Abstract
Cocaine blocks plasma membrane monoamine transporters and increases extracellular levels of dopamine (DA), norepinephrine (NE), and serotonin (5-HT). The addictive properties of cocaine are mediated primarily by DA, while NE and 5-HT play modulatory roles. Chronic inhibition of dopamine β-hydroxylase (DBH), which converts dopamine (DA) to norepinephrine (NE), increases the aversive effects of cocaine and reduces cocaine use in humans, and produces behavioral hypersensitivity to cocaine and D2 agonism in rodents, but the underlying mechanism is unknown. We found a decrease in β-arrestin2 (βArr2) in the nucleus accumbens (NAc) following chronic genetic or pharmacological DBH inhibition, and overexpression of βArr2 in the NAc normalized cocaine-induced locomotion in DBH knockout (Dbh −/−) mice. The D2/3 agonist quinpirole decreased excitability in NAc medium spiny neurons (MSNs) from control, but not Dbh −/− animals, where instead there was a trend for an excitatory effect. The Gαi inhibitor NF023 abolished the quinpirole-induced decrease in excitability in control MSNs, but had no effect in Dbh −/− MSNs, whereas the Gαs inhibitor NF449 restored the ability of quinpirole to decrease excitability in Dbh −/− MSNs, but had no effect in control MSNs. These results suggest that chronic loss of noradrenergic tone alters behavioral responses to cocaine via decreases in βArr2 and cellular responses to D2/D3 activation, potentially via changes in D2-like receptor G protein coupling in NAc MSNs.
Keywords: norepinephrine, dopamine, cocaine, dopamine β-hydroxylase, D2 receptor, mice
Introduction
Dopamine β-hydroxylase (DBH) is the enzyme that converts dopamine (DA) to norepinephrine (NE) in noradrenergic neurons, thereby controlling NE production and the DA/NE ratio (Weinshilboum, 1978). DBH is of clinical interest in cocaine dependence because (1) polymorphisms in the human DBH gene that are associated with reduced serum DBH enzymatic activity lead to greater cocaine-induced paranoia (Cubells et al., 2000; Kalayasiri et al., 2007), and (2) inhibition of DBH by the alcoholism medication, disulfiram, or the selective DBH inhibitor, nepicastat (Kapoor et al., 2011; Stanley et al., 1997), alters the subjective effects of cocaine and reduces cocaine use in humans (Gaval-Cruz and Weinshenker, 2009; Stanley et al., 1997) (K. Cunningham, personal communication). Genetic (DBH knockout; Dbh −/−) or pharmacological (disulfiram, nepicastat) DBH inhibition produces hypersensitivity to cocaine-induced locomotion, stereotypy, place preference, and place aversion in mice; it also enhances the discriminitive stimulus effects of cocaine and attenuates cocaine-, cue-, and stress-induced reinstatement of cocaine seeking in rats (Gaval-Cruz et al., 2012; Manvich et al., 2013; Schank et al., 2006; Schroeder et al., 2013; Schroeder et al., 2010)
Because Dbh −/− mice are hypersensitive to the D2/3 agonist, quinpirole, but not the D1 agonist, SKF81297, cocaine hypersensitivity would appear to be mediated by alterations in the D2 pathway (Schank et al., 2006; Weinshenker et al., 2002). These phenotypes are likely driven by compensatory responses in DA signaling following the chronic decrease in extracellular DA availability when noradrenergic excitatory drive on the mesocorticolimbic system is missing. We initially reported an increase in the abundance of high-affinity state D2 receptors in the striatum of Dbh −/− mice, which could explain the cocaine and D2 hypersensitivity (Schank et al., 2006). However, subsequent work failed to confirm this finding (Skinbjerg et al., 2010), suggesting a contribution from downstream signaling molecules. Indeed, the behavioral alterations in Dbh −/− mice were accompanied by a rise in striatal pERK and ΔFosB protein levels (Rommelfanger et al., 2007).
The goals of the present study were to determine the molecular and cellular mechanisms behind the D2- and psychostimulant-induced hypersensitivity that follow chronic DBH inhibition. First, we found a decrease of β-arrestin2 (βArr2), a protein involved in D2 desensitization and signaling (Beaulieu and Gainetdinov, 2011), in the NAc of Dbh −/− mice and mice treated chronically with nepicastat. We next used viral-mediated overexpression to determine whether increasing βArr2 levels in the NAc could normalize cocaine-induced behavior in Dbh −/− mice. Finally, we assessed electrophysiological responses to quinpirole in MSNs from the NAc of control and Dbh −/− mice in the presence and absence of Gαi and Gαs inhibitors.
