Abstract
Background
The timing of prenatal exposure to tobacco cigarette smoking can be crucial for the developing fetus. Pushing the field beyond prior pregnancy trimester-focused smoking estimates, we estimated month-specific prevalence proportions for tobacco cigarette smoking among pregnant and non-pregnant women of the United States (US), with consideration of tobacco dependence (TD) as well. In advance, we posited that pregnancy onset might prompt smoking cessation in early months, before the end of the 1st trimester, and that TD might account for sustained smoking in later months, especially months 8-9, when there are added reasons to quit.
Methods
Estimates are from the 2002-2009 National Surveys on Drug Use and Health Restricted-Data Analysis System (R-DAS), with large nationally representative samples of US civilians, including 12-44 year old women (n~70,000) stratified by pregnancy status and month of pregnancy, with multi-item assessment of TD as well as recently active smoking. Age was held constant via the Breslow-Day indirect standardization approach, a methodological detail of potential interest to other research teams conducting online R-DAS analyses.
Results
Among 12-44 year old women in Month 1 of pregnancy, as well as non-pregnant women, just over one in four was a recently active smoker (26%-27%), and approximately one-half of these smokers qualified as a TD case (52%). Corresponding estimates for women in Month 3 were 17.6% and two-thirds, respectively, lending some support for our advance hypotheses. Nonetheless, our a priori TD hypothesis about Months 8-9 seems to be contradicted: an increased concentration of TD among smokers surfaced early in pregnancy.
Conclusions
Evidence of a possible ameliorative pregnancy effect on smoking prevalence as well as TD's effect on smoking persistence might be seen quite early in pregnancy. Substitution of a month-specific view for the traditional trimester view sheds new light on how pregnancy might shape smoking behavior before the end of trimester 1, with TD seeming to thwart a public health goal of 100% cessation, early in pregnancy.
Keywords: Tobacco, Cigarettes, Pregnancy, Drug use, Prevalence, Smoking
1. Introduction
The timing of prenatal tobacco smoking exposure can be crucial for pregnancy outcomes and fetal development, with potential smoking-affected epigenetic processes as early as the embryonic period of development and before Month 2 of pregnancy has ended (1-4). Low birth weight (LBW) provides one illustration of timing issues. There is tangible smoking-attributable excess LBW risk for offspring of mothers who smoke through all nine months of the pregnancy, but apparently no such excess risk for offspring when the mothers quit smoking by Month 5 (5). In past research, trimester-specific estimates of tobacco smoking before and during pregnancy suggested beneficial effects on smoking behavior, and raise a possibility that early discovery that one is pregnant might function as a precipitant of smoking cessation (6-8). In an attempt to bring the human data into greater alignment with pre-clinical lines of research on suspected consequences of prenatal exposure to nicotine and tobacco smoking, in this study, we have substituted a month-specific approach for the traditional trimester-specific approach. We are presenting estimates for tobacco cigarette smoking proportions among women age 12-44 years old, stratified by pregnancy status and month of pregnancy, as well as corresponding month-specific estimates for Tobacco Dependence (TD) prevalence among pregnant smokers. Our advance expectation was that we would see evidence consistent with pregnancy-attributable smoking cessation as early as Months 2-3 of the pregnancy (i.e., possibly not in Month 1, but before the end of the first trimester), and a relatively greater concentration of TD cases among pregnant smokers in Months 8-9. We did not expect smoking prevalence to be appreciably different in the contrast of non-pregnant women versus women in the earliest weeks of pregnancy; they might have not had enough time to quit smoking during those weeks. We forecast a greater TD prevalence in Months 8-9 because TD might help explain why knowledge of the pregnancy had not been enough to promote successful smoking cessation by those months.
With advantages of recent nationally representative sample surveys and standardized data collection approaches, the objective was to check whether the possibly ameliorative effect and reduced smoking prevalence could be seen within the first months of pregnancy (i.e., before the first trimester has ended), and to add new evidence on TD as a determinant of smoking prevalence in late pregnancy. This study's smoking prevalence estimates for independently sampled pregnant mothers, month by month, allowed us to learn from the more fine-grained temporal month-wise approach (i.e., more fine-grained than the traditional trimester approach). Eiden and colleagues previously used a month-wise approach when studying 215 urban pregnant females, but we have found no nationally representative month-wise estimates in any previously published article (9). In past research, samples for the trimester-specific approach generally have been limited to pregnant women, most often assessed perinatally and retrospectively across the months of pregnancy (10, 11). This approach might have introduced special threats to data validity when women have been told not to smoke during pregnancy. A US government report in 2009, based on the National Surveys on Drug Use and Health (NSDUH) data, constrained this threat to validity via the Audio Computer Assisted Self-Interview (ACASI) system described below, with samples including both non-pregnant and pregnant women, but the research team took the traditional trimester-specific approach to derive smoking estimates (6).
