Abstract
Tissue-specific stem cells are found throughout the body and, with proper intervention and environmental cues, these stem cells exercise their capabilities for differentiation into several lineages to form cartilage, bone, muscle, and adipose tissue in vitro and in vivo. Interestingly, it has been widely demonstrated that they do not differentiate with the same efficacy during lineage-specific differentiation studies, as the tissue-specific stem cells are generally more effective when differentiating toward the tissues from which they were derived. This review focuses on four mesodermal lineages for tissue-specific stem cell differentiation: adipogenesis, chondrogenesis, myogenesis, and osteogenesis. It is intended to give insight into current multilineage differentiation and comparative research, highlight and contrast known trends regarding differentiation, and introduce supporting evidence which demonstrates particular tissue-specific stem cells’ superiority in lineage-specific differentiation, along with their resident tissue origins and natural roles. In addition, some epigenetic and transcriptomic differences between stem cells which may explain the observed trends are discussed.
Keywords: Tissue-specific stem cell, Osteogenesis, Adipogenesis, Chondrogenesis, Myogenesis, Epigenetics, Transcriptomics
Introduction
Humans and other higher eukaryotes utilize various populations of stem cells throughout the developmental process and into adulthood. The vast repertoire of functional stem cell populations is imperative to normal cellular and tissue renewal. Despite possessing a high degree of pluripotency and proliferative potential (1), embryonic stem cell research has been met with various ethical concerns and strict regulations, especially in the United States, restricting the use of such stem cells in research and clinical settings. These obstructions have forced scientists to search for alternative approaches in stem cell therapy, shifting research focus to the utilization of somatic stem cells for regenerative medicine and tissue engineering.
Somatic stem cells, commonly referred to as adult stem cells (ASCs) or tissue-specific stem cells, are present throughout various tissues in the body (2). Tissue-specific stem cells are multipotent and self-renewing cells which possess endogenous functions for tissue renewal and repair at their respective resident tissues (3). Although ASCs seem to exist ubiquitously throughout a variety of tissues, current literature suggests that not all are necessarily created equal in their differential and proliferative capacities, or their ability to respond to outside influences such as microenvironments. In reality, ASCs have inherent properties which greatly contribute to their ability to undergo successful single lineage-specific differentiation. Great variability in the differential capacity certainly exists between tissue-specific stem cells, which may vary within the same cell type. Populations and subpopulations of cells derived from the same tissue may exhibit slight variations in surface marker expression or in their expression of a single gene, which may alter their tendency to engage in uniform lineage-specific differentiation. For researchers investigating stem cell-based tissue engineering, it is necessary to choose the most appropriate type of ASCs naturally suited to the research goals and objectives. Many times, the inherent properties of tissue-specific stem cells are overlooked. This review focuses on four mesodermal lineages for ASC differentiation: adipogenesis, chondrogenesis, myogenesis, and osteogenesis. It is intended to review the current multilineage differentiation and comparative research, highlight and contrast known trends regarding differentiation, and introduce supporting evidence which demonstrates particular ASCs’ superiority in lineage-specific differentiation, concomitant with their resident tissue origins and natural roles. In addition, some epigenetic and transcriptomic differences between stem cells which may explain the observed trends are discussed.
Tissue-Specific Stem Cells Benefiting Lineage-Specific Differentiation
Adipogenesis
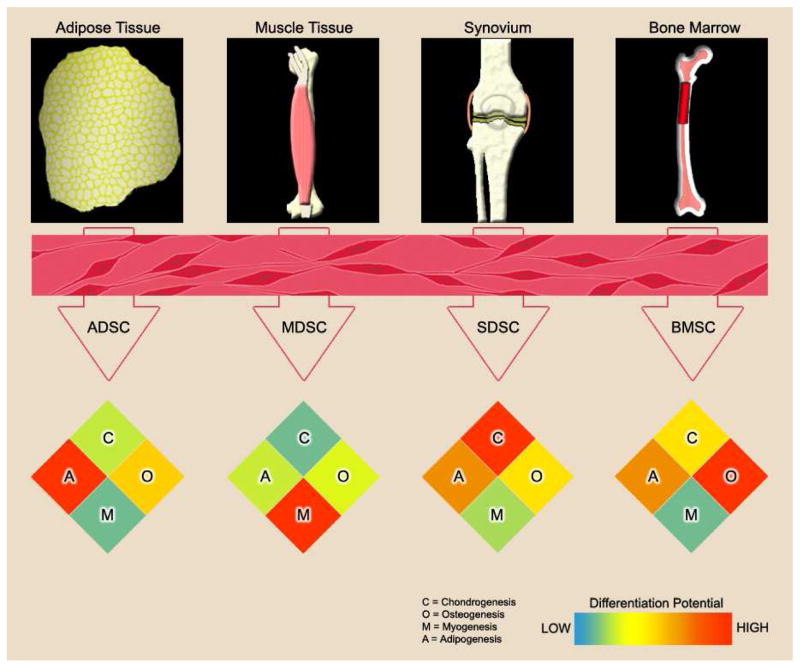
Although commonly removed via liposuction surgery, newly differentiated ASCs from adipose tissue have therapeutic potential in cosmetic surgery (2), as well as tissue grafts for burn victims and autologous transplantation (4). The use of adipose-derived stem cells (ADSCs) in lineage-directed studies has been established, with their greatest success demonstrated along the adipogenic lineage (Figure 1).
Figure 1.
Adult stem cells can be derived from various tissues in the body. These viable and undifferentiated stem cell populations can be expanded in vitro and induced to undergo lineage-specific differentiation for chondrogenesis (C), osteogenesis (O), myogenesis (M), or adipogenesis (A). Although the cells may appear similar in morphology upon harvest, they are anything but identical. From the data presented in the section “Tissue-Specific Stem Cells Benefiting Lineage-Specific Differentiation”, the efficacy of ASCs in lineage-specific differentiation is greatly affected by the type of resident tissue from which they are harvested. In the heatmap, the differentiation capacity is visualized by color ranging from low differentiation (blue) to high differentiation (red).
In a multilineage comparison study by Yoshimura and colleagues using murine ASCs, the greatest adipogenic potential was observed using Oil-Red-O staining in the groups from both synovial-derived stem cells (SDSCs) and ADSCs compared to those from muscle-derived stem cells (MDSCs), periosteum-derived stem cells, and bone marrow-derived stem cells (BMSCs). These findings were supported by reverse transcription polymerase chain reaction (RT-PCR) results for adipogenic markers [peroxisome proliferator-activated receptor gamma (PPARG) and CCAAT/enhancer binding protein alpha (CEBPA)] after four days of adipogenic lineage induction (5). These conclusions were consistent with the findings by Sakaguchi and colleagues. They found that the SDSC and ADSC groups represented the only groups with each of their three colonies stained positive for lipid accumulation; the BMSC group had one colony with a staining rate greater than 80%. In contrast, the periosteum and MDSC groups had zero colonies possessing a rate of Oil-Red-O staining greater than 80%, which is indicative of being highly inferior for adipogenesis (6). These results are further supported by the work of Mochizuki and colleagues, where differences between SDSCs harvested from fibrous synovium, SDSCs from adipose synovium, and subcutaneous ADSCs were indistinguishable in Oil-Red-O staining (7). In a multilineage study by Peng and colleagues, rat ADSCs exhibited the greatest normalized PPARG and lipoprotein lipase (LPL) levels at day 7 in an adipogenic induction regimen, demonstrating superior adipogenic potential of ADSCs to BMSC and cartilage-derived stem cell groups, which was further confirmed by densitometric analysis of Oil-Red-O stained cultures (8). Based on these studies, it appears that SDSCs and ADSCs can each undergo successful adipogenic differentiation. More studies need to be conducted in order to determine if definitive adipogenic superiority exists between the two cell types.
