconstriction or by increasing the circulating volume. Evidence from renal transplantation, diuretic action and renal Na+ transporter mutations support Guyton's hypothesis that long term regulation of blood pressure depends on fractional renal sodium reabsorption.18-20 Ultimately, excess Na+ reabsorption drives counteracting natriuretic responses to restore Na+ output to match Na+ intake at the expense of elevated BP.18 “Pressure-natriuresis” responses suggest Na+ transporter inhibition, e.g. mediated by transporter trafficking out of active domains, and/or decreases in abundance or activity/transporter. 21-24 For example, two week AngII infusion activates distal Na+ transporters (cortical NKCC, NCC and ENaC), and depresses proximal transporters (NHE3, NaPi2, and medullary NKCC) in Sprague Dawley rats.25 Our current study aimed to investigate the role of cytokine production during AngII infusion hypertension by testing the hypothesis that IFN-γ and IL-17A either facilitate AngII stimulation of distal transporters or blunt the pressure-natriuretic depression of proximal transporters. Our results demonstrate that: AngII mediated hypertension and anti-natriuresis are blunted similarly in both IFN- γ−/− and IL-17A−/− mice, that IFN-γ, but not IL-17A is required for AngII stimulation of distal transporters, and that both IFN- γ and IL-17A blunt the pressure-natriuretic depression of proximal transporters.
Methods
Animals and blood pressure measurement
The institutional animal care and use committees at Vanderbilt University and Keck School of Medicine approved the animal protocols. C57BL/6J and IFN- γ −/− mice were obtained from Jackson Laboratories. IL-17A −/− mice were generated as described. 26 Osmotic minipumps (Alzet, 2004) were implanted into 10-12 wks old mice for subcutaneous infusion of AngII (490 ng/kg/min) or saline for 14 days. Blood pressure was recorded by telemetry, as described previously.27
Saline challenge test
As previously described,6 mice were injected i.p. with a volume of warmed isotonic saline equivalent to 10% of their body weight and placed immediately in metabolic cages for urine collection. Results are expressed as the percentage of the injected sodium and volume excreted over 4 hr.
Transporter profiling
After euthanasia, immunoblotting was carried out as described. 10, 25 Kidneys were homogenized in a buffer containing protease and phosphatase inhibitors, and a 2,000 × g supernatant, i.e., “homogenate,” was isolated and flash frozen in single use aliquots. Each sample was analyzed at 1 and ½ amounts to verify linearity of the detection system (one amount shown in Figures), as detailed in Table S1. Signals were analyzed with Odyssey Infrared Imaging System (Li-COR) and software. Arbitrary density units were normalized to mean intensity of control group, defined as 1.0.
Statistical Analyses
WT versus IFN-γ−/− and WT versus IL-17A−/− were compared, IFN-γ−/− was not compared to IL-17A−/−. Data are expressed as mean ± standard error of the mean. For blood pressure and saline challenge results over time, one way ANOVA followed by Neuman-Keuls post-hoc test was used. For immunoblot abundance, Student's t-test was implemented. A p value of <0.05 was considered significant.
See the online Data Supplement for an expanded more detailed Material and Methods section.
Results
Blood pressure and anti-natriuretic responses to AngII infusion are blunted in both IFN-γ−/− and IL-17A −/−mice
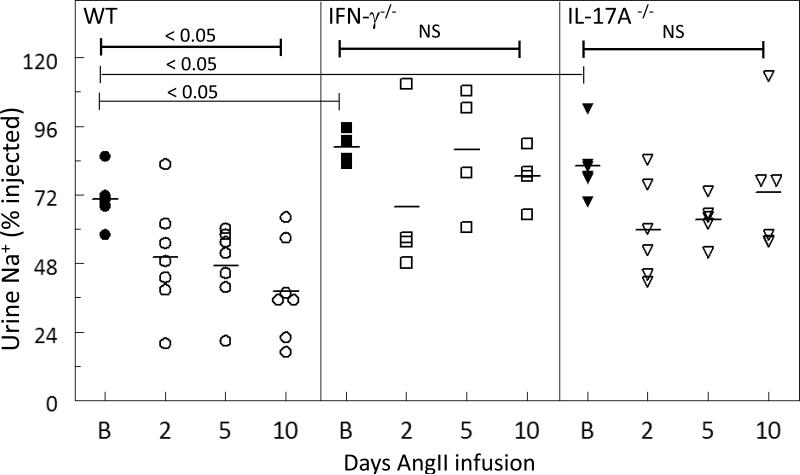
Infusing AngII into C57BL/6J (wild type, WT) mice for 14 days raised mean systolic blood pressure about 40 mmHg: from 126 ± 1 mmHg at baseline to 170 ± 4 mmHg during the last two days of Ang II infusion, (means ± SEM, n=5, p<0.001, Table S2). AngII infusion also significantly depressed the diuretic and natriuretic responses to a normal saline challenge (10% of body weight injected i.p.): untreated WT mice excreted 70% of the injected sodium (Figure 1) and 85% of the injected volume (diuretic responses to saline challenge in Figure S1) over the 4 hr collection period; after 2, 5, and 10 days of AngII infusion the percentage of sodium and volume excreted over 4 hr decreased to 40% and 56% of the injected amounts, respectively. While all animals will eventually excrete the Na+ and volume infused in the saline challenge, the rate at which the saline is excreted is a function of renal Na+ transporter activity. These results indicate that chronic AngII infusion activates Na+ transporters which depresses the fraction of the saline excreted during the initial 4 hrs.