The present study pushes the field beyond these trimester-specific views in order to see what can be learned by studying tobacco smoking experience with a more fine-grained month-wise view of potential variation of estimates within trimesters. In our opinion, the use of a more fine-grained approach should add value to what has been published in the past. This work might stimulate new research with month-specific assessments in lieu of the more traditional trimester-specific assessments.
2. Materials and methods
The NSDUH Restricted-Data Analysis System (R-DAS), appropriately weighted for pooled 2002-2009 analyses, yield nationally representative sample survey estimates for the US non-institutionalized civilian population age 12 years and older, as described elsewhere (12). Briefly, each year, the NSDUH secures a new sample using a cross-sectional multi-stage area probability sampling survey research approach, with oversampling of subgroups (e.g., youth and young adults) in order to provide more precise weighted estimates for these subgroups. Generally, NSDUH survey response levels exceed 70%. In addition to applying weights to reflect the probability of selection and to adjust for non-response, post-stratification adjustment factors are used -- for example, to bring survey estimates into balance with US Census distributions for age, sex, and race-ethnicity (13). The NSDUH study protocol have been reviewed and approved by cognizant institutional review boards for protection of human subjects in research.
For this project on smoking in pregnancy, the pertinent NSDUH study sample encompasses females aged 12-44 years and an effective sample size of more than 70,000 women. However, some designated participants had missing or invalid responses to key study variables, reducing effective sample size to just under 70,000 women, with slightly more than 4,200 assessed during pregnancy. The numbers of pregnant women were roughly equal across months 2-8 of pregnancy (11%-13% of all pregnant females), whereas 7 % of pregnant women were 1 month pregnant and 7.4 % of pregnant women were 9 months pregnant.
NSDUH assessments involve the confidential ACASI system that covers drug use and other health characteristics in pre-worded and generally precoded standardized multi-item modules. The ACASI system provides a highly private means of responding to questions, designed to increase the level of honest reporting of sensitive conditions such as extra-medical drug use. The tobacco module precedes the pregnancy module by at least 10 minutes, which might promote accuracy and completeness of the smoking history relative to alternatives (e.g., versus an approach that asks initial items on pregnancy, immediately followed by smoking items).
The key response variable in this study is tobacco cigarette smoking in the 30 days prior to the self-report assessment date, based a question about smoking cigarettes “within the past 30 days”. NSDUH self-reports also assess pregnancy status and the month of pregnancy through these questions “Are you pregnant?” and “How many months pregnant?”. As for tobacco dependence, the R-DAS TD variable is based on a composite NDSS/TTFC TD index combining multi-item information from the Nicotine Dependence Syndrome Scale (NDSS) (14) and Fagerstrom's 'Time To First Cigarette' item (TTFC) (15), as described in an online appendix. Whereas some researchers have expressed concern about validity of smoking self-reports in pregnancy research (16, 17), in the national survey context there is evidence of acceptable validity and reliability (18).
R-DAS online data analysis software allows contingency table analyses, but no regression modeling or other advanced analysis approaches. Crude smoking estimates can be misleading when there are important imbalances in often correlated variables such as age. As explained in the appendix, in the current study we used an indirect age standardization approach devised by Breslow and Day (19), with non pregnant females 12-44 years of age specified as the referent population. All estimates are based on the R-DAS analysis weights for pooled data, with Taylor series linearization for variance estimation, given the complex study design.
3. Results
Among women age 12-44 years who are in the 1st month of pregnancy, the estimated prevalence of recently active tobacco cigarette smoking is 26.6% (see Table 1). This smoking prevalence estimate for Month 1 is not appreciably different from the estimate for all 12-44 year old non-pregnant women, 26.7% (95% CI = 26.4%, 27.1%, data not shown in a table). Evidence of a possible ameliorative effect of pregnancy on active smoking is not seen in Months 1-2, but can be seen in Table 1's estimate for women in Month 3 of pregnancy, when fewer than 20% are active smokers. There is no appreciable variation across subsequent months; the smallest estimate is seen among mothers in Month 9 (11.3%; Table 1).