Materials and methods
Animals
Adult control (Dbh +/−) and Dbh−/− mice were generated as previously described (Schank et al., 2006; Thomas et al., 1998). Dbh −/− males were bred to Dbh +/− females. Pregnant Dbh +/− mice were given the AR agonists isoproterenol and phenylephrine (20 μg/ml each) + vitamin C (2 mg/ml) from E9.5-E14.5, and L-3,4-dihydroxyphenylserine (DOPS; 2 mg/ml + vitamin C 2 mg/ml) from E14.5-birth in their drinking water to rescue the embryonic lethality associated with the homozygous Dbh −/− mutation. Because of this treatment, NE and epinephrine were present in both Dbh −/− animals before but not after birth. They were maintained on a mixed C57BL/6J and 129SvEv background and group-housed, and food and water were available ad libitum throughout the course of the study. Both sexes were used due to the extreme measures required to breed sufficient numbers of knockout mice for the experiments (Thomas et al., 1998; Thomas et al., 1995). Comparable numbers of male and female knockouts were used for each experiment, and sex-matched Dbh +/− littermates were used as controls. Although the studies were not powered sufficiently to rigorously detect sex differences, no obvious ones were observed. The Dbh +/−mice were used as controls because their brain catecholamine levels and behavior is indistinguishable from wild-type (Dbh +/+) mice (Bourdelat-Parks et al., 2005; Mitchell et al., 2006; Thomas et al., 1998). Some wild-type C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were also used as controls for the electrophysiology experiments.
All animals were treated in accordance with the National Institutes of Health (NIH) Intramural Animal Care and Use Program guidelines. The experiments described in this article followed the UTSA and Emory University Division of Animal Resources’ Guide for the Care and Use of Laboratory Animals and were approved by the UTSA and Emory Institutional Animal Care and Use Committee.
Chronic nepicastat treatment
Nepicastat was administered to Dbh +/− mice via daily i.p. injections (western blots) or osmotic minipumps (locomotor activity). For the i.p. administration, Dbh +/− mice received vehicle or nepicastat (50 mg/kg, i.p. × 3, each injection spaced 2 h apart) for 5 consecutive days. This dosing regimen reduces brain NE levels by ~ 75% and produces cocaine hypersensitivity (Gaval-Cruz et al., 2012). Mice were euthanized by CO2 asphyxiation 11 days later, and their brains were removed, dissected on ice, and stored at −80°C. For the minipump administration, nepicastat was dissolved in 50% saline and 50% DMSO and loaded into Alzet osmotic minipumps (Model #2004, 0.25μL/hour, 28 days; Durect, Cupertino, CA) to achieve a dose of 50 mg/kg/d. All pumps were placed in a sterile 37°C saline bath for 1 d before implantation. Mice were anesthetized with isoflurane, and minipumps implanted in the intraperitoneal cavity. Buprenorphine (2.5mg/kg, s.c.) was given immediately after surgery. Cocaine-induced locomotion was recorded 21 d after pump implantation.
Locomotor recordings
Mice were placed in locomotion recording chambers (transparent Plexiglas cages placed into a rack with 7 infrared photobeams spaced 5 cm apart; San Diego Instruments Inc., La Jolla, CA) and allowed to habituate for 30 min before receiving a single injection of cocaine (10 or 15 mg/kg, i.p.). Novelty-induced locomotion was defined as ambulations during the first 10 min of the habituation period. Ambulations (consecutive beam breaks) were recorded for an additional 1–2 h following drug administration.
Western blotting
Mouse brain tissue was homogenized in 500 μl harvest buffer (10 mM HEPES, 50 mM NaCl, 5 mM EDTA, pH 7.4, supplemented with protease inhibitors) using a sonicator. Laemmli sample buffer containing SDS, β-mercaptoethanol, glycerol, Tris-Cl, and bromophenol blue was added to samples after measuring protein concentrations with a BCA Assay (Thermo Fisher Scientific, Rockford, IL). Samples were resolved by SDS-PAGE on 4–20% Tris-Glycine precast gels followed by transfer to nitrocellulose membranes. Following transfer, membranes were incubated with Ponceau staining to assess even protein loading, then rinsed with distilled water. Membranes were then incubated in blocking buffer (10 mM HEPES, 50 mM NaCl, 1% Tween-20, 2% dry milk, pH 7.4, for most antibodies; (1X TBS, 0.1% Tween-20 with 5% w/v nonfat dry milk, for pAKT, GSK3β, and pGSK3β) for 30 min, and then incubated with primary antibody overnight at 4°C. The primary incubation buffer was the same as blocking buffer for all antibodies except pAKT, GSK3β, and pGSK3β. For these, the primary incubation buffer was 1X TBS, 0.1% Tween-20 with 5% BSA. The membranes were washed 3 times in blocking buffer and incubated with either a fluorescent (1:10000) or HRP-conjugated secondary (1:4000) antibody (Invitrogen, Carlsbad, CA) for 30 min, washed more times, and then visualized using either the Odyssey imaging system (Li-Cor) or via ECL reagent (Thermo Fisher Scientific, Rockford, IL), followed by exposure to film. Membranes were stripped for 20 min at 37°C and 10 min at room temperature with stripping buffer and re-probed for α-actin to confirm equal loading of samples. Blots were analyzed by densitometry using Image J Software. A mean density value was calculated for the “control” group (i.e. Dbh +/− mice were the control for Dbh −/− mice, vehicle was the control for nepicastat, etc), and data were expressed as % control.