Figure 1. Physiologic responses to AngII infusion (490 ng/kg/min × 14 d) are blunted in IFN-γ−/− and IL-17A −/− mice.
At baseline (B), 2, 5, and 10 days of AngII infusion, WT, IFN-γ−/− and IL-17A −/− mice were challenged with an i.p. bolus of warmed saline equivalent of 10% of their body weight and placed in metabolic cages for urine collection. Results are expressed as the fraction of the amount injected (the challenge) excreted over the 4 hr collection period. AngII infusion significantly reduced the natriuretic responses in WT, but not in the IFN-γ −/− or IL-17A −/− mice. Data are expressed as mean ± SEM, n= 4-7 per group. *P < 0.05
Diuretic responses are displayed in Figure S1.
In mice lacking the ability to produce IFN-γ, the responses to 14 day AngII infusion were blunted: mean systolic blood pressure rose from 136 ± 4 to 150 ± 3 mmHg (n=5, p< 0.05 versus baseline, p=0.001 versus AngII infused WT, Table S2). In response to a saline challenge at baseline, IFN-γ−/− mice excreted 89% of the injected sodium (Figure 1), which is significantly more than excreted in the wild type mice at baseline (p=0.004), and 91% of the injected volume (Figure S1); in response to 2-10 days AngII infusion there was no change in the rate of excreting the saline challenge (Figure 1, S1). In mice lacking the ability to produce IL-17A, the responses to 14 day AngII infusion was, similarly, blunted: mean systolic blood pressure rose from 127 ± 3 to 143 ± 4 mmHg (n=8, p = 0.01 versus baseline, p<0.001 versus AngII infused WT, Table S2). In response to a saline challenge, IL-17A−/− mice excreted 83% of the injected sodium, significantly more sodium excreted than measured in the wild type mice (p=0.02) (Figure 2) and 95% of the injected volume (Figure S1); in response to 2-10 days AngII infusion there was no change in the rate of excreting the saline challenge (Figure 1, S1). The results indicate an improved propensity to excrete salt in both IL17A−/− and IFN-g−/− at baseline as well as during AngII infusion along with a blunted hypertensive response to AngII. The results also provide evidence that these cytokines independently promote the anti-natriuretic and diuretic efficacy of AngII in WT mice.