In another comparative study using several types of tissue-specific stem cells, ADSCs were directly compared with BMSCs after seeding on collagen scaffolds. Despite similar trilineage differentiation overall (chondrogenic, adipogenic, and osteogenic) between both groups, there was a significantly greater and more rapid upregulation of adipogenic genes in ADSCs and osteogenic genes in BMSCs after in vitro induction (9). A transcriptomics study by Monaco and colleagues aimed to compare the differentially expressed genes of ADSCs derived from adult porcine subcutaneous adipose tissue and BMSCs derived from the femur before and after osteogenic and adipogenic differentiation (10). Just as Vishnubalaji and colleagues observed (11), Monaco and colleagues found that ADSCs had greater lipid metabolism than BMSCs while BMSCs had an increased osteogenic and proliferative capacity; ADSCs exhibited significantly lower expression for osteopontin (OPN) than BMSCs, which was also confirmed by quantitative RT-PCR. Based upon their functional analyses, it is reasonable to suggest that ADSCs naturally progress toward the adipogenic lineage with greater propensity than BMSCs and vice versa (10).
Chondrogenesis
Producing healthy, viable human cartilage for surgical repair through autologous transplantation has widespread therapeutic potential, especially for patients in the aging populations. The synovium has proved to be a valuable source of ASCs for effective induction of chondrogenesis and the production of high-quality cartilage in vitro (12, 13) and in vivo (14), but it has also been investigated in osteogenic, adipogenic, and myogenic experiments (Figure 1).
SDSCs have a tendency to progress toward the chondrogenic lineage more effectively than other stem cells. Mochizuki and colleagues found that human SDSCs from both fibrous and adipose synovium exhibited similar superiority over subcutaneous ADSCs in chondrogenic potential (7). Another study comparing various human ASCs from separate sources was performed by Sakaguchi and colleagues, where SDSCs were once again the most superior source for stem cell chondrogenesis over ADSCs and MDSCs; the SDSC group yielded pellets with the largest size and the highest intensity for toluidine blue cartilage matrix staining (6). Similar conclusions were supported by Yoshimura and colleagues, who reported that rat SDSCs exhibited the greatest efficiency and growth kinetics, producing the heaviest chondrogenic pellets due to matrix formation (5). Compared to BMSCs, ADSCs exhibited a reduced chondrogenic potential under standard culture conditions driven by transforming growth factor beta (TGFβ). Hennig and colleagues found that human ADSCs had reduced expression of bone morphogenetic protein-2 (BMP2), -4 (BMP4), and -6 (BMP6) mRNA and did not express TGFβ-receptor-1 protein. BMP6 treatment induced TGFβ-receptor-1 expression and combined application of TGFβ and BMP6 eliminated the reduced chondrogenic potential of ADSCs inducing a gene expression profile similar to differentiated BMSCs. Similar to BMSCs, chondrogenesis of ADSCs was associated with hypertrophy according to premature collagen X (COL10A1) expression, upregulation of ALP activity, and in vivo calcification of spheroids after ectopic transplantation in SCID mice (15). Although this study did not use SDSCs (in addition to BMSCs and ADSCs) to similarly compare their hypertrophy or calcification fates, SDSCs have been evaluated in other studies. In a report using an osteogenic induction medium, SDSCs exhibit a 5- to 10-fold decrease compared to BMSCs in the levels of osteocalcin (OCN) and ALP (16), which are known to contribute to calcification and pro-osteoblast activity; however, the generation of articular cartilage without hypertrophic terminal differentiation still remains a current challenge in the field (17).
Several studies have compared the in vivo efficacy and capabilities of SDSCs for cartilage regeneration and repair of osteochondral defects in rabbit models. After initially demonstrating that SDSCs were superior stem cells for chondrogenesis, Koga and colleagues transplanted donor-matched ASCs to repair cartilage defects created in a rabbit model and found that SDSCs and BMSCs produced significantly greater amounts of cartilage matrix than other cells of adipose and muscle tissue origins; when SDSCs were transplanted at a higher cell density and with a periosteal patch, more abundant cartilage matrix was observed. They also noted that SDSCs had a clear advantage in terms of proliferative potential, giving SDSCs an additional edge over BMSC counterparts for therapeutic applications (18). In another similar in vivo experiment, Pei and colleagues set out to repair full-thickness rabbit cartilage defects via allogeneic in vitro engineered SDSC cartilage constructs. Six months after implantation of SDSC-based constructs, the femoral condyle defects were filled with smooth hyaline-like cartilage, did not exhibit collagen I, and possessed high levels of collagen II and glycosaminoglycan (GAG), with well integrated new tissue formation. These results are contrasted by control groups which possessed fibrous tissue (14). A third study using a rabbit model for defective articular cartilage repair, Lee and colleagues also tested SDSCs’ in vivo effectiveness. For this study, SDSCs were seeded in a platelet-rich plasma (PRP) gel, which could be injected into the femoral defect. After 24 weeks, results very similar to the Pei et al. study were obtained, with fibrous tissue in the control group and hyaline cartilage in both the PRP group and the PRP-SDSC group. The PRP with seeded SDSCs possessed greater GAG content than the non-SDSC groups, as well as the greatest collagen II expression (19). In another relevant study, hydrogel encapsulated porcine SDSCs, BMSCs, and ADSCs were compared for in vitro and in vivo chondrogenesis, SDSCs were once again found to be the most chondrogenic. SDSCs yielded mechanically stiffer constructs and as others have found, SDSC hydrogels exhibited the greatest GAG and collagen expression of any group (20).
SDSCs’ success in chondrogenesis seems to lie in their inherent cellular properties and growth characteristics (17, 21). One study found that chondrocytes and intraarticular tissue stem cells (including SDSCs) from human donors exhibited a higher expression of proline arginine-rich end leucine-rich repeat protein (PRELP), a connective tissue glycoprotein of the leucine-rich repeat family abundant in cartilage rather than in cultured fibroblasts, which was absent in extraarticular tissue stem cells, such as ADSCs and MDSCs; BMSCs increased PRELP expression during in vitro chondrogenesis (22). After many passages, ASCs tend to undergo a process marked by telomere shortening and replicative senescence, leading to impaired ability to differentiate into specific tissues (23). SDSCs retain multipotency for up to ten passages with limited cell senescence and retained chondrogenic capacity (24). This characteristic presents a reasonable explanation for SDSCs’ remarkable ability to successfully differentiate into cartilaginous tissue and, to a lesser degree, yet notably, the ability to produce muscle, bone, and adipose tissue. It is also notable that the in vitro microenvironment can influence SDSC differentiation toward chondrogenesis, particularly the extracellular matrix (ECM). ECM deposited by SDSCs has been shown to improve SDSC expansion in vitro and shift the SDSCs at a greater propensity toward the chondrogenic lineage, while decreasing osteogenesis and adipogenesis (25, 26).
Myogenesis
Several potential therapeutic applications for myogenically differentiated stem cells exist, including dystrophic diseases and orthopaedic surgery (27). Therapies which produce viable muscle tissue have the potential to aid against the pathogenicity of muscle diseases and elucidate natural mechanisms for muscle repair via ASCs. The contribution of MDSCs to myogenic differentiation in vitro have been investigated, as well as their ability to contribute to muscle tissue in vivo. Following a similar trend as other tissue-specific stem cells, MDSCs seem to most effectively undergo myogenesis than other types of lineage specification (Figure 1).
Several muscle progenitor populations have been identified in muscle which do not express satellite cell markers such as Pax7, and some of these populations have been shown to be myogenic in vivo and in vitro. One such population located in the interstitium of postnatal muscle, expressing PW1, a cell stress mediator, is referred to as PW1+/Pax7− interstitial cells (PICs). Mitchell and colleagues found that PICs exhibited comparable levels of myogenesis to that of satellite cells in vivo and engaged in the stem cell process of self-renewal. Interestingly, PICs require Pax7 for myogenic specification, as none of the Pax7-deficient PICs was deemed myogenic (28). Differing from the commonly researched and highly myogenic populations of Pax7+ muscle satellite cells (27, 29), other MDSCs can be multipotent and have the capacity to differentiate into many cell types such as myocytes, chondrocytes, adipocytes, and osteocytes under the necessary conditions (30). Aside from PICs, other muscle-derived cell populations have been discovered and evaluated for their contributions to muscle repair (31–34). Some of these muscle-derived side populations may be able to form new myotubes and contribute to muscle repair and regeneration (35). It should be noted that, despite the fact that several populations expressing mesenchymal stem cell markers have been identified and can engage in multilineage differentiation, their stem cell status is currently debated in the field; however, it is accepted that non-satellite muscle cells accumulate in the interstitium following muscle injury and can contribute to muscle repair in the presence of necessary environmental or outside factors (31), leading some investigators to speculate about their roles in the repair of damaged muscle.