Table 1.
Estimated prevalence of recently active smoking among pregnant women age 12-44 years, by month of pregnancy, before and after indirect age standardization. Data for the United States based on the R-DAS online analysis system of the National Surveys on Drug Use and Health, 2002-2009.
| Panel A (Point estimates) | ||||
|---|---|---|---|---|
| Observed Prevalence | Expected prevalence* | Prevalence ratio+ | Age standardized smoking ratio | |
| 1 month | 26.6% | Referent | Referent | Referent |
| 2 months | 24.5% | 26.1% | 0.9 | 0.9 |
| 3 months | 17.6% | 26.4% | 0.7 | 0.7 |
| 4 months | 13.1% | 25.9% | 0.5 | 0.4 |
| 5 months | 14.6% | 26.3% | 0.5 | 0.6 |
| 6 months | 14.5% | 25.8% | 0.5 | 0.6 |
| 7 months | 14.5% | 26.1% | 0.5 | 0.6 |
| 8 months | 13.7% | 25.6% | 0.5 | 0.5 |
| 9 months | 11.3% | 25.9% | 0.4 | 0.4 |
| Panel B (95% Confidence Intervals) | ||||
|---|---|---|---|---|
| Observed Prevalence (%) | Expected prevalence (%)* | Prevalence ratio+ | Age standardized smoking ratio | |
| 1 month | (21.6, 32.2) | Referent | Referent | Referent |
| 2 months | (20.7, 28.7) | (22.2, 30.4) | (0.7, 1.2) | (0.7, 1.2) |
| 3 months | (14.4, 21.4) | (22.6, 30.6) | (0.5, 0.9) | (0.5, 0.9) |
| 4 months | (10.5, 16.3) | (22.3, 29.8) | (0.4, 0.7) | (0.4, 0.7) |
| 5 months | (12.0, 17.8) | (22.8, 30.0) | (0.4, 0.7) | (0.4, 0.7) |
| 6 months | (11.5, 18.2) | (22.0, 30.2) | (0.4, 0.7) | (0.4, 0.7) |
| 7 months | (11.9, 17.5) | (22.7, 29.7) | (0.4, 0.7) | (0.4, 0.7) |
| 8 months | (11.0, 16.9) | (22.1, 30.0) | (0.4, 0.7) | (0.4, 0.7) |
| 9 months | (8.5, 14.9) | (21.7, 30.5) | (0.3, 0.6) | (0.3, 0.6) |
Expected values are based on age specific estimates from the referent population (Females 12-44 years in the first month of pregnancy categorized into 12-20, 21-30 and 31-44 age groups).
PR= Observed prevalence (%) among pregnant, divided by observed prevalence (%) among females in the first month of pregnancy with the CI derived through Wald method.
The other columns in Table 1 show expected prevalence for pregnant women if they had had age-specific smoking prevalence at levels seen for pregnant women in the 1st month, as well as the crude prevalence ratio, and the indirectly standardized comparison that holds age constant. Each element of evidence is consistent with reduced smoking prevalence before the end of the 1st trimester but after pregnancy months 1-2. [Interested readers may wish to view the online supplementary appendix table illustrating indirect age standardization, with an alternative reference population (i.e., non-pregnant women), leading to conclusions not appreciably different from those obtained in our primary analysis.]
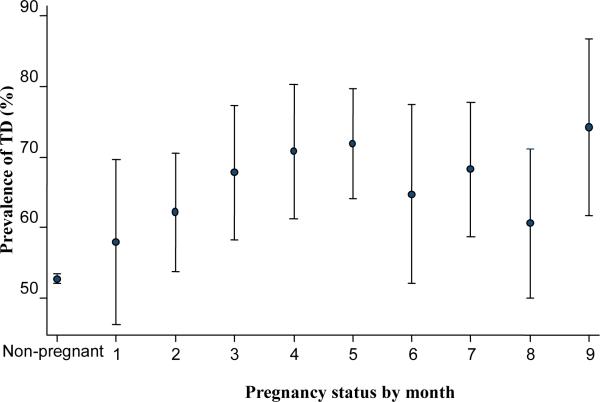
Figure 1 displays estimates for month-specific TD prevalence among smokers, based on the NDSS/TTFC approach, with TD found to affect just over one-half of non-pregnant 12-44 year old female smokers (52.7%), and with evidence that TD might be affecting smoking persistence in early pregnancy, as gauged by tangibly greater TD concentration among smokers in the later months of pregnancy (i.e., >60% prevalence from Month 3 onward). Upon seeing these estimates, we made an ex post facto contrast of pregnant smokers in Months 3-9 versus non-pregnant smokers, using a prevalence odds ratio (POR) approach explained in the online appendix, and found excess TD odds among the pregnant smokers in Months 3-9 (POR = 1.3; 95% CI = 1.2, 1.4), with no appreciable variation month-by-month among pregnant smokers. The appendix also provides estimates based on other TD case definitions that some TD experts might prefer to use; the results are not too distant from what can be seen in our primary analyses.