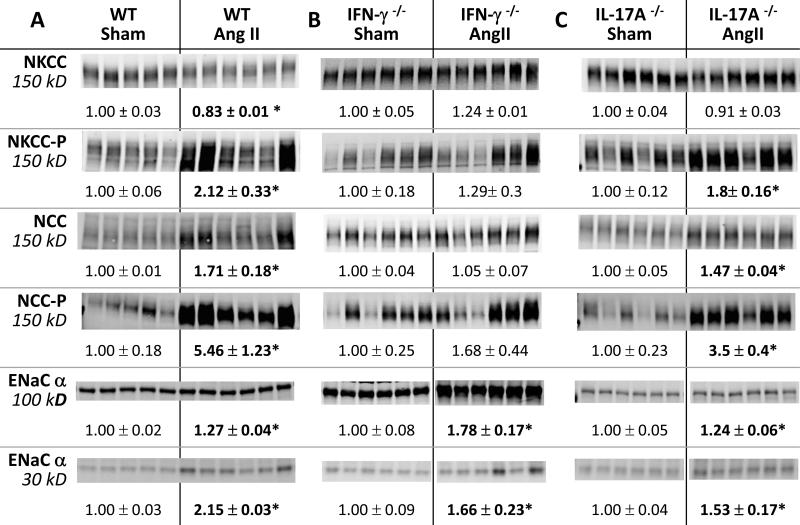
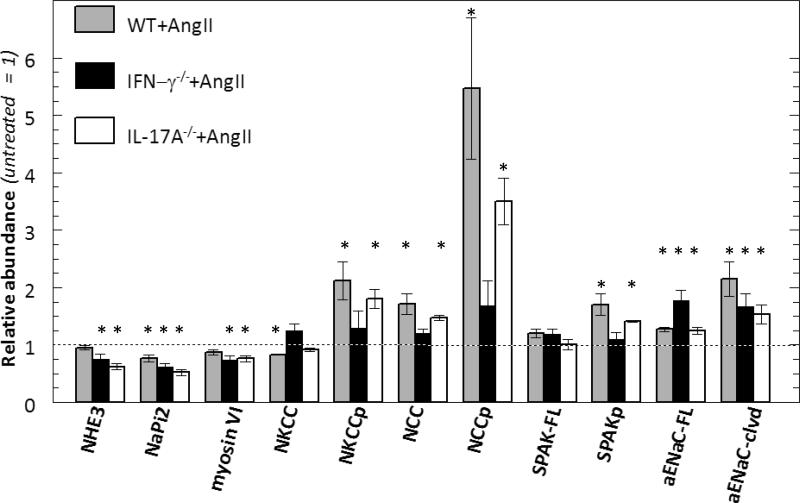
Figure 2. Effects of IFN-γ and IL-17A on NKCC, NCC and ENaC abundance regulation during AngII infusion.
Transporter abundance was analyzed in whole renal tissue homogenates from: A. wild-type (WT), B. IFN-γ −/−, and C. IL-17A −/− mice 2 weeks after sham or Ang II infusion. Immunoblots were performed with a constant amount of protein per lane and ½ amounts (not shown) to verify linearity of detection (details provided in Table S1). Relative abundance indicated as mean ± SEM. n = 5-6/group. *P < 0.05.
Distal nephron transporter responses to AngII are blunted in IFN-γ−/−, but not in IL-17A −/− mice
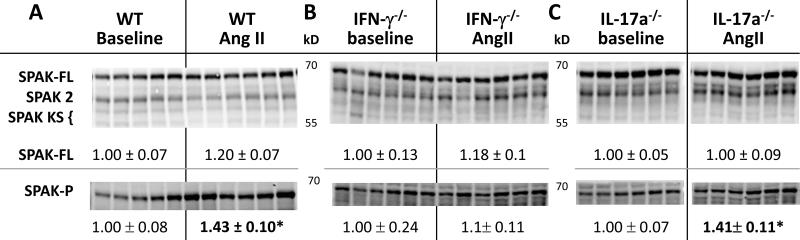
Since both the IFN-γ −/− and IL-17A −/− mice excreted a saline load more rapidly than the WT during AngII infusion, we tested the hypothesis that AngII driven Na+ transporter activation was blunted in these genotypes. Baseline patterns of sodium transporter abundance, phosphorylation and cleavage, measured before treatment, were not significantly different (Figure S3). We have previously reported that AngII infusion into rats and mice activates distal nephron sodium transporters. 9, 10, 25, 28 The findings in the AngII infused WT group closely correspond with our previous reports: abundance of phosphorylated NKCC (NKCC-P) and NCC doubled and NCC-P increased 5.5 fold (Figure 2). The 15% decrease in total NKCC (p=0.004) is not unexpected as we have previously reported decrease in medullary NKCC and in this sample set we did not separate cortex from medulla. The kinase SPAK is expressed along the TALH and DCT where it can phosphorylate and activate NKCC and NCC. In AngII treated WT, full length SPAK kinase total abundance was not significantly altered (nor were the SPAK 2 kidney or kidney specific SPAK isoforms), yet the activated form, SPAK-P, was increased 40% (Figure 3), paralleling the increases in the SPAK substrates NKCC-P and NCC-P. AngII infusion increased the relative abundance of αENaC by 30% and doubled the abundance of the cleaved (proteolytically activated) α subunit.29 Taken together, these findings provide confirmatory evidence for sodium transporter activation in the TALH and distal nephron of WT mice during AngII infusion as previously reported (Figure 2,3).10, 25 In interferon γ deficient mice, AngII infusion did not significantly increase the abundance of NKCC-P, NCC, NCC-P, or SPAK-P (Figures 2, 3). AngII did increase collecting duct ENaC α subunit abundance and proteolytic cleavage, as in WT (Figure 2). Thus, IFN-γ production is important for TALH and DCT transporter activation during AngII infusion, but not for activation of epithelial Na+ channel. In contrast, in AngII infused IL-17A deficient mice the activation of distal nephron NKCC, NCC, SPAK and ENaC (abundance, phosphorylation and cleavage was essentially the same as in the WT mice suggesting that IL-17A does not play a role in activating distal transporters during AngII infusion under these conditions.