In a study by Meligy and colleagues, ADSCs, BMSCs, and skeletal MDSCs were harvested from six-week-old rats for in vitro myogenic comparative studies. Flow cytometry data showed that all stem cells exhibited positive expression of CD90 and CD44 and lacked expression of CD35, CD41, and CD34. Under myogenic induction, the greatest myogenic marker expression was exhibited by the skeletal MDSC population with peak myogenin expression of 93% in the myogenically differentiated MDSCs, 83.3% in the BMSCs, and 77% in the ADSCs (36). The similarity of myogenic potential between BMSCs and ADSCs was also demonstrated in another report using rats. After four passages, investigators observed high expression of CD90 in both ADSCs and BMSCs and a reduction of CD44 expression in ADSCs. They also observed significantly higher expression of myogenic differentiation 1 (MyoD1) in BMSCs compared to ADSCs (37). In a related comparative study conducted by Lei and colleagues, after a 28-day myogenic induction, higher expression levels of skeletal muscle-specific genes were observed in adult mouse MDSCs than fetal counterparts (p < 0.01) and the lowest expression levels were demonstrated in ADSCs (p < 0.01). All stem cells were detected for both CD29 and CD90 positive and CD45 negative phenotype, and exhibited fibroblast-like spindle morphology in cell cultures. In addition, muscle-specific cadherin (M-Cad) and myosin heavy chain (MyHC) expressions in ADSCs were not detected by immunofluorescence or quantitative real-time PCR (38). These results suggest that some inherent properties may exist in non-satellite MDSC populations, which allowing the MDSC populations to more readily upregulate myogenic genes and progress along the myogenic lineage than stem cells from alternative sources.
Satellite cells and other MDSC side populations seem to be the most natural choice for producing quality myotubes; however, other stem cells have been utilized, despite the fact that ADSCs appear to be a poor choice. In two separate experiments by De Bari and colleagues, SDSCs were evaluated for myofiber incorporation and myogenic capacity (24, 39). In the earlier study from 2001, five SDSC clones were evaluated for adipogenic, myogenic, chondrogenic, osteogenic, and myogenic differentiation capacity. All clones were determined to be fully capable of chondrogenesis, adipogenesis, and osteogenesis; however, this myogenic differentiation was described as a “few scattered, rudimentary myotubes” (24). In their later 2003 study, using the in vivo mdx mouse model and tibialis anterior muscle injection of human SDSCs, they found that SDSCs possessed the capacity to contribute to myofiber formation, independent of fusion with muscle cells. Successful myogenesis occurred and the implanted SDSCs were able to contribute to the local satellite cell population (39). More research is needed to truly elucidate the complete differential capabilities of non-satellite muscle-derived cell populations, as well as an accurate method for classifying the status of these populations as stem cells.
Osteogenesis
In conjunction with cartilage engineering studies, experiments which aim to produce bone tissue are crucial and invaluable to the medical community. Bone constructs produced from stem cells can be used in fracture repair, as well as treating bone tissue defects (40). Although BMSCs have been evaluated for multilineage potential, especially chondrogenesis, they most effectively undergo osteogenic differentiation (Figure 1).
Several in vitro studies have been performed which demonstrate the superior capabilities of BMSCs to differentiate into bone tissue. A comparison study by Im and colleagues set out to determine the difference between the chondrogenic and osteogenic capacity of ADSCs and BMSCs by differentiating these cells on a monolayer culture. Based on the results of Von Kossa matrix mineralization assay and alkaline phosphatase (ALP) staining for osteoblastic differentiation, the BMSCs proved to be superior to the ADSCs (41). This conclusion was also in agreement with another comparative study by Vishnubalaji and colleagues (11). In a study which took a less common approach to evaluate osteogenesis, Park and colleagues used Chip-Based assays to measure osteogenic markers and gene expression to compare the potential of human BMSCs and ADSCs for bone formation. Using hydraulic pressure to add cell stress, they saw increases in bone matrix formation in both cell types; however, stimulated BMSCs showed greater staining in Alizarin Red S and ALP assays which is indicative of osteogenesis. They concluded that BMSCs were more susceptible to changes in osteogenic differentiation under mechanical stimulation than ADSCs (42). This conclusion seems reasonable when one considers the weight bearing responsibility and mechanical stability demand of the human skeletal system, which is likely a manifestation of the susceptibility and responsiveness of osteoblastic precursor and BMSC populations to such mechanical forces.
These growth and differentiation characteristics may also contribute to their natural and specific inclination toward the osteogenic lineage, as well as their role as effective ASCs for bone growth and formation. This idea was tested by Muraglia and colleagues with BMSCs for osteogenesis, chondrogenesis, and adipogenesis after producing non-immortalized clones. In two clone groups, 60% and 80% of clones in each respective group were bipotent toward the osteochondrogenic lineage. They found that some groups of BMSC clones do in fact possess trilineage potential at the clonal level; however, the BMSCs studied seem to favor the osteogenic lineage, as they shed their multipotency and all clones progressed toward osteogenic differentiation. All clones exhibited this osteogenic bias. Notably, certain clonal phenotypes were not observed in the study, such as clones which expressed the chondrogenic or adipogenic phenotypes exclusively (43).
Other support for BMSCs’ superiority in osteogenesis is highlighted in a report that, compared with ADSCs and SDSCs, equine BMSCs exhibited significant five-fold increases in runt-related transcription factor 2 (RUNX2) levels on day 7 of osteogenic differentiation and a six-fold increase in expression by day 14; levels of osteoblast-specific marker Osterix (OSX), were much higher (greater than 10 times) at basal levels in BMSCs versus ADSC and SDSC cell groups and Osteomodulin (OSM), a protein found in mature osteoblasts which links cells to the ECM, showed levels that were twice as high in BMSC cultures as well (44). Another earlier study by Jansen and colleagues found that there were large differences between the genetic profiles of ASCs derived from differing sources; human BMSCs appear to be more genetically prepared to undergo skeletal development than human ADSCs (45). With the consideration of apparent differences in gene expression in predifferentiated states of various ASCs, as well as unique features based solely upon harvest location and cell type, questions regarding genetic predisposal and natural capability are valid.
Although ADSCs and MDSCs can differentiate into osteoblasts in vitro, they have not been demonstrated as contributing to bone repair in vivo (46), although some controversy exists as to whether muscle may also contribute stem cells to repair. Cells derived from adipose and muscle tissues that are more accessible can potentially serve as autologous transplants. ADSCs have been expanded in vitro and tested in vivo for cartilage and bone formation (47). When transplanted in muscle, ADSCs induce ectopic bone (48). In a canine defect model, ADSCs did not have a significant effect on repair when transplanted locally even after osteogenic differentiation; however, ADSCs could augment bone regeneration after genetic modification to overexpress BMP2 (49). Shen et al. demonstrated that MDSCs expressing BMP4 could heal a critical-sized skull bone defect in immunocompetent mice; MDSCs could still be found in the repair site at 3 weeks post implantation, but were mostly gone by 4 weeks, although some of the cells appeared to differentiate into osteoblasts in the new bone (50). Thus, MDSCs and ADSCs can act mainly as carriers, producing osteogenic factors to recruit endogenous cells.
In addition to the in vitro experiments which lend support to the osteogenic success of BMSCs, in vivo studies have also proven similar conclusions. In experiments testing the ability of BMSCs to repair bone defects in the mid-diaphysis of rabbits, the BMSC treatment groups, either from an autologous or allogeneic source, were determined to be more effective in osteogenesis and bone formation in vivo (51). Sato and colleagues obtained similar results, with successful administration of BMSCs to rabbit periosteal distraction. BMSCs significantly contributed to increases in bone height, volume, mineral density, and bone mineral content (52). Success of BMSCs was not only demonstrated in animal models, but also in an earlier clinical study by Quarto and colleagues. They used bone marrow progenitors harvested from bone marrow and expanded the cells ex vivo to repair large bone defects in three patients. Implants were aided by macroporous hydroxyapatite scaffolds. In all patients, radiography and computed tomography confirmed successful bone-implant integration and callus formation at the repair sites (53). Just as with SDSCs and chondrogenesis, the ECM microenvironment can help dictate differentiation. A BMSC-based ECM enhanced osteogenesis of BMSCs expanded on this ECM, which seems to reflect the ASCs’ capacities for differentiation toward their “intended” lineages based on their individual matrix properties as tissue-specific stem cells (54). With successful integration into the bone tissue for in vivo repair, undeniable successful differentiation, and studies suggesting their favoritism toward the osteogenic lineage in vitro, BMSCs appear to be an ideal choice for ASC osteogenesis.