Antibodies
The antibodies used and their working dilutions were as follow: βArr2 (anti-rabbit; 1:2500; Cell Signaling Technology, Danvers, MA, CS3857); α-actin (anti-mouse; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, SC58671); pAkt-Ser473 (anti-rabbit; 1:1000; Cell Signaling CS9271); Akt (anti-mouse; 1:500; Santa Cruz Biotechnology; SC5298); pGSK3β-Ser9 (anti-rabbit; 1:1000; Cell Signaling CS9322); pGSK3β (anti-rabbit; 1:1000; Cell Signaling CS9315); fosB (anti-rabbit; 1:1000; Cell Signaling CS2551); FosB (anti-rabbit; 1:1000; Cell Signaling CS9890).
β-arrestin2 viral vectors
The original βArr2 plasmid (rat sequence) was obtained from Sudha Shenoy in the lab of Dr. Robert Lefkowitz. The Duke Neurotransgenic Laboratory then removed the βArr2 open reading frame, and the insert was cloned into a pCMVShuttle plasmid (AdEasy System, Stratagene, Santa Clara, CA, USA). The AdEasy βArr2 recombinant plasmid was generated per Stratagene instructions, and the βArr2 adenoviral vector was expanded and purified. The viruses were harvested with a titer of 2×1012/μL (βArr2) and 5×109/μL (GFP control).
β-arrestin2 viral infusions
Mice (n = 16 for each treatment group: βArr2 overexpression adenovirus and GFP adenovirus) were anesthetized using isoflurane and placed in a stereotaxic frame with a nose bar. The animal’s scalp was opened and bregma and lambda aligned to flat-skull position. The stereotaxic arm was then lowered to the NAc core. The core subregion was chosen because it has been implicated in cocaine-induced locomotion and behavioral sensitization to cocaine. The anteroposterior (AP) and mediolateral (ML) coordinates of the NAc core in relation to bregma were AP = 1.4 mm, ML= ± 1.0 mm, and a small hole was drilled in the skull at these coordinates. A 5-μl Hamilton microsyringe was lowered to target the NAc core (dorsoventral coordinate = −4.2 mm). The 26-gauge beveled tip of the Hamilton needle was precoated with 2% anti-bovine serum (BSA) prior to loading the virus to prevent molecular interactions between the syringe and the viral vectors. Animals received 1 μl of virus per side, injected at a rate of 0.2 μl/min, and the needle remained in place for 5 min after the injection and removed slowly. The skin was glued together using Vetbond tissue glue. All animals received meloxicam (0.5 mg/kg) for post-operative pain and water/liquid ibuprofen (0.1 mg/ml).
Ten days after the infusion of βArr2 overexpression and GFP control vectors, all mice were placed in locomotor chambers, and their basal locomotion was recorded for 30 min before receiving an injection of cocaine (15 mg/kg, i.p.), and cocaine-induced locomotion was recorded for 2 h. Mice were anesthetized and transcardially perfused with saline and 4% paraformaldehyde 24–48 h later, their brains removed, stored in 4% paraformaldehyde for 4 days, and then transferred to 30% sucrose. Brains were sectioned and stained with antibodies against GFP or βArr2, and expression in the NAc was assessed. Three mice that received the βArr2 virus and 2 mice that received GFP virus were removed from the analysis due to incorrect placement of viral infusion.
Electrophysiological recordings of nucleus accumbens neurons
C57BL/6J, Dbh +/−, and Dbh −/− mice were used for electrophysiological recordings. C57BL/6J mice were used to (1) confirm that Dbh +/− mice NAc MSNs were similar to wild-type NAc MSNs, and (2) increase the number of cells in a few experiments when not enough appropriately sex- and age-matched Dbh +/− control animals were available. Mice were anesthetized with a lethal dose of isoflurane and decapitated. The brains were quickly removed and placed into an ice-cold, oxygenated cutting solution containing (in mM): 110 choline Cl, 2.5 KCl, 1.25 NaH2PO4, 4 MgCl2, 2 CaCl2, 10 dextrose, 25 NaHCO3, 1.3 ascorbic acid, 2.4 sodium pyruvate, and 0.05 glutathione. Parasagittal brain slices containing the nucleus accumbens (250 μm) were cut using a vibrating tissue slicer (Microm HM 650V). The slices were then transferred to an incubation chamber containing warm (35°C) artificial cerebral spinal fluid (ACSF) for 1 h prior to recordings, and then stored at room temperature. The slices were transferred to a recording chamber for the experiments, where they were submerged in oxygenated ACSF. The ACSF was equilibrated with 95% O2–5% CO2, had a pH of 7.2 and contained (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, 10 dextrose, 25 NaHCO3, 1.3 ascorbic acid, and 2.4 sodium pyruvate. The slices were superfused with 34–36°C ACSF at a rate of 2 ml/min.