Figure 3. Effects of IFNγ and IL-17A on Ste20/SPS1-related Proline/Alanine-rich Kinase (SPAK) abundance and regulation during AngII infusion (490 ng/kg/min × 14 d).
SPAK and SPAK-P abundance (analyzed as described in Figure 2 and Table S1) from: A. wild-type (WT) , B. IFN-γ−/− ,and C. IL-17A −/− mice 2 weeks after sham or Ang II infusion. SPAK 2 and kidney specific SPAK (SPAK KS) indicated but not quantified. Relative abundance indicated as mean ± SEM, n = 5-6/group. *P < 0.05.
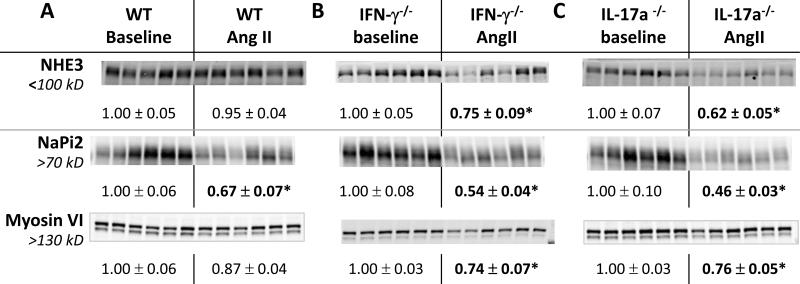
Proximal nephron transporters are significantly decreased during AngII infusion in both IFN-γ−/− and IL-17A −/− mice
The proximal tubule normally reabsorbs 2/3 of the filtered load, largely through the apical brush border sodium hydrogen exchanger isoform 3 (NHE3). The sodium phosphate transporter isoform 2 (NaPi2) is another apical brush border transporter and both are activated by AngII and suppressed by hypertension.22, 25, 30 The atypical molecular motor myosin VI appears necessary to drive NHE3 and NaPi2 to the base of the microvilli.30-32 The hypertensive and anti-natriuretic responses to AngII can, theoretically, be blunted by either reducing distal transporter activation10 or augmenting proximal tubule and TALH mediated pressure natriuresis.28 We compared the regulation of PT NHE3, NaPi2 and myosin VI abundance in the three genotypes during AngII infusion (Figure 4). In the WT mice, AngII infusion hypertension did not change NHE3 or myosin VI abundance, but decreased NaPi2 abundance by 35%, consistent with a response to elevated blood pressure. In IFN-γ−/− mice, AngII infusion decreased NHE3, NaPi2 and myosin VI abundance by 25%, 50%, and 25%, respectively. In IL17A −/− mice,AngII infusion decreased NHE3, NaPi2 and myosin VI abundance by 40%, 50%, and 25%, respectively. These results indicate that removing IFN-γ or IL-17A production during AngII hypertension promotes a significant homeostatic reduction in proximal tubule NHE3 and NaPi2 abundance, (likely secondary to the hypertension caused by elevated distal sodium reabsorption), which contributes to the consistent natriuretic response to a saline challenge and lower blood pressure. That is, the production of IFN-γ and IL-17A during AngII infusion in WT mice counteracts the homeostatic suppression of proximal transporters NHE3 and NaPi2 and their associated regulator myosin VI.