Mechanistic Explanations of Niche Specific Lineage Preference by Adult Stem Cells
Several studies have found proteonomic, transcriptonomic (16, 55–60), and epigenomic (61, 62) heterogeneity in stem cells from different tissues which may account for the source-dependent lineage preferences. In an effort to provide plausible mechanistic explanations for the lineage preferences of ASCs discussed above, we reviewed studies that compared the molecular properties of stem cells taken from different tissues and, when possible, discussed the significance of these differences in the context of stem cell differentiation. Given the number of recent reviews on this topic (63–65), the question has not been addressed: “Why do adult stems cells from different tissues preferentially differentiate into different lineages?”, which is central to the premise of this paper. Consequently, we focused on reviewing the molecular differences between stem cells taken from different tissues, drawing parallels, where possible, to studies that have investigated the mechanistic impact of the genes, proteins, and mRNA that vary in the stem cells based on tissue of origin.
Differences in Gene Expression
The stem cells from different sources have unique genetic profiles that inherently affect their ability to differentiate along various lineages (45). Investigation of genetic differences in ASCs has revealed differences in the expression of several genes, some of which have been directly implicated in differentiation mechanisms. For instance, the expression of the osteogenic genes OSX and OPN was higher in human BMSCs than in human ADSCs, while the expression of the adipogenic genes LEPTIN and ADIPSIN was highest in ADSCs, which has led some to conclude that ASC lineage preference is affected by their tissue of origin (66). Furthermore, a comparison of human BMSCs with cord blood-derived stem cells and ADSCs demonstrated that expression of ALP and RUNX2 was the greatest in BMSCs at all stages of osteogenic differentiation (65). Although BMSCs expressed the highest levels of collagen I (COL1A1), osteonectin (ON), and BMP2 during osteogenic induction, it was observed that ADSCs expressed higher levels of COL1A1, ON, and BMP2 prior to differentiation, which suggests that the expression profile of “resting” stem cells is not necessarily predictive of lineage preference (67). The findings are in accord with earlier studies that, under osteogenic induction, elevated osteocalcin (OCN, an osteogenic, non-collagenous protein) levels and ALP (a ubiquitously used marker of osteogenesis) activities per DNA in rat BMSCs were observed in comparison with ADSCs; further in vivo study by subcutaneously implanting the composites of these cells and hydroxyapatite ceramics into syngeneic rats for 6 weeks demonstrated that the bone volume of BMSC composites was more than that of ADSC composites (p < 0.001), quantified by micro-computed tomographic analysis (68). Moreover, Djouad et al. observed a statistically insignificant increase in the upregulation of collagen II (COL2A1) and aggrecan (ACAN) during chondrogenesis by human SDSCs relative to BMSCs, and a statistically significant increase in the upregulation of OCN and ALP during osteogenesis of human BMSCs relative to human SDSCs (16). It has also been reported that human SDSCs exhibited greater expression of platelet-derived growth factor receptor alpha (PDGFRα) than human BMSCs; due to human serum containing high levels of PDGF, neutralizing PDGF decreased the proliferation of SDSCs with autologous human serum (69), while human ADSCs expressed higher levels of integral membrane protein 2A (ITM2A) than human BMSCs, and forced expression of ITM2A inhibited chondrogenesis in a murine mesenchymal stem cell line (C3H10T1/2) (70).
Noel and colleagues observed differential expression between human BMSCs and ADSCs of genes (WNT11, WNT7B, and SOX6) involved in Wnt signaling and differentiation, an interesting finding in light of the osteogenic function of Wnt signaling (55). Canonical Wnt signaling elevates intracellular levels of β-catenin, which transposes to the nucleus and heterodimerizes with lymphoid enhancer-binding factor/T cell factor (LEF/TCF), eventually triggering translation of genes that affect lineage choice (71), while non-canonical Wnt signaling is independent of β-catenin (65). Both, however, are widely regarded to be mostly osteogenic (72), suggesting that the differential expression of Wnt signals may help predispose BMSCs toward osteogenesis. This supposition is in line with a recent report that the signaling pathways enriched in human BMSC-TERT [transduced with human telomerase reverse transcriptase gene (hTERT)] included pathways involved in bone formation (e.g. Wnt, TGFβ) and mitogen-activated protein kinase (MAPK) signaling while signaling pathways enriched in human ADSCs belonged to adipocyte-relevant metabolic functions (e.g. steroid hormone biosynthesis and linoleic acid metabolism) (73). This finding is in agreement with other studies of human ASCs, which have found greater expression of genes relevant to bone formation or osteoblast differentiation in BMSCs relative to ADSCs, and a higher expression of genes relevant to lipid metabolism in ADSCs relative to BMSCs (74, 75). The studies referenced above demonstrate that heterogeneity in gene expression exists in stem cells from different tissues, and the tissue specific profile of gene expression correlates with differentiation preference.
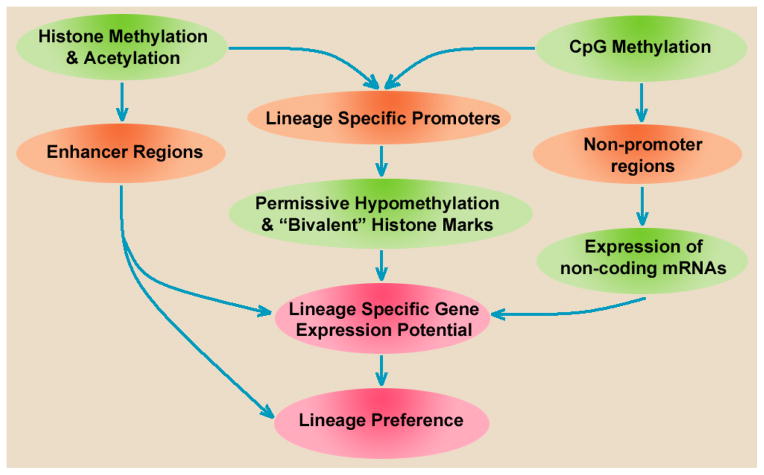
Differences at the Epigenetic Level
The heterogeneity of ASC epigenetics may explain the differences in gene expression among ASCs of differing origins (Figure 2). Collas and colleagues noted hypomethylation in the promoters of four adipogenic ADSC genes (76) and asked whether ASCs were pre-programmed toward a certain lineage by DNA methylation (77). At least in part, the answer to the above question appears to be in the affirmative; as Boquest et al. noted, human ADSCs are hypermethylated in the promoters for the myogenic differentiation gene myogenin (MYOG) and the endothelial genes CD31 and CD144 (also called vascular endothelium cadherin or CDH5), and, relative to adipogenic genes, are also hypermethylated at the promoter of the osteogenic gene osteoglycin (OGN) (76–78). Mouse BMSCs were shown to undergo demethylation and gene upregulation at the OPN promoter following mechanical stimulation (a well-recognized osteogenic stimulus) and it was hypothesized that the absence of epigenetic changes to OCN and COL1A1 promoters resulted from these regions having already been primed for osteogenesis by methylations occurring prior to mechanical stimulation (79). In human SDSCs, it was found that 10 of 11 chondrogenic genes tested were promoter hypomethylated (80), which may partially account for the preference of human SDSCs to differentiate into chondrocytes (6).
Figure 2.
Epigenetic determination of niche-specific lineage preference. CpG methylation of promoters creates a permissive, but non-predictive state, while non-promoter CpG methylation, histone modifications, and differentially expressed miRNAs may combine to determine lineage preference.
Furthermore, it was shown that promoters of osteogenic transcription factors are hypermethylated in the murine myoblast C2C12 cell line relative to promoters of myogenic transcription factors, and that chemically induced demethylation enhances osteogenesis and adipogenesis of C2C12 cells. Hupkes et al. postulated that DNA methylation preprogramming could underlie the default differentiation of C2C12 cells toward the myogenic lineage (81). Collas described 400–700 hypermethylated genes specific to ADSCs, BMSCs, and muscle progenitor cells (MPCs) and commented that these methylation patterns might be determined by the tissue-specific stem cell niche (82).