Figure 1.
Estimated month-specific tobacco dependence prevalence* for female smokers age 12-44 years in relation to pregnancy status. Data for the United States based on the R-DAS online analysis system of the National Surveys on Drug Use and Health, 2002-2009.
4. Discussion
Studying month-specific estimates of active smoking among non-pregnant and pregnant women who participated in very recent nationally representative surveys in the United States, we found evidence that an ameliorative effect of pregnancy might be occurring before the end of the first trimester, perhaps observable as early as Month 3 and as soon as a woman becomes aware that she is pregnant. We also discovered evidence of a possible tobacco dependence effect emerging earlier in pregnancy than we had anticipated, which might help explain why some pregnant smokers do not quit smoking. A noteworthy 11.3% smoking prevalence estimate was seen in Month 9, and this relatively low value might be explained by processes described later in this discussion.
Before any additional discussion of this evidence, several limitations of the study merit attention. The NSDUH study design is not longitudinal. It yields cross-sectional snapshots of non-pregnant women versus all women in the 1st month of pregnancy, and versus all women in each of the subsequent months of pregnancy. In addition, all study variables were by self-report (pregnancy month and recently active smoking), raising a possibility of measurement errors (16, 17), which can be addressed in future research via bio-assays to confirm pregnancy and recent smoking status (18). The main response variable, smoking in the past 30 days, did not fully capture all facets of smoking, and the NSDUH TD assessments are more akin to a screening approach versus a formal diagnostic approach. The restriction to simple R-DAS crosstabular analyses placed limits on study of confounding variables other than age. Finally, NSDUH assessments did not cover the timing of smoking cessation or counseling during pregnancy.
Notwithstanding limitations such as these, the study findings are of interest due to large nationally representative samples, with standardized independent modularized assessments of smoking, month by month, separated from later questions in a module about pregnancy month, and with no requirement for long-term recall and reporting, as is typical when post-partum mothers are asked to provide retrospective smoking reports over the entire span of pregnancy. These estimates have disclosed for the first time the month-specific prevalence of smoking during pregnancy in the US, with age-standardized comparisons to women in the first month of pregnancy, yielding three useful discoveries: (i) the possibly ameliorative pregnancy effect on smoking prevalence might emerge as early as Month 2 of a pregnancy; (ii) TD's effect on smoking persistence also might be seen quite early in pregnancy; (iii) smoking prevalence seemed to drop during the last month of a pregnancy. A comparison of the NSDUH estimates with corresponding estimates from recent birth certificates and the Pregnancy Risk Assessment Monitoring System (11) suggests that the NSDUH ACASI approach might be superior to other methods in terms of completeness and accuracy, although there is a remote possibility of NSDUH keystroke mistakes and subsequent misclassified smoker identification. The similarity in smoking prevalence estimate for 12-44 year old non-pregnant women and those in Month 1 pregnancy was perhaps because some of the smoking days occurred before the women in Month 1 knew that they had become pregnant (20). Moreover, the lower smoking prevalence of 11.3% in Month 9 of pregnancy might reflect greater misclassification of self-reports in that month (especially falsely negative reports), a smoking-attributable increased risk of pre-term birth (3, 21), or positive effects of mid-late pregnancy counseling or behavioral change intended to have dual benefits: (i) smoking cessation, and (ii) increased likelihood of planning for and initiation of breastfeeding (22). With the exception of the 11.3% value in Month 9, we learned that tobacco smoking prevalence among mothers-to-be did not vary appreciably across pregnancy months after the first trimester. It might well be that TD helped to account for the relative stability in these estimates. This study's observed TD prevalence values among pregnant smokers might foster more aggressive screening and outreach approaches for TD cases among pregnant women who have not quit before Month 3, as well as more general smoking cessation efforts along a continuum of services from pre-conception to post-partum (23). Similarly, whereas the relatively lower TD prevalence in the final months of pregnancy (Month 6-8) might reflect a TD-attributable increased risk of pre-term birth as noted (24), we acknowledge that the true explanation might be sampling variability, with no need for a speculation.