Figure 4. Effects of IFNγ and IL-17A on NHE3, NaPi-2 and myosin VI abundance regulation during AngII infusion.
Abundance (analyzed as described in Figure 2 and Table S1) from: A. wild-type (WT) , B. IFN-γ −/− , and C. IL-17A −/− mice 2 weeks after sham or Ang II infusion. Relative abundance values indicated as mean ± SEM. n = 5-6/group. *P < 0.05.
Discussion
Two weeks of AngII infusion provoked hypertension in WT C57BL/6J mice, as well as in mice lacking production of the specific cytokines IFN-γ or IL-17A. However, the final blood pressures were lower, by 20-25 mmHg, in IFN-γ −/− and IL-17A −/− mice. Additionally, while the natriuretic response to a saline challenge was reduced 30% in WT mice infused with AngII, the natriuresis was not significantly reduced by AngII in either IFN-γ−/− or IL-17A−/−. Interestingly, both the IFN-γ−/− and the IL-17A−/− excreted a larger percentage of the sodium injected over 4 hours at baseline (Figure 1). This capacity was not reflected in significant differences in sodium transporter abundance at baseline (Figure S3), but may reflect less inflammation potential throughout the animal. Taken together, these results suggest that the higher compensatory natriuresis and diuresis responses during AngII infusion in IFN-γ−/− and IL-17A−/− mice contribute to their lower blood pressure.
The effects of 2 week AngII infusion on the transporter profiles of all three genotypes is summarized and compared in Figure 5 (from data in Figures 2 - 4). From these distinct profiles it is evident that: 1) in mice lacking either IFN-γ or IL-17A cytokine production, proximal transporters, that reabsorb the majority of the filtered sodium, are depressed during AngII infusion more than in WT mice, 2) In mice lacking IFN-γ, AngII does not activate sodium transporters or SPAK in the TALH or DCT, 3) ENaC α is activated during AngII infusion independent of IFN-γ or IL-17A production. What is the evidence that the differences in the responses to a saline load are mediated at the level of tubular reabsorption? The saline volume expansion has been reported to raise GFR (limited by tubuloglomerular feedback), and to decrease PT fractional Na+ reabsorption, which leads to a partial compensatory increase in distal Na+ reabsorption, culminating in a significant decrease in fractional Na+ reabsorption.33, 34 Our previous study in mice with no proximal tubule AT1 receptors also reported decreased abundance of PT NHE3 and NaPi2 during AngII treatment (similar to decreases in IL-17A −/− and IFN−/−) associated with significantly lower PT fractional Na+ reabsorption rates measured by free flow micropuncture (corrected for single nephron GFR).28 The excretion of a saline load would be expected to be hampered by the anti-natriuretic influence of AngII in the PT as well as elevated transporters in the distal nephron; in contrast, the lower abundance of PT transporters in the IFN-γ−/− and IL-17A−/− would facilitate the excretion of the saline challenge, as observed. Overall, compared to AngII infused WT mice, the IFN-γ −/− genotype exhibits suppressed proximal, TALH and DCT transporters and SPAK-P, while IL-17A −/− genotype exhibits only PT transporter suppression, yet, blood pressure during AngII infusion is suppressed to the same extent in both IFN-γ−/− and IL-17A−/− mice supporting the importance and compensatory potential of the proximal tubule pressure natriuresis response.
Figure 5. Renal transporter profiles of WT C57BL/6J, IFN-γ−/− and IL-17A−/− mice during AngII infusion.
Data summarized from Figures 2-4 is displayed as relative abundance of AngII infused mice (490 ng/kg/min × 14 d) relative to abundance in control mice of the same genotype infused with saline, defined as abundance =1. *P<0.05 versus saline infused mice of the same genotype.