While CpG methylation is a well-studied epigenetic modification to DNA, research indicates poor correlation between gene expression and promoter methylation, suggesting that other epigenetic mechanisms may also be important determinants for lineage preference (77, 81, 83). Additionally, many studies have discovered general hypomethylation of lineage-specific promoter regions in mesenchymal and non-mesenchymal ASCs, regardless of origin (61, 84, 85). These observations helped clarify the role of CpG island methylation in lineage-specific promoters; it appears that hypomethylation of these promoters is permissive, but not necessarily predictive, of lineage preference (62, 82).
The functional significance of methylation patterns outside promoter regions is incomplete and poorly understood. Irizarry et al. showed that most tissue-specific methylation changes do not occur in CpG islands, but rather in nearby “CpG island shores”, and that gene expression is tightly linked with these methylation patterns (86). However, others have found that tissue-specific methylation often occurs within coding sequences or entirely downstream of known genes (87) and have postulated that such sites might contain standard methyl-sensitive repressor elements that are able to operate at a distance to silence adjacent promoters (87). Intragenic methylation may also enhance transcription of noncoding RNA (87), a theory with interesting implications in light of our growing appreciation for the roles of micro RNA (miRNA or miR) in stem cell differentiation (88–90).
Histone modifications may also play a large role in influencing the lineage preference of ASCs (91). In human BMSCs, the promoter regions of the master adipogenic transcription factor, PPARG, is histone 3 lysine 9 (H3-K9) methylated, an epigenetic modification that repressed transcription, leading Tan et al. to hypothesize, “adipogenic lineage-specific genes regulated by PPARG may be silenced by the H3-K9 hypermethylation at their promoter regions” (92). Later research indicates the promoters of 70% of underexpressed genes in human BMSCs were indeed H3-K9 methylation enriched (93). In human ADSCs, both the permissive H3-K4M3 and repressive H3-K27M3 marks have been noted on promoters for lineage-specific genes (94), which led Collas et al. to theorize that adipogenic promoters are preprogrammed for activation upon adipogenic stimulation (83). Human BMSCs are also hypomethylated as well as H3-K4M3 and H3-K27M3 enriched (61) and this pattern may also regulate myogenesis (95). It is believed that this “bivalent” histone modification pattern positions a cell to rapidly respond to differentiation inducing stimuli; the loss of this bivalent pattern may correspond to diminished stem cell potency and differentiation (61, 85, 96, 97).
A recent study by Ragni et al. compared the miRNA profiles of human ASCs taken from sources including bone marrow, adipose tissue, and umbilical cord blood. The authors noted that the miRNA expression patterns between ASCs from unmatched donors were mostly consistent (98). In contrast to earlier studies which found only a single miRNA, miR-424, differentially expressed between human ADSCs and BMSCs (96), Ragni and coworkers concluded that, although the miRNA expression patterns of the various ASC types are similar, there appear to be at least 20 differentially expressed miRNAs between human ADSCs and human BMSCs (66). Interestingly, they noted expression differences in several miRNAs that may be involved in lineage choice.
First, they noted that the expression of miR-135b is nearly 48 times higher in BMSCs than ADSCs (66). Studies have suggested that miR-135b was downregulated in unrestricted somatic stem cell osteogenic differentiation (99); mesenchymal stem cells from multiple myeloma patients exhibited an abnormal upregulation of miR-135b, showing meanwhile impaired osteogenic differentiation and a decrease of mothers against decapentaplegic homolog 5 (SMAD5) expression, which is the target of miR-135b involved in osteogenesis (100). As reviewed by Cook et al., SMADs 1, 5, and 8 usually transmit BMP signaling, which activates distal-less 5 (DLX5), resulting in the downstream activation of RUNX2 and OSX (63). miR-135 targeting SMAD5 could effectively inhibit osteogenesis (88, 101).
Second, it was found that miR-138 was 11 times more highly expressed in BMSCs than in ADSCs (66). miR-138 has been implicated in inhibiting adipogenesis (102) as well as osteogenesis (103). Focal adhesion kinase (FAK), which regulated the osteogenesis of stem cells (104), has been identified as a target of miR-138 in human BMSCs (103). Another miRNA of interest, miR-31, was expressed at fivefold greater levels in BMSCs than in ADSCs. miR-31 downregulated the adipogenic CEBPA (88, 105) as well as osteogenic OSX (106). Deng and colleagues investigated the role of miR-31 in rat ADSCs and concluded that miR-31, which was suppressed by elevated Runx2 expression, inhibits osteogenesis, possibly by decreasing the translation of special AT-rich sequence-binding protein 2 (Satb2) (107), a conclusion similar to earlier work demonstrating that miR-31 was diminished in osteo-differentiated BMSCs relative to BMSCs and that transfection with antisense miR-31 increased expression of Runx2 and BMP receptor 2 (BMPR2), promoting osteogenesis (108).
Finally, Gao et al. showed that miR-424, which was expressed 5.5 times more in ADSCs than BMSCs (66), was diminished in osteo-differentiated BMSCs, and predicted that miR-424 played a role in inhibiting osteogenesis (108). The combination of these findings suggests that, although much of the tissue-specific miRNA expression in stem cells functions to prevent the premature differentiation of these stem cells, the unique profile of different tissue-specific stem cells may also help to determine lineage preference. Ragni and colleagues concluded that differential expression of miRNA may provide a molecular explanation of stem cell niche memory (66). Although the above findings may not be sufficient to completely explain, mechanistically, the observation that stem cells from different tissues exhibit lineage preferences, it is clear that there is extensive epigenetic variability between stem cells based upon origin, and it seems likely that these differences, such as the restrictive promoter hypermethylation or the repression of a signaling molecule implicated in differentiation (FAK, for example), play a role in the mechanisms underlying lineage preference.
Differences at the Protein Level
There is evidence that stem cells from different tissues differ in their expression of ECM proteins and secreted factors. Researchers compared the surface proteins of stem cells from different origins and concluded that, while expression of many surface markers is similar, differences do exist. For example, CD146 was more highly expressed in human BMSCs than human ADSCs (73), and CD49d was less pronounced in adult human BMSCs than perinatal human stem cells from amniotic membrane, though this finding could be influenced by donor age (60). Further, ADSCs have been found to express CD34 after isolation, while BMSCs do not (109).
Mesenchymal stem cells’ secretion and responses to soluble factors may vary depending on the tissue of origin. A comparison of human BMSCs and ADSCs revealed that, at early passages (P2-P4 or up to 14–15 in vitro population doublings), BMSCs secreted more vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF-1), monocyte chemotactic protein-1 (MCP-1), and TGFβ1 than ADSCs did (110). TGFβ1 is of particular interest given its important role in regulating stem cell differentiation. TGFβ1 signals through multiple pathways, including SMAD2/3, MAPK, and Wnt. Zhao and Hantash have provided a thorough review of TGFβ1 regulation of BMSC differentiation to which the reader is referred for a detailed discussion. In short, TGFβ1 inhibited adipogenesis in fibroblasts (and possibly BMSCs), but stimulated chondrogenesis and osteogenesis of BMSCs (111). TGFβ1 may also trigger chondrogenesis in human ADSCs (112), but the chondrogenic commitment of TGFβ1-treated ADSCs is delayed relative to their BMSC counterparts (113). Afizah and colleagues demonstrated that human BMSCs synthesized more GAG and collagen II following TGFβ3 treatment than donor-matched ADSCs (114). It has further been shown that dexamethasone augmented the TGFβ1-induced chondrogenesis in 4-month-old bovine BMSCs, but not in 4-month-old bovine SDSCs (115). Human BMSCs exhibited greater expression of HLA-DR (an MHC class II cell surface receptor encoded by the human leukocyte antigen complex on chromosome 6 region 6p21.31) than stem cells from amniotic membrane after stimulation by tumor necrosis factor alpha and interferon gamma (60). The combination of heterogeneous receptor profiles and secretomes exhibited by stem cells from different tissues may underlie the previously discussed differences in sensitivity to differentiation-inducing stimuli, while also contributing, mechanistically, to lineage preference.