The cells were visualized using gradient contrast illumination through a 40X water-immersion lens attached to an Olympus BX51 (Olympus) upright microscope. Patch pipettes were pulled from borosilicate glass (o.d. – 1.5 mm, i.d. – 0.84 mm) using a P-97 Flaming/Brown electrode puller (Sutter Instruments). Pipettes were filled with a solution containing (in mM): 138 K-gluconate, 10 HEPES, 0.0001 CaCl2, 0.2 EGTA, 4 NaATP, 0.4 NaGTP, 2 MgCl2. with an osmolarity of 270–275 mOsm and adjusted to a pH of 7.3 with KOH. Recordings were made using a MultiClamp 700B amplifier (Molecular Devices). Signals were digitized at 15–30 kHz and saved to a hard drive for analysis using the software program, AxoGraph X (AxoGraph Scientific).
Spiny neurons in the NAc core were identified as having the following properties: a hyperpolarized membrane potential (< − 70 mV), a low input resistance (< 350 MΩ), and delayed spiking upon current injection. Drugs were applied to the slice by superfusion at the indicated concentration. All experiments were performed in the presence of: 5 μM NBQX (AMPA antagonist), 25 μM D-APV (NMDA antagonist), 100 μM picrotoxin (GABAa antagonist), and 10 μM SCH 23390 (D1 antagonist). The drug NF023 (10 μM) was applied internally. For NF 449 (1 μM) application, the slices were incubated in the Gαs antagonist for 1 h prior to recording, and then continuously exposed to NF449 throughout the recording process. All drugs were obtained from Tocris Bioscience (Bristol, UK) or Sigma-Aldrich (St. Louis, MO). In current-clamp configuration, current was injected for 200 ms at 100-pA step intervals (100–500 pA) with 5 s between each pulse, until the cell was depolarized and spikes were evoked. An input/output curve was obtained under baseline conditions before and after superfusing 10 ml of a 5 μM solution of quinpirole for approximately 5 min. Action potentials were detected using an amplitude threshold, and spike frequency was calculated as the reciprocal of the inter-spike interval.
Statistical analysis
Western blot data were analyzed by t-test using GraphPad Prism 6.0 for Macintosh. Behavioral data were analyzed by two-way repeated measures ANOVA (RMANOVA), followed by Bonferroni posthoc tests, where appropriate, using Prism. Electrophysiological data were analyzed by RMANOVA with a generalized estimating equation (GEE). In our data, the number of observations differed between different current steps across cells. Due to the unbalanced design, the classic RMANOVA was therefore not an appropriate test. We used the RMANOVA with a GEE approach with an exchangeable correlation structure to take into account the unbalanced design, as well as correlated observations. For each test, GEE uses a robust test (Wald Chi-squared test based on robust variance estimators) for each effect. These analyses were performed using R (www.r-project.org).
Results
Dbh −/− mice have decreased β-arrestin2 in the nucleus accumbens
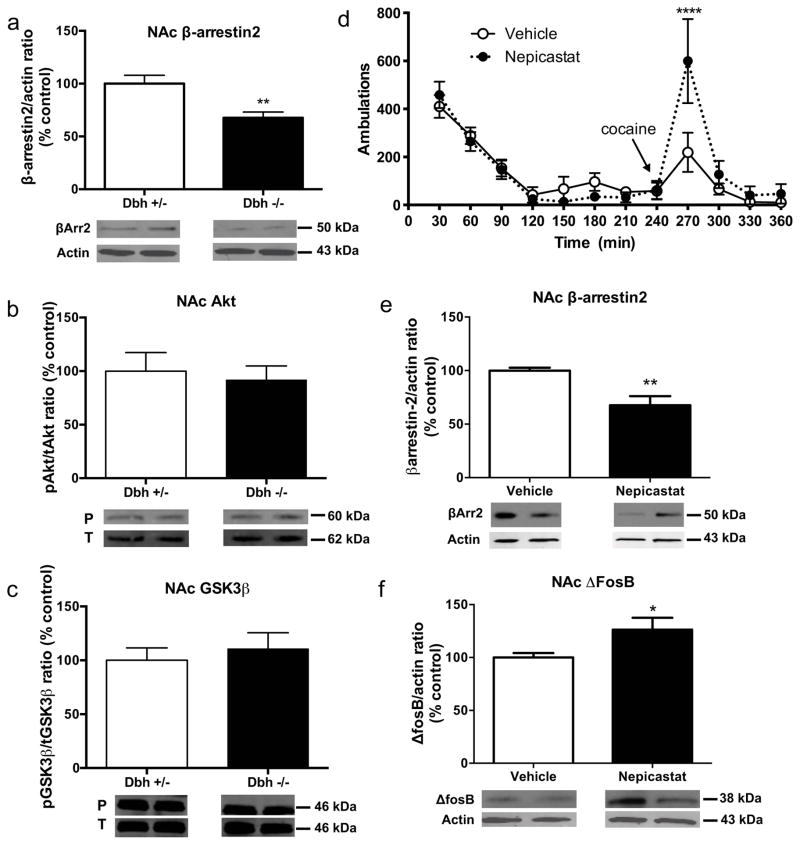