Angiotensin II hypertension has proven to be a useful model to assess the relationship between blood pressure regulation, renal sodium transport, and renal pathology. The studies of Crowley and Coffman20 provide very strong evidence that impaired renal sodium excretion is central to the development of hypertension, whether the initiating cause involves vascular, neural or inflammatory responses. These findings are also in keeping with recent observations of Trott et al, demonstrating an oligoclonal expansion of CD8+ T cells in kidney ( not spleen or vasculature) during Ang II infusion.6 Acutely, AngII stimulates sodium transporters along the nephron by increasing their abundance in plasma membranes via trafficking. 30, 35 After three days infusion of a non-pressor dose of AngII, total abundance of NHE3, NKCC, NCC and SPAK increase;36 and after two weeks of the pressor dose used in this study, the distal transporters are further activated, while proximal tubule and medullary TALH transporters decrease to baseline or below, presumably driven by the hypertension, which effects compensatory natriuresis that balances Na+ output to Na+ intake. In our previous reports in wild type rodents treated with AngII we have observed both a return to baseline NHE310 as well as a suppression below NHE3 baseline.25, 28 We attribute the difference in responses to the dose and time of AngII treatment, as well as to whether the cortical NHE3 was dissected from the medullary NHE3. In any case, long term AngII hypertension fails to stimulate proximal transporters as it does over the short term. The hypertensive phase is accompanied by inflammation, immune cell infiltration, accumulation of intrarenal AngII and increased abundance of markers of renal injury. In contrast, if high plasma AngII is physiologically provoked for the same period by a low salt diet, Na+ transporter abundance and activation are increased without the accompanying negative sequelae listed above.37 For this reason, it has been suggested that release of proinflammatory signals (cytokines) during experimental hypertension compromises the kidneys’ ability to turn down Na+ reabsorption to accomplish sufficient pressure natriuresis. Thus, the blood pressure has to rise further to generate a sufficient natriuretic response to balance Na+ output to intake.38 Studies have detailed the effects of IL-17A15 and the lack of IL-17A14 on the vasculature, and the accumulation of IFN-γ in untreated hypertensives as well as a role in hypertensive cardiac and renal injury. 16, 17, 39 Our results suggest that, ultimately, the proinflammatory cytokines released contribute to elevated blood pressure by activating sodium transporters and/or blunting pressure natriuresis responses of proximal sodium transporters. Are cytokines brought into the kidney via immune cell infiltration or produced in the kidney during AngII treatment? Recent studies provide evidence for both possibilities. The Harrison group reported that re-introducing CD8+ T cells restores AngII hypertension to Rag-1−/− mice, and that CD8+ cells secrete IFN-γ, suggesting that immune cell production of cytokines is sufficient. 6, 12 In contrast, the Crowley group reported that the blunting of hypertension observed in TNF-α knockout mice, during an AngII plus uninephrectomy protocol, was due to TNF-α specifically produced in the kidney.40 Additionally, whether the cytokines work directly on cytokine receptors in renal epithelial cells, or indirectly by stimulating hormonal and/or neural activation, to inappropriately raise sodium transport has not been addressed in this study and both possibilities warrant serious consideration. These issues include consideration of multiple cell types in the kidney, raising the potential for paracrine cytokine regulation as well as for intrarenal synthesis of AngII, associated with inflammation, known to raise sodium transporter activity.9, 10
The transporter profile of the AngII infused IFN-γ −/− mice is indistinguishable from the pattern we reported in AngII infused mice with no kidney angiotensin converting enzyme (ACE 10/10).10 The ACE 10/10 mice have blunted intrarenal AngII production, blunted hypertensive response to AngII infusion, and no AngII-stimulated increases in NKCC-P, NCC-P or SPAK-P. The similarity suggests that intrarenal AngII accumulation may be blunted or lacking in the IFN-γ−/− mouse. These findings are in line with studies showing diminished intrarenal angiotensin II accumulation in immunodeficient mice in response to hypertensive stimuli.8, 41, 42
The transporter profile of the IL-17A −/− mouse is similar to the profile reported in mice with a proximal tubule specific deletion of the AT1 receptor,28 notably, 50-60% decrease in abundance of proximal tubule NHE3 and NaPi2 as well as reduced fluid reabsorption in response to AngII infusion. Thus, IL-17A production may contribute to AngII hypertension by specifically stimulating NHE3 and NaPi2 which counteracts pressure natriuresis. This suggestion is in line with the recent studies associating impaired pressure natriuresis with tubulointerstitial immune cell infiltration in the kidney.42
Similarities between the transporter profiles of the IFN-γ and IL-17A knockout mice are also informative. ENaC stimulation by AngII infusion, evident in the WT genotype, is not reduced in the AngII infused knockout mice , nor in the ACE 10/10 mice, suggesting that plasma AngII or aldosterone, rather than these cytokines, produced during local inflammation, are responsible for ENaC stimulation.43, 44 In addition, this persistent ENaC stimulation may, at least in part, be responsible for the residual elevation in blood pressure in the two knockout genotypes during AngII infusion. In addition, NHE3, NaPi2 and myosin VI are reduced similarly in both the IFN-γ and IL-17A knockout genotypes compared to WT during AngII hypertension, albeit to a lesser extent in the IFN-γ −/− mice (Figure 4). It is surprising that blood pressure is reduced similarly in the two knockout genotypes since distal transporter activation by AngII is not reduced in the IL-17A knockout mice. This pattern suggests that the reductions in proximal NHE3, NaPi2 and myosin VI are key to improving pressure natriuresis and diuresis and strong enough to both counteract the distal NKCC and NCC transport stimulation during AngII hypertension and to reduce the rise in blood pressure necessary to maintain effective circulating volume.