Conclusions and Perspective
Stem cell therapies are undoubtedly treatment options in various areas of the biomedical field; however, each stem cell population’s characteristics and source are commonly overlooked factors. Overall, we can conclude that ASCs are best suited for differentiation along their natural prospective lineages for the formation of quality bone, cartilage, adipose, and muscle tissues. The theory that various multipotent stem cell subpopulations exist within a given tissue, as well as subpopulations possessing various capacities for quality differentiation, also supports the idea that ASCs from their resident locations are the most effective contributors to a particular lineage. These populations are naturally functioning and thriving in vivo with respect to the surrounding tissues in which they exist. Although the current literature offers expansive support for this idea based on fundamental in vitro data, there is still an overall lack of in vivo studies which compare a wide variety of ASCs for multilineage differentiation capacity. In order to draw more conclusive results from in vitro experiments, it would be beneficial to utilize ASC clones; these clones can eliminate the possibility of progenitor cell heterogeneity which can skew the results of multilineage studies. Another great deficiency in current multilineage studies is the lack of in vivo and in vitro studies which investigate multilineage characteristics from the same donor. Studies of this nature could give insight into variability between organisms and, more importantly, highlight, strengthen, and uncover trends and tendencies of tissue-specific stem cells as they progress toward lineages outside their respective conventional differentiation fates. Although studies have independently demonstrated the ability of ASCs for cross-differentiation to other lineages, studies which compare several ASCs directly, rather than retrospectively, are generally more valuable. Direct comparison methods and using multiple cell types simultaneously can offer more direct assessment and circumvent experimental variability to produce more reliable conclusions regarding the differentiation potential of ASCs from separate tissues. Through our current knowledge about the specific properties of each type of ASC and future in vivo experimentation, the possibility of elucidating and revealing a cellular hierarchy for ASCs and lineage-specific differentiation is feasible.
Despite noteworthy advancement in the study of niche-specific regulation of ASC lineage preference, mechanistic research remains active. Further comparative analysis of tissue-specific miRNA expression, histone modifications to lineage-specific genes, and non-promoter methylation patterns is needed. For instance, to our knowledge, the miRNA expression profile of SDSCs has not been thoroughly compared to ADSCs or BMSCs. We believe that such studies could yield important findings, especially in light of the extensively demonstrated success in SDSC chondrogenesis studies. Additionally, it is interesting to note that tissue-specific histone modifications are far more abundant in enhancer regions than in promoter regions (116), and these modifications are made prior to cell fate commitment (117), raising the possibility that lineage preference, at least in part, is a consequence of enhancer modifications. It remains likely that tissue-specific epigenetic patterns play a role in the preference of ASCs for certain lineages (91) and such modifications may underlie the differential lineage preference of ASCs derived from various anatomical tissues. Finally, although the studies considered earlier have demonstrated that stem cells from different tissues are not identical in their responses to chemical differentiation stimuli, the molecular explanation for this observation is incomplete. It would be interesting to compare, at a molecular level, the responses of stem cells from various tissues to important differentiation factors, such as BMP, Wnt, and insulin-like growth factor (IGF), as evidence exists that stem cells from different tissues might respond in subtly different ways to the same molecular stimulus. Studies further addressing differences in signaling cascades, secretion of soluble factors, and matrix receptors might help elucidate the underlying molecular heterogeneity among stem cells of different tissues. The implications and impacts of such discoveries would certainly span a wide array of biomedical disciplines and help shape the future of stem cell therapy and regenerative medicine.
Acknowledgments
The authors thank Suzanne Danley and Kayla Branyan for help in editing the manuscript. This project was partially supported by Research Grants from the AO Foundation (S-12-19P) and NIH R03 (no. 1 R03 AR062763-01A1).
Footnotes
Disclosure of potential conflict of interest
The authors indicate no potential conflicts of interest.
Author contributions:
T.P.: collection and assembly of data, data analysis and interpretation, and manuscript writing; K.L.: collection and assembly of data, data analysis and interpretation, and manuscript writing; M.P.: conception and design, administrative support, manuscript writing, and final approval of the manuscript.
References
- 1.Hammerick KE, Huang Z, Sun N, Lam MT, Prinz FB, Wu JC, et al. Elastic properties of induced pluripotent stem cells. Tissue Engineering Part A. 2011;17(3–4):495–502. doi: 10.1089/ten.tea.2010.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moroni L, Fornasari PM. Human mesenchymal stem cells: a bank perspective on the isolation, characterization and potential of alternative sources for the regeneration of musculoskeletal tissues. Journal of Cellular Physiology. 2013;228(4):680–687. doi: 10.1002/jcp.24223. [DOI] [PubMed] [Google Scholar]
- 3.van der Kooy D, Weiss S. Why Stem Cells? Science. 2000;287(5457):1439–1441. doi: 10.1126/science.287.5457.1439. [DOI] [PubMed] [Google Scholar]
- 4.Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. The Lancet. 2013;382(9898):1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and Tissue Research. 2007;327(3):449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis & Rheumatology. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis & Rheumatology. 2006;54(3):843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 8.Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells and Development. 2008;17(4):761–773. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Zhang N, Marsano A, Vunjak-Novakovic G, Zhang Y, Lopez MJ. In vitro mesenchymal trilineage differentiation and extracellular matrix production by adipose and bone marrow derived adult equine multipotent stromal cells on a collagen scaffold. Stem Cell Reviews. 2013;9(6):858–872. doi: 10.1007/s12015-013-9456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monaco E, Bionaz M, Rodriguez-Zas S, Hurley WL, Wheeler MB. Transcriptomics comparison between porcine adipose and bone marrow mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. PloS One. 2012;7(3):3248–3258. doi: 10.1371/journal.pone.0032481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell and Tissue Research. 2012;347(2):419–427. doi: 10.1007/s00441-011-1306-3. [DOI] [PubMed] [Google Scholar]
- 12.Pei M, He F, Kish VL, Vunjak-Novakovic G. Engineering of functional cartilage tissue using stem cells from synovial lining: a preliminary study. Clinical Orthopaedics Related Research. 2008;466(8):1880–1889. doi: 10.1007/s11999-008-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76(10):1044–1056. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei M, He F, Boyce BM, Kish VL. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis and Cartilage. 2009;17(6):714–722. doi: 10.1016/j.joca.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. Journal of Cellular Physiology. 2007;211(3):682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 16.Djouad F, Bony C, Häupl T, Uzé G, Lahlou N, Louis-Plence P, et al. Transcriptional profiles discriminate bone marrow derived and synovium derived mesenchymal stem cells. Arthritis Research & Therapy. 2005;7(6):1304–1315. doi: 10.1186/ar1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei M. Can synovium-derived stem cells deposit matrix with chondrogenic lineage-specific determinants? Journal of Tissue Science and Engineering. 2012;3(3):1–3. [Google Scholar]
- 18.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell and Tissue Research. 2008;333(2):207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee JC, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 2013;29(6):1034–1046. doi: 10.1016/j.arthro.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Engineering Part A. 2012;18(11–12):1161–1170. doi: 10.1089/ten.tea.2011.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Engineering Part B Reviews. 2012;18(4):301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 22.Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. Journal of Orthopaedic Research. 2009;27(4):435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Engineering Part B. 2012;18(4):270–287. doi: 10.1089/ten.TEB.2011.0583. [DOI] [PubMed] [Google Scholar]
- 24.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis & Rheumatology. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Engineering Part A. 2009;15(12):3809–3821. doi: 10.1089/ten.TEA.2009.0188. [DOI] [PubMed] [Google Scholar]
- 26.He F, Pei M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(1):73–84. doi: 10.1002/term.505. [DOI] [PubMed] [Google Scholar]
- 27.Rinaldi F, Perlingeiro RC. Stem cells for skeletal muscle regeneration: therapeutic potential and roadblocks. Translational Research. 2014;163(4):409–417. doi: 10.1016/j.trsl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nature Cell Biology. 2010;12(3):257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 29.Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. Journal of Histochemistry & Cytochemistry. 2011;59(1):33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Developmental Biology. 2000;218(2):115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 31.Boppart MD, De Lisio M, Zou K, Huntsman HD. Defining a role for non-satellite stem cells in the regulation of muscle repair following exercise. Frontiers in Physiology. 2013;4:310. doi: 10.3389/fphys.2013.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle MJ, Zhou S, Tanaka KK, Pisconti A, Farina NH, Sorrentino BP, et al. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. Journal of Cell Biology. 2011;195(1):147–163. doi: 10.1083/jcb.201103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. Journal of Cell Biology. 2002;157(5):851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motohashi N, Uezumi A, Yada E, Fukada S, Fukushima K, Imaizumi K, et al. Muscle CD31(-)CD45(-) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. American Journal of Pathology. 2008;173(3):781–791. doi: 10.2353/ajpath.2008.070902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. Journal of Applied Physiology. 2001;91(2):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 36.Meligy FY, Shigemura K, Behnsawy HM, Fujisawa M, Kawabata M, Shirakawa T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In vitro Cellular & Developmental Biology-Animal. 2012;48(4):203–215. doi: 10.1007/s11626-012-9488-x. [DOI] [PubMed] [Google Scholar]
- 37.Bayati V, Hashemitabar M, Gazor R, Nejatbakhsh R, Bijannejad D. Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study. Anatomy & Cell Biology. 2013;46(2):113–121. doi: 10.5115/acb.2013.46.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei H, Yu B, Huang Z. Comparative analysis of mesenchymal stem cells from adult mouse adipose, muscle, and fetal muscle. Molecular Biology Reports. 2013;40(2):885–892. doi: 10.1007/s11033-012-2129-3. [DOI] [PubMed] [Google Scholar]
- 39.De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. Journal of Cell Biology. 2003;160(6):909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthopaedics & Traumatology: Surgery & Research. 2014;100(1 Suppl):S107–S112. doi: 10.1016/j.otsr.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13(10):845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Park SH, Sim WY, Min BH, Yang SS, Khademhosseini A, Kaplan DL. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PloS One. 2012;7(9):466–89. doi: 10.1371/journal.pone.0046689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. Journal of Cell Science. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 44.Pantaleoni Andrietti A, Stewart MC. Comparative osteogenesis of equine mesenchymal stem cells isolated from bone marrow, adipose tissue and synovium. 2012 http://hdl.handle.net/2142/31213.