We showed previously that ΔFosB, which is induced in the NAc by chronic drug exposure and is known to promote psychostimulant-induced behaviors (Kelz et al., 1999), is elevated in the striatum of drug-naïve Dbh −/− mice (Rommelfanger et al., 2007). As part of a larger survey to identify potential upstream mediators of the cocaine hypersensitivity that follows chronic DBH inhibition, we found that Dbh −/− mice had significantly less βArr2 in the NAc (t6 = 3.493, p < 0.05) (Fig. 1a). Besides its role in G-protein-coupled receptor desensitization, βArr2 can signal through a protein kinase B/glycogen synthase kinase-3β (Akt/GSK3β) pathway (Del’guidice et al., 2011); however, we detected no genotype differences in the proportion of phosphorylated Akt and GSK3β proteins compared to the total protein levels when comparing Dbh −/− mice to control Dbh +/− mice (Fig. 1b, 1c).
Figure 1. Behavioral and neurochemical phenotypes of mice with chronic DBH deficiency.
Western blot data (mean ± SEM above, representative blot below) for (a) βArr2:actin ratio, (b) protein kinase B (Akt; phospho:total ratio) and (c) glycogen synthase kinase-3β (GSK-3β; phosphor:total ratio) in the nucleus accumbens (NAc) of Dbh +/− and Dbh −/− mice (n = 8 per group). *p < 0.05 compared to Dbh +/− mice. (d) Cocaine-induced (10 mg/kg, i.p.) locomotion, (e) βArr2:actin ratio, and (f) ΔFosB:actin ratio in the NAc of Dbh +/− control mice treated chronically with the selective DBH inhibitor nepicastat. *p < 0.05, **p < 0.01, ****p < 0.0001 compared to vehicle.
Nepicastat-treated mice are hypersensitive to cocaine and have decreased β-arrestin2 and increased ΔFosB in the nucleus accumbens
We next determined whether the cocaine hypersensitivity observed in Dbh −/− mice could be mimicked by chronic pharmacologic DBH inhibition in control mice. Dbh +/− mice with normal NE content that received chronic nepicastat (via osmotic minipump or daily i.p. injections) had no change in locomotion induced by a novel environment, but displayed increased cocaine-induced stereotypy (Gaval-Cruz et al., 2012) and/or locomotion (Fig. 1d), reminiscent of Dbh −/− mice (Gaval-Cruz et al., 2012; Schank et al., 2006; Weinshenker et al., 2002). Two-way ANOVA revealed a main effect of time (F11,110 = 17.55, p < 0.0001) and a treatment x time interaction (F11,110 = 2.64, p < 0.01). Posthoc tests showed that peak cocaine-induced locomotion was significantly enhanced by chronic nepicastat administration. Acute DBH inhibition, in contrast, does not augment cocaine responses and can even inhibit them (Haile et al., 2003; Maj et al., 1968; Schroeder et al., 2013). These results indicate that the hypersensitivity to psychostimulants seen in Dbh −/− mice cannot be attributed to developmental alterations produced solely by DBH knockout, but likely result from downstream changes in the signaling pathways that occur following prolonged deficits in NE.
Because Dbh −/− mice have decreased βArr2 in the NAc and increased ΔFosB in the striatum, we measured the relative levels of these proteins in the NAc of control mice following chronic treatment with nepicastat. We found that nepicastat-treated mice had decreased βArr2 (t14 = 3.49, p < 0.01; Fig. 1e) and increased ΔFosB (t14 = 2.69, p < 0.05; Fig. 1f), confirming that genetic and pharmacological inhibition of NE synthesis produce similar alterations in DA signaling proteins in the ventral striatum.