Perspectives
Previous animal studies focusing on the role of immune cytokines in AngII hypertension have examined the role of IFN-γ and other immune cytokines in vascular dysfunction. Others have shown a clear link between a hypertensive response and T cell infiltration into the kidney. This study connects the immune cytokines IFN-γ and IL-17A to sodium transporter regulation in the kidney, a key determinant of blood pressure and hypertension. Our results suggest the hypotheses, for further study, that 1) interferon gamma may indirectly stimulate transporters via activation of intrarenal AngII production, and that 2) both IFN-γ and IL-17A directly interfere with pressure natriuretic driven decreases in proximal tubule transporters during hypertension. Thus, this study makes a direct link between the immune system and the Guytonian model of the regulation of effective circulating volume and blood pressure. The results suggest new therapeutic targets for treating this widespread and chronic disease.
Supplementary Material
Novelty and Significance Box.
What is new:
Lack of IFN-γ blunts Ang II hypertension as well as anti-natriuretic responses to AngII, prevents significant activation of NKCC, NCC and SPAK, and amplifies the decrease in PT NHE3, NaPi2 and myosin VI. We conclude that production of IFN-γ during AngII hypertension blunts the pressure-natriuresis decrease in PT transporters NHE3 and NaPi2 and is required for stimulation of NKCC, NCC and SPAK.
Lack of IL-17A blunts AngII hypertension as well as the anti-natriuretic responses to AngII and amplifies the decrease in PT transporters. We conclude that production of IL-17A during AngII hypertension blunts the decrease in PT transporters NHE3 and NaPi2, but does not affect distal transporter activation.
ENaC stimulation during AngII is independent of cytokine production, thus, appears to be a direct effect of AngII or aldosterone.
What is relevant:
Novel connections are established between immune cell cytokines and renal Na+ transport activation. Interfering with these connections blunts experimental hypertension, suggesting therapeutic targets to improve pressure natriuresis response of proximal tubule transporters to lower blood pressure.
Locations (direct or indirect) regulated by cytokines are pinpointed to: both IL-17A and IFN-γ blunting proximal tubule natriuretic responses, and IFN-γ activation in the distal nephron.
Summary: Our results suggest that during AngII hypertension both IFN-γ and IL-17A production interfere with the pressure natriuretic decrease in proximal tubule sodium transport which leads to further hypertension. IFN-γ production is also necessary for activation of distal sodium transporters, perhaps via stimulation of intrarenal RAS. Both findings warrant future investigation for therapeutic potential.
Acknowledgements
Donna Lee Ralph for investigator training.
Sources of Funding.
Wright Foundation Keck School of Medicine Dean's Research Scholars Program, (NVK), American Society of Nephrology Medical Student Scholar program (NVK), AHA Post-Doctoral Fellowship (13POST14440041),NIH grants: DK083785 (AAMcD), GM074771 and DK093501 (ED), R01HL039006, P01HL058000, P01HL095070, P01GM015431, R01HL108701, and R01HL105294 (DGH), and K08HL121671 (MM).