- 45.Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells and Development. 2010;19(4):481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 46.Colnot C. Cell sources for bone tissue engineering: insights from basic science. Tissue Engineering Part B Reviews. 2011;17(6):449–457. doi: 10.1089/ten.teb.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levi B, Longaker MT. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells. 2011;29(4):576–582. doi: 10.1002/stem.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Engineering. 2006;12(7):1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochemical and Biophysical Research Communications. 2007;356(4):836–842. doi: 10.1016/j.bbrc.2007.02.165. [DOI] [PubMed] [Google Scholar]
- 50.Shen HC, Peng H, Usas A, Gearhart B, Cummins J, Fu FH, et al. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone. 2004;34(6):982–992. doi: 10.1016/j.bone.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 51.Udehiya RK, Amarpal, Aithal HP, Kinjavdekar P, Pawde AM, Singh R, et al. Comparison of autogenic and allogenic bone marrow derived mesenchymal stem cells for repair of segmental bone defects in rabbits. Research in Veterinary Science. 2013;94(3):743–752. doi: 10.1016/j.rvsc.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Sato K, Haruyama N, Shimizu Y, Hara J, Kawamura H. Osteogenesis by gradually expanding the interface between bone surface and periosteum enhanced by bone marrow stem cell administration in rabbits. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, Endodontics. 2010;110(1):32–40. doi: 10.1016/j.tripleo.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. New England Journal of Medicine. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 54.Pei M, He F, Kish VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Engineering Part A. 2011;17(23–24):3067–3076. doi: 10.1089/ten.tea.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, et al. Cell specific differences between human adipose derived and mesenchymal stromal cells despite similar differentiation potentials. Experimental Cell Research. 2007;314(7):1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 56.Miranda HC, Herai RH, Thomé CH, Gomes GG, Panepucci RA, Orellana MD, et al. A quantitative proteomic and transcriptomic comparison of human mesenchymal stem cells from bone marrow and umbilical cord vein. Proteomics. 2012;12(17):2607–2617. doi: 10.1002/pmic.201200111. [DOI] [PubMed] [Google Scholar]
- 57.Panepucci RA, Siufi JL, Silva WA, Jr, Proto-Siquiera R, Neder L, Orellana M, et al. Comparison of Gene Expression of Umbilical Cord Vein and Bone Marrow Derived Mesenchymal Stem Cells. Stem Cells. 2004;22(7):1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 58.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue derived versus bone marrow derived mesenchymal stem and stromal cells. Stem Cells and Development. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 59.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Experimental Hematology. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Wegmeyer H, Broske A, Leddin M, Kuentzer K, Nisslbeck A, Hupfeld J, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells and Development. 2013;22(19):2606–2618. doi: 10.1089/scd.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sorensen A, Jacobsen B, Reiner A, Andersen IS, Collas P. Promoter DNA methylation patterns of differentiated cells are largely programmed at the progenitor stage. Molecular Biology of the Cell. 2010;21(12):2066–2077. doi: 10.1091/mbc.E10-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorensen AL, Timoskainen S, West FD, Vekterud K, Boquest AC, Ahriund-Richter L, et al. Lineage-specific promoter DNA methylation patterns segregate adult progenitor cells types. Stem Cells and Development. 2010;19(8):1257–1266. doi: 10.1089/scd.2009.0309. [DOI] [PubMed] [Google Scholar]
- 63.Cook D, Genever P. Regulation of Mesenchymal Stem Cell Differentiation. Advances in Experimental Medicine and Biology. 2013;786:213–29. doi: 10.1007/978-94-007-6621-1_12. [DOI] [PubMed] [Google Scholar]
- 64.Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World Journal of Stem Cells. 2013;5(4):136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.James A. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo) 2013;2013:1155–1170. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ragni E, Montemurro T, Montelatici E, Lavazza C, Viganò M, Rebulla P, et al. Differential microRNA signature of human mesenchymal stem cells from different sources reveals an “environmental-niche memory” for bone marrow stem cells. Experimental Cell Research. 2013;319(10):1562–1574. doi: 10.1016/j.yexcr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Shafiee A, Seyedjafari E, Soleimani M, Ahmadbeigi N, Dinarvand P, Ghaemi N. A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnology Letters. 2011;33(6):1257–1264. doi: 10.1007/s10529-011-0541-8. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcified Tissue International. 2008;82(3):238–247. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 69.Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis & Rheumatology. 2008;58(2):501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 70.Boeuf S, Börger M, Hennig T, Winter A, Kasten P, Richter W. Enhanced ITM2A expression inhibits chondrogenic differentiation of mesenchymal stem cells. Differentiation. 2009;78(2–3):108–115. doi: 10.1016/j.diff.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Kim W, Kim M, Jho E. Wnt/B-catenin signaling: from plasma membrane to nucleus. Biochemical Journal. 2013;450(1–2):9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 72.Takada I, Kouzmenko A, Kato S. Wnt and PPARg signaling in osteoblastogenesis and adipogenesis. Nature Reviews Rheumatology. 2009;5(8):442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 73.Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Review. 2013;9(1):32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boeuf S, Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Research & Therapy. 2010;1(4):31. doi: 10.1186/scrt31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25(3):750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 76.Noer A, Sorensen AL, Boquest AC, Collas P. Stable CpG hypomethylation of adipogenic promoters in freshly isolated cultured and differentiated mesenchymal stem cells from adipose tissue. Molecular Biology of the Cell. 2006;17(8):3543–3556. doi: 10.1091/mbc.E06-04-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boquest A, Noer A, Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Review. 2006;2(4):319–329. doi: 10.1007/BF02698059. [DOI] [PubMed] [Google Scholar]
- 78.Boquest AC, Noer A, Sorensen AL, Vekterud K, Collas P. CpG methylation profiles of endothelial cell specific gene promoter regions in adipose tissue stem cells suggest limited differentiation protential towards the endothelial cell lineage. Stem Cells. 2007;25(4):852–861. doi: 10.1634/stemcells.2006-0428. [DOI] [PubMed] [Google Scholar]
- 79.Arnsdorf EJ, Tummala P, Castillo AB, Zhang F, Jacobs CR. The epigenetic mechanism of mechanically induced osteogenic differentiation. Journal of Biomechanics. 2010;43(15):2881–2886. doi: 10.1016/j.jbiomech.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ezura Y, Sekiya I, Koga H, Muneta T, Noda M. Methylation status of CpG islands in the promoter regions of signature genes during chondrogenesis of human synovium derived mesenchymal stem cells. Arthritis & Rheumatology. 2009;60(5):1416–1426. doi: 10.1002/art.24472. [DOI] [PubMed] [Google Scholar]
- 81.Hupkes M, Someren EP, Middelkamp SH, Piek E, van Zoelen EJ, Dechering KJ. DNA methylation restricts spontaneous multi-lineage differentiation of mesenchymal progenitor cells, but is stable during growth factor-induced terminal differentiation. Biochimica Biophysica Acta. 2011;1813(5):839–849. doi: 10.1016/j.bbamcr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 82.Collas P. Programming differentiation potential in mesenchymal stem cell. Epigenetics. 2010;5(6):476–482. doi: 10.4161/epi.5.6.12517. [DOI] [PubMed] [Google Scholar]