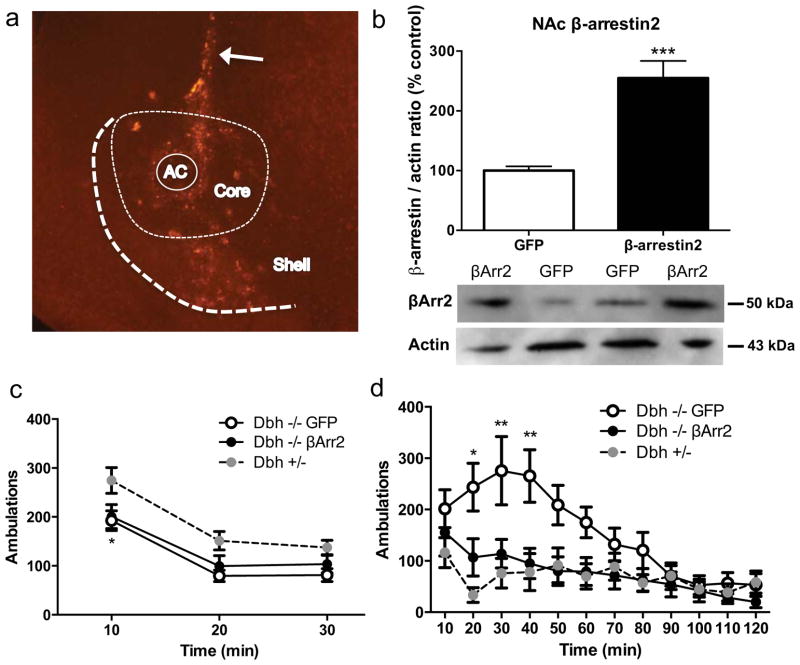
Overexpression of β-arrestin2 in the nucleus accumbens reverses cocaine hypersensitivity in Dbh −/− mice
While ΔFosB is induced in the NAc by chronic drug exposure and is known to promote psychostimulant-induced behaviors (Kelz et al., 1999), the role of βArr2 is less clear. To determine whether the decreased βArr2 in the NAc of mice with chronic NE deficiency contributes to their behavioral hypersensitivity to cocaine, we overexpressed green fluorescent protein (GFP) or βArr2 in the NAc of Dbh −/− mice using adenoviral vectors and assessed novelty- and cocaine-induced locomotor activity. High levels of βArr2 immunoreactivity were evident along the needle track and in both the core and shell subregions (Fig. 2a) and βArr2 protein levels were doubled in the Dbh −/− NAc as assessed by western blot (Fig. 2b) 7–10 days following viral vector injection, indicating that βArr2 overexpression was achieved and that the antibody we used was detecting βArr2. βArr2 overexpression had no effect on the reduced novelty-induced locomotor activity of Dbh −/− mice (Fig. 2c). Two-way ANOVA revealed a main effect of time (F2,66 = 99.44, p < 0.0001) and genotype (F2,33 = 4.46, p < 0.05). Posthoc tests showed that Dbh −/− mice overexpressing GFP or βArr2 had decreased novelty-induced locomotion compared to Dbh +/− controls with normal NE content at the 10-min time point. As expected, Dbh −/− mice that were infused with the GFP virus were hypersensitive to cocaine-induced locomotion compared to mice with normal NE content. By contrast, overexpression of β Arr2 in the NAc core of Dbh −/− mice completely normalized their cocaine response (Fig. 2d). Two-way ANOVA revealed a main effect of time (F11,286 = 9.62, p < 0.0001), genotype (F2,26 = 5.20, p < 0.05), and a time x genotype interaction (F22,286 = 2.88, p < 0.0001). Posthoc tests showed that Dbh −/− mice overexpressing GFP displayed increased locomotion compared with Dbh +/− mice and Dbh −/− overexpressing βArr2 at the 20-, 30-, and 40-min time points following cocaine administration, whereas there were no apparent differences between Dbh +/− mice and Dbh −/− mice overexpressing βArr2 in the NAc at any time point. These results suggest that the cocaine hypersensitivity conferred by chronic DBH inhibition is mediated, at least in part, by reduced βArr2 levels in the NAc.
Figure 2. β-arrestin2 overexpression in the nucleus accumbens restores normal cocaine sensitivity to Dbh −/− mice.
(a) Representative picture of βArr2 overexpression in the NAc of a Dbh −/− mouse that received the βArr2 virus. (b) Western blot data (mean ± SEM above, representative blot below) for βArr2:actin ratio in the NAc of Dbh −/− mice that received GFP virus (n = 6) or βArr2 virus (n = 7). ***p < 0.001 compared to Dbh −/− with GFP virus. (c) Novelty-induced (drug-free state) and (d) cocaine-induced (15 mg/kg, i.p., administered after 30 min in chamber) locomotor activity in Dbh +/− mice (n = 8), Dbh −/− mice that received GFP virus (n = 10), and Dbh −/− mice that received βArr2 virus (n = 11). *p < 0.05, **p < 0.01 compared to Dbh +/− controls. AC, anterior commissure; Core, NAc core; Shell, NAc shell; arrow, needle track.