Abbreviations and Acronyms
- AngII
angiotensin II
- C57BL/6J
wild type mice (WT)
- CD
collecting duct
- DCT
distal convoluted tubule
- ENaC
epithelial Na+
- IL-17A
cytokine interleukin 17A
- IFN-γ
cytokine interferon gamma
- NaPi2
Na+-phosphate transporter isoform 2
- NCC
Na+-Cl− cotransporter
- NCC-P
NCC phosphorylated at Ser 71
- NHE3
Na+/H+ exchanger isoform 3
- NKCC
apical Na+-K+-2Cl− cotransporter
- NKCC-P
NKCC phosphorylated at Thr 96, Thr 101
- PT
proximal tubule
- RAS
renin-angiotensin system
- SPAK
Ste20/SPS-1 related proline-alanine rich kinase
- SPAK-P
SPAK phosphorylated at Ser 373
- TALH
thick ascending limb of Henle's loop
- WT
wild type C57BL/6J mice
Footnotes
COI Disclosures: - none
References
- 1.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension. 2013;62:226–230. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clinical and Experimental Pharmacology and Physiology. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. The Journal of Experimental Medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. Dc isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin ii concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 9.Giani JF, Janjulia T, Kamat N, Seth DM, Blackwell WL, Shah KH, Shen XZ, Fuchs S, Delpire E, Toblli JE, Bernstein KE, McDonough AA, Gonzalez-Villalobos RA. Renal angiotensin-converting enzyme is essential for the hypertension induced by nitric oxide synthesis inhibition. J Am Soc Nephrol. 2014;25:2752–63. doi: 10.1681/ASN.2013091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trott DW, Harrison DG. The immune system in hypertension. Advances in Physiology Education. 2014;38:20–24. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thabet SR, Chen W, Harrison D. Role of CD8+ T lymphocytes in the genesis of angiotensin II-induced hypertension. FASEB J. 2010;24:lb564. [Google Scholar]
- 13.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2012;60:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 18.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 19.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: Importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–1941. doi: 10.1016/j.febslet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Crowley SD, Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest. 2014;124:2341–2347. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010;298:R851–861. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol. 2004;287:F896–906. doi: 10.1152/ajprenal.00160.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Riquier AD, Yang LE, Leong PK, Maunsbach AB, McDonough AA. Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles. Am J Physiol Renal Physiol. 2009;296:F810–818. doi: 10.1152/ajprenal.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D, Navar LG. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension. 2008;52:137–142. doi: 10.1161/HYPERTENSIONAHA.108.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during Ang II-dependent hypertension: Distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol. 2013;305:F510–519. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in Il-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 27.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 28.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1a angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: Regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riquier-Brison AD, Leong PK, Pihakaski-Maunsbach K, McDonough AA. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am J Physiol Renal Physiol. 2010;298:F177–186. doi: 10.1152/ajprenal.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaine J, Okamura K, Giral H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M. PTH-induced internalization of apical membrane NaPi2a: Role of actin and myosin VI. Am J Physiol Cell Physiol. 2009;297:C1339–1346. doi: 10.1152/ajpcell.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol. 2005;16:2890–2896. doi: 10.1681/ASN.2005040366. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein SW, Szyjewicz J. Single-nephron function and renal oxygen consumption during rapid volume expansion. Am J Physiol. 1976;231:1166–1172. doi: 10.1152/ajplegacy.1976.231.4.1166. [DOI] [PubMed] [Google Scholar]
- 34.Blantz RC, Singh P, Deng A, Thomson SC, Vallon V. Acute saline expansion increases nephron filtration and distal flow rate but maintains tubuloglomerular feedback responsiveness: Role of adenosine A(1) receptors. Am J Physiol Renal Physiol. 2012;303:F405–411. doi: 10.1152/ajprenal.00329.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. Ang II provokes acute trafficking of distal tubule Na+-Cl− cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen MTH,J, McDonough AA. Renal responses to short term non-pressor AngII +/− intrarenal RAS blockade in rats. The FASEB Journal. 2013;27:909.902. [Google Scholar]
- 37.Shao W, Seth DM, Prieto MC, Kobori H, Navar LG. Activation of the renin-angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. Am J Physiol Renal Physiol. 2013;304:F505–514. doi: 10.1152/ajprenal.00587.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Iturbe B, Franco M, Johnson RJ. Impaired pressure natriuresis is associated with interstitial inflammation in salt-sensitive hypertension. Curr Opin Nephrol Hypertens. 2013;22:37–44. doi: 10.1097/MNH.0b013e32835b3d54. [DOI] [PubMed] [Google Scholar]
- 39.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-alpha produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension. 2014;64:1275–81. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 42.Franco M, Tapia E, Bautista R, Pacheco U, Santamaria J, Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol. 2013;304:F982–990. doi: 10.1152/ajprenal.00463.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 44.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension. 2013;62:1111–1122. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.