- 83.Collas P, Noer A, Sorensen A. Epigenetic basis for the differentiation potential of mesenchymal and embryonic stem cells. Transfusion Medicine and Hemotherapy. 2008;35(3):205–215. doi: 10.1159/000127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohn F, Schubeler D. Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends in Genetics. 2009;25(3):129–136. doi: 10.1016/j.tig.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 85.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 86.Irizarry R, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nature Genetics. 2009;41(2):178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Straussman R, Nejman D, Roberts D, Steinfeld I, Blum B, Benvenisty N, et al. Developmental programming of CpG island methylation profiles in the human genome. Nature Structural & Molecular Biology. 2009;16(5):564–571. doi: 10.1038/nsmb.1594. [DOI] [PubMed] [Google Scholar]
- 88.Guo L, Zhao R, Wu Y. The role of microRNAs in self renewal and differentiation of mesenchymal stem cells. Experimental Hematology. 2011;39(6):608–616. doi: 10.1016/j.exphem.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 89.Perdiguero E, Sousa-Victor P, Ballestar E, Muñoz-Cánoves P. Epigenetic regulation of myogenesis. Epigenetics. 2009;4(8):541–550. doi: 10.4161/epi.4.8.10258. [DOI] [PubMed] [Google Scholar]
- 90.Romao J, Jin W, Dodson M, Hausman GJ, Moore SS, Guan LL. MicroRNA regulation in mammalian adipogenesis. Experimental Biology and Medicine. 2011;236(9):997–1004. doi: 10.1258/ebm.2011.011101. [DOI] [PubMed] [Google Scholar]
- 91.Teven C, Liu X, Hu N, Tang N, Kim SH, Huang E, et al. Epigenetic regulation of mesenchymal stem cells: a focus on osteogenic and adipogenic differentiation. Stem Cells International. 2011;2011:201371. doi: 10.4061/2011/201371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan J, Huang H, Huang W, Li L, Guo J, Huang B, et al. The genomic landscapes of histone H3-Lys9 modifications of gene promoter regions and expression profiles in human bone marrow mesenchymal stem cells. Journal of Genetics & Genomics. 2008;35(10):585–593. doi: 10.1016/S1673-8527(08)60079-X. [DOI] [PubMed] [Google Scholar]
- 93.Tan J, Lu J, Huang W, Dong Z, Kong C, Li L, et al. Genome-wide analysis of histone H3 lysine9 modifications in human mesenchymal stem cell osteogenic differentiation. PLoS One. 2009;4:e6792. doi: 10.1371/journal.pone.0006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noer A, Linderman L, Collas P. Histone H3 modifications associated with differentiation and long term culture of mesenchymal adipose stem cells. Stem Cells and Development. 2009;18(5):725–736. doi: 10.1089/scd.2008.0189. [DOI] [PubMed] [Google Scholar]
- 95.Dilworth FJ, Blais A. Epigenetic regulation of satellite cell activation during muscle regeneration. Stem Cell Research Therapy. 2011;2(2):18. doi: 10.1186/scrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aranda P, Agirre X, Ballestar E, Andreu EJ, Román-Gómez J, Martin-Subero JI, et al. Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS One. 2009;4(11):7809. doi: 10.1371/journal.pone.0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Voigt P, Tee W, Reinberg D. A double take on bivalent promoters. Genes & Development. 2013;27(12):1318–1338. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pandey AC, Semon JA, Kaushal D, O’Sullivan RP, Glowacki J, Gimble JM, et al. MicroRNA profiling reveals age dependent differential expression of nuclear factor kB and mitogen activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Research Therapy. 2011;2(6):49. doi: 10.1186/scrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schaap-Oziemlak AM, Raymakers RA, Bergevoet SM, Glissen C, Jansen BJ, Adema GJ, et al. MicroRNA has-miR-135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cells and Development. 2010;19(6):877–885. doi: 10.1089/scd.2009.0112. [DOI] [PubMed] [Google Scholar]
- 100.Xu S, Santini GC, Veirman KD, Broek IV, Leleu X, Becker AD, et al. Upregulation of miR-135b is involved in the impaired osteogenenic differentiation of mesenchymal stem cells derived from multiple myeloma patients. PLoS One. 2013;8(11):e79752. doi: 10.1371/journal.pone.0079752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, et al. A microRNA signature for a BMP2 induced osteoblast lineage commitment program. Proceedings of the National Academy of Sciences USA. 2008;105(37):13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Z, Bian C, Zhou H, Huang S, Wang S, Liao L, et al. MicroRNA has-miR-138 inhibits adipogenic differentiation of human adipose tissue derived mesenchymal stem cells through adenovirus EID-1. Stem Cells and Development. 2011;20(2):259–267. doi: 10.1089/scd.2010.0072. [DOI] [PubMed] [Google Scholar]
- 103.Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, et al. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proceedings of the National Academy of Sciences USA. 2011;108(15):6139–6144. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mathieu P, Loboa E. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Engineering Part B. 2012;18(6):436–444. doi: 10.1089/ten.teb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun F, Wang J, Pan Q, Yu Y, Zhang Y, Wan Y, et al. Characterization of function and regulation of miR-24-1 and miR-31. Biochemical and Biophysical Research Communications. 2009;380(3):660–665. doi: 10.1016/j.bbrc.2009.01.161. [DOI] [PubMed] [Google Scholar]
- 106.Baglìo SR, Devescovi V, Granchi D, Baldin N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527(1):321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 107.Deng Y, Zhou H, Zou D, Xie Q, Bi X, Gu P, et al. The role of miR-31-modified adipose tissue derived stem cells in repairing rat critical sized calvarial defects. Biomaterials. 2013;34(28):6717–6728. doi: 10.1016/j.biomaterials.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 108.Gao J, Yang T, Han J, Yan K, Qui X, Zhou Y, et al. MicroRNA expression during osteogenic differentiation of human multipotent mesenchymal stromal cells from bone marrow. Journal of Cellular Biochemistry. 2011;112(7):1844–1856. doi: 10.1002/jcb.23106. [DOI] [PubMed] [Google Scholar]
- 109.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow and subcutaneous adipose tissue derived mesenchymal stem cells: Differences and similarities. Cell Cycle. 2012;11(2):377–383. doi: 10.4161/cc.11.2.18858. [DOI] [PubMed] [Google Scholar]
- 111.Zhao L, Hantash B. TGF-B1 regulates differentiation of bone marrow mesenchymal stem cells. Vitamins & Hormones. 2011;87:127–141. doi: 10.1016/B978-0-12-386015-6.00042-1. [DOI] [PubMed] [Google Scholar]
- 112.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue derived stromal cells in vitro and in vivo. Biochemical and Biophysical Research Communications. 2002;290(2):763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 113.Mehlhorn AT, Niemeyer P, Kaiser S, Finkenzeller G, Stark GB, Sudkamp NP, et al. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Engineering. 2006;12(10):2853–2862. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- 114.Afizah H, Yang Z, Hui JH, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Engineering. 2007;13(4):659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 115.Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. European Cells and Materials. 2011;22:302–319. doi: 10.22203/ecm.v022a23. [DOI] [PubMed] [Google Scholar]
- 116.Heintzman N, Hon G, Hawkins R, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell type specific gene expression. Nature. 2009;459(7243):108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ong CT, Corces VG. Enhancers: emerging roles in cell fate specification. EMBO Reports. 2012;13(5):423–430. doi: 10.1038/embor.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]