Dbh −/− nucleus accumbens medium spiny neurons have aberrant responses to quinpirole
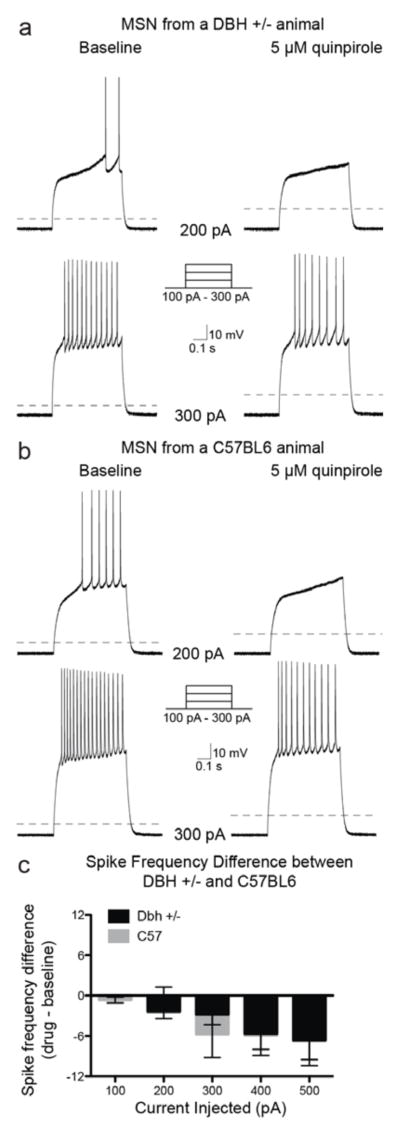
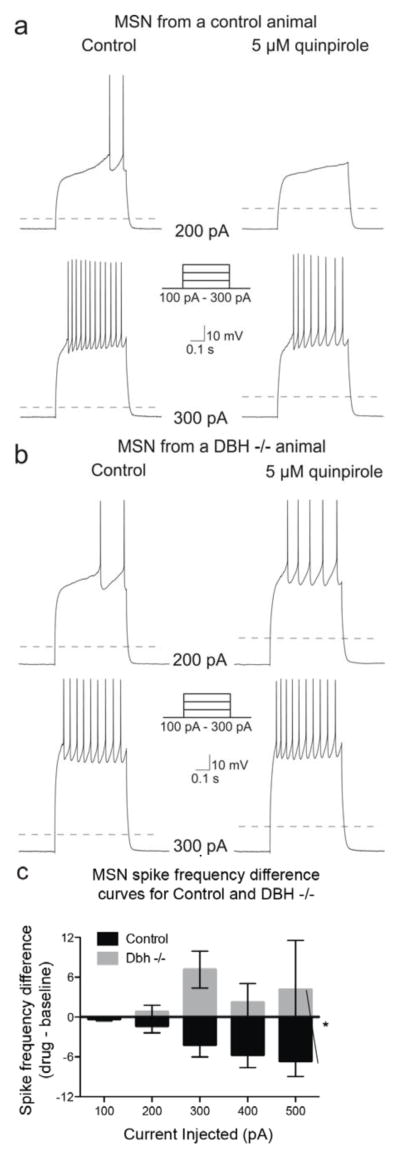
To uncover the cellular underpinnings of the D2 and cocaine hypersensitivity following chronic DBH inhibition, we measured the spike frequency of trains of action potentials elicited by the injection of current steps in NAc MSNs both at baseline and following bath application of quinpirole (5 μM) from control and Dbh −/− mice. The control group consisted of both Dbh +/− and wild-type C57Bl/6J mice since results comparing baseline and quinpirole responses in these groups were not significantly different (n = 22 Dbh −/−, 24 C57Bl/6J; Chi-square (df = 1) = 0.1, p = 0.75) (Fig. 3). We found no genotype differences between control and Dbh −/− mice in baseline firing rate in untreated MSNs (F4, 160 = 0.43; p = 0.79; data not shown), and activation of D2 receptors by quinpirole did not significantly change the input resistance or the resting membrane potential in either control or Dbh −/− mice (control ΔRin = 5.84 ± 4.28 MΩ, Dbh −/− ΔRin = 6.57 ± 8.55 MΩ, p = 0.54; control ΔVrest = −0.71 ± 1.80 mV, Dbh −/− ΔVrest = −1.36 ± 1.04 mV, p = 0.43). As reported previously and expected for a Gαi/o-coupled receptor (Perez et al., 2006; Surmeier and Kitai, 1993; Yasumoto et al., 2002; Zamponi and Snutch, 1998), activation of D2 receptors by quinpirole (5 μM) reduced evoked mean spike frequency in MSNs from control animals (Chi-square (df = 1) = 5.2, p = 0.02) (Fig. 4a, 4c). However, the D2-mediated reduction in MSN excitability seen in control mice was absent in MSNs recorded from Dbh −/− mice. Instead, quinpirole tended to have an excitatory effect in the knockout neurons, but it did not quite reach significance (Chi-square (df = 1) = 3.5, p = 0.06) (Fig. 4b, 4c). There was a highly significant difference between the spike frequency difference curves between MSNs from controls compared to MSNs from Dbh −/− animals (Chi-square (df = 1) = 20.99, p < 0.0001) (Fig. 4c), indicating the excitability of MSNs is increased in Dbh −/− animals compared to controls.