In conclusion, this study's month-specific estimates from nationally representative samples shed new light and add useful new epidemiological facts about smoking in pregnancy as an important aspect of women's health, drawing attention to a possible ameliorative effect of pregnancy on smoking prevalence, observable as early as Month 3, which cannot be seen when the traditional trimester approach is used. Most women in the US recognize their pregnancy and initiate prenatal care by the twelfth gestational week (20), which is consistent with our results. When we observe these active smoking estimates, month-by-month during pregnancy, as well as the relatively large TD prevalence among pregnant smokers, we are reminded of a need for more aggressive smoking cessation initiatives integrated with health services for women in the childbearing years. It should not be difficult to probe into the implications of these cross-sectional observations as part of ongoing prospective and longitudinal studies of pregnant women, within which comparative randomized controlled trials for smoking cessation are being conducted. Clearly, for the many smoking mothers-to-be who qualify as TD cases, there is need for specialist attention, with anticipated benefits and improvements in relation to the overall health of the mothers and their offspring (25).
Supplementary Material
ACKNOWLEDGMENTS
This work is completed during OA's first year of postdoctoral epidemiology fellowship, supported by the National Institute on Drug Abuse (T32DA021129) and JCA's NIDA Senior Scientist and Mentorship Award (K05DA015799), and by Michigan State University. The content is the sole responsibility of the authors and does not necessarily represent the official views of Michigan State University, the National Institute on Drug Abuse, or the National Institutes of Health. In addition, we would like to thank the United States Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality for sponsoring the National Surveys on Drug Use and Health and making the datasets available for public use to allow research of this nature.
ABBREVIATIONS
- CI
confidence interval
- NSDUH
National Surveys on Drug Use and Health
- R-DAS
implemented Restricted-use Data Analysis System
- FTND
Fagerstrom Test of Nicotine Dependence
References
- 1.Juarez SP, Merlo J. Revisiting the effect of maternal smoking during pregnancy on offspring birthweight: a quasi-experimental sibling analysis in Sweden. PLoS One. 2013;8(4):e61734. doi: 10.1371/journal.pone.0061734. PubMed PMID: 23616908. Pubmed Central PMCID: Pmc3629140. Epub 2013/04/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of fetal exposure to maternal smoking. Am J Public Health. 1994 Jul;84(7):1127–31. doi: 10.2105/ajph.84.7.1127. PubMed PMID: 8017537 Pubmed Central PMCID: Pmc1614741. Epub 1994/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C Embryo Today. 2008 Mar;84(1):1–15. doi: 10.1002/bdrc.20119. PubMed PMID: 18383133. Epub 2008/04/03. eng. [DOI] [PubMed] [Google Scholar]
- 4.Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking in pregnancy and risk for congenital malformations: results of a Danish register-based cohort study. Acta Obstet Gynecol Scand. 2014 May 23; doi: 10.1111/aogs.12433. PubMed PMID: 24861914. Epub 2014/05/28. Eng. [DOI] [PubMed] [Google Scholar]
- 5.Tominey E. Maternal Smoking During Pregnancy and Early Child Outcomes. Centre for Economic Performance, LSE; 2007. Contract No.: dp0828. [Google Scholar]
- 6.United States. Department of Health and Human Services . The NSDUH Report: Substance Use among Women During Pregnancy and Following Childbirth. Rockville, MD: Substance Abuse and Mental Health Services Administration. Office of Applied Studies. p. 2009. [Google Scholar]
- 7.Muhuri PK, Gfroerer JC. Substance use among women: associations with pregnancy, parenting, and race/ethnicity. Matern Child Health J. 2009 May;13(3):376–85. doi: 10.1007/s10995-008-0375-8. PubMed PMID: 18566878 Epub 2008/06/21. eng. [DOI] [PubMed] [Google Scholar]
- 8.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990 May;80(5):541–4. doi: 10.2105/ajph.80.5.541. PubMed PMID: 2327529. Pubmed Central PMCID: Pmc1404636. Epub 1990/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiden R, Homish G, Colder C, Schuetze P, Gray T, Huestis M. Changes in Smoking Patterns During Pregnancy. Substance Use & Misuse. 2013. 2013;48(7):513–22. doi: 10.3109/10826084.2013.787091. PubMed PMID: WOS:000318433000006. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao G, Ford ES, Tsai J, Li C, Ahluwalia IB, Pearson WS, et al. Trends in health-related behavioral risk factors among pregnant women in the United States: 2001-2009. J Womens Health (Larchmt) 2012 Mar;21(3):255–63. doi: 10.1089/jwh.2011.2931. PubMed PMID: 22047097. Epub 2011/11/04. eng. [DOI] [PubMed] [Google Scholar]
- 11.Tong VT, Dietz PM, Farr SL, D'Angelo DV, England LJ. Estimates of smoking before and during pregnancy, and smoking cessation during pregnancy: comparing two population-based data sources. Public Health Rep. 2013 May-Jun;128(3):179–88. doi: 10.1177/003335491312800308. PubMed PMID: 23633733. Pubmed Central PMCID: Pmc3610070. Epub 2013/05/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seedall RB, Anthony JC. Risk estimates for starting tobacco, alcohol, and other drug use in the United States: male-female differences and the possibility that ‘limiting time with friends’ is protective. Drug Alcohol Depend. 2013 Dec 1;133(2):751–3. doi: 10.1016/j.drugalcdep.2013.06.035. PubMed PMID: 24018318. Epub 2013/09/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States. Substance Abuse and Mental Health Services Administration . Results from the 2011 NSDUH: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. [Google Scholar]
- 14.Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob Res. 2004 Apr;6(2):327–48. doi: 10.1080/1462220042000202481. PubMed PMID: 15203807. Epub 2004/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 15.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007 Nov;9(Suppl 4):S555–70. doi: 10.1080/14622200701673480. PubMed PMID: 18067032. Pubmed Central PMCID: 2933747. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn't enough. Nicotine Tob Res. 2004 Apr;6(Suppl 2):S141–51. doi: 10.1080/14622200410001669141. PubMed PMID: 15203817. Epub 2004/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 17.England LJ, Grauman A, Qian C, Wilkins DG, Schisterman EF, Yu KF, et al. Misclassification of Maternal Smoking Status and its Effects on an Epidemiologic Study of Pregnancy Outcomes. Nicotine & Tobacco Research. 2007;9(10):1005–13. doi: 10.1080/14622200701491255. [DOI] [PubMed] [Google Scholar]
- 18.Harrison LD, Martin SS, Enev T, Harrington D. Comparing drug testing and self-report of drug use among youths and young adults in the general population. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2007. [Google Scholar]
- 19.Breslow NE, Day NE. Indirect standardization and multiplicative models for rates, with reference to the age adjustment of cancer incidence and relative frequency data. J Chronic Dis. 1975 Jun;28(5-6):289–303. doi: 10.1016/0021-9681(75)90010-7. PubMed PMID: 1141424. eng. [DOI] [PubMed] [Google Scholar]
- 20.Ayoola AB, Nettleman MD, Stommel M, Canady RB. Time of pregnancy recognition and prenatal care use: a population-based study in the United States. Birth. 2010 Mar;37(1):37–43. doi: 10.1111/j.1523-536X.2009.00376.x. PubMed PMID: 20402720. eng. [DOI] [PubMed] [Google Scholar]
- 21.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004 Apr;6(Suppl 2):S125–40. doi: 10.1080/14622200410001669187. PubMed PMID: 15203816. Epub 2004/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 22.Wambach KA, Aaronson L, Breedlove G, Domian EW, Rojjanasrirat W, Yeh HW. A randomized controlled trial of breastfeeding support and education for adolescent mothers. West J Nurs Res. 2011 Jun;33(4):486–505. doi: 10.1177/0193945910380408. PubMed PMID: 20876551. Epub 2010/09/30. eng. [DOI] [PubMed] [Google Scholar]
- 23.Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology, and maternal smoking during pregnancy. Am J Public Health. 2008 Mar;98(3):448–53. doi: 10.2105/AJPH.2006.102772. PubMed PMID: 17600245. Pubmed Central PMCID: Pmc2253564. Epub 2007/06/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickstrom R. Effects of Nicotine During Pregnancy: Human and Experimental Evidence. Curr Neuropharmacol. 2007 Sep;5(3):213–22. doi: 10.2174/157015907781695955. PubMed PMID: 19305804. Pubmed Central PMCID: 2656811. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012 Nov;55(Suppl:S33-40) doi: 10.1016/j.ypmed.2011.12.016. PubMed PMID: 22227223. Pubmed Central PMCID: Pmc3399924. Epub 2012/01/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.