Figure 3. Activation of D2 receptors similarly inhibits evoked firing of NAc MSNs from Dbh +/− and C57BL/6J mice.
Example traces of a MSN from a Dbh +/− (a) and a wild-type C57BL/6J (b) animal at baseline and following bath application of the D2/3 agonist, quinpirole (5 μM), while action potentials were evoked with a series of current steps. Dashed line indicates −70 mV. (c) Population data (mean ± SEM) showing the effects of D2 receptor activation on spike frequency differences (quinpirole – baseline) from cells recorded from Dbh +/− (n = 22) and C57BL/6J (n = 24) mice.
Figure 4. Quinpirole inhibits evoked firing of NAc MSNs from control, but not Dbh −/− mice.
Example traces of a MSN from a control (a) and Dbh −/− (b) animal at baseline, and following bath application of the D2/3 agonist, quinpirole (5 μM), while action potentials were evoked with a series of current steps. Dashed line indicates −70 mV. (c) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole – baseline) from cells recorded from control (n = 46) and Dbh −/− (n = 15) mice. * indicates significant difference (p < 0.05) between control and Dbh −/− population data.
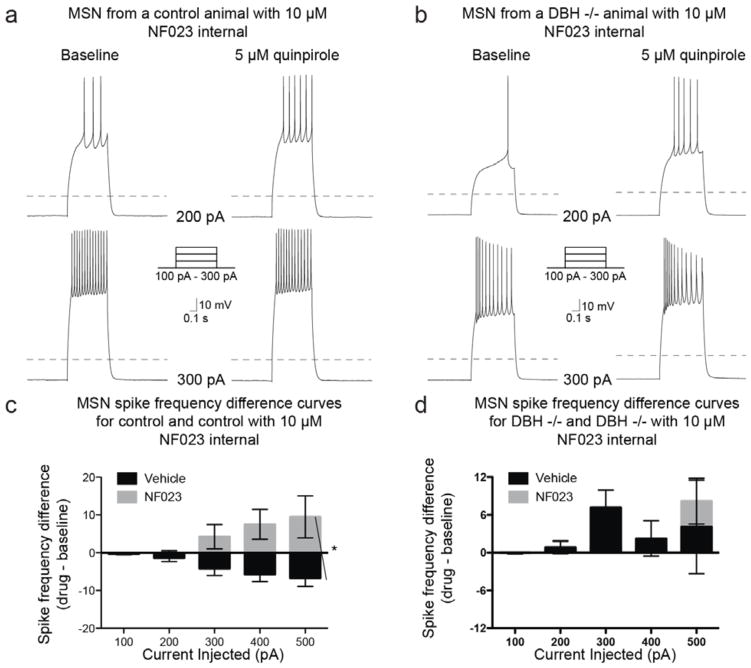
Because D2-like receptor abundance is normal in Dbh −/− mice but the cellular response to quinpirole is altered, we suspected that D2 receptors might be aberrantly coupled in NAc MSNs of Dbh −/− mice. To test this idea, we assessed the effects of quinpirole on MSN excitability in the presence of either a Gαi (NF023; 10 μM), or Gαs (NF449; 1 μM) inhibitor (Freissmuth et al., 1996; Hohenegger et al., 1998). As expected for a Gαi-coupled receptor like D2, application of NF023 to control MSNs abolished the quinpirole-induced decrease in excitability (Chi-square (df = 1) = 1.2, p = 0.27) and resulted in a significant difference in the spike frequency difference curve compared to the spike frequency difference curve for MSNs treated with quinpirole alone (Chi-square (df = 1) = 16.5; p < 0.0001) (Fig. 5a, 5c). By contrast, the spike frequency difference curve obtained from Dbh −/− MSNs after application of NF023 was not significantly different from the spike frequency difference curve obtained from Dbh −/− MSNs treated with quinpirole alone (Chi-square (df = 1) = 1.57, p = 0.21) (Fig. 5b, 5d).
Figure 5. Gαi inhibition abolishes quinpirole-mediated inhibition of MSN spike excitability in control mice, but has no effect in Dbh −/− mice.
Example traces from MSNs recorded from a control (a) and a Dbh −/− (b) animal at baseline or following bath application of quinpirole (5 μM) with the Gαi inhibitor NF023 (10 μM) applied internally, while action potentials were evoked with a series of current steps. Dashed line indicates −70 mV. (c) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole – baseline) in the presence of vehicle (n = 46) or NF023 (n = 10) from cells recorded from control mice. (d) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole – baseline) in the presence of vehicle (n = 15) or NF023 (n = 17) from cells recorded from Dbh −/− mice. * indicates significant difference (p < 0.05) between vehicle and NF023 population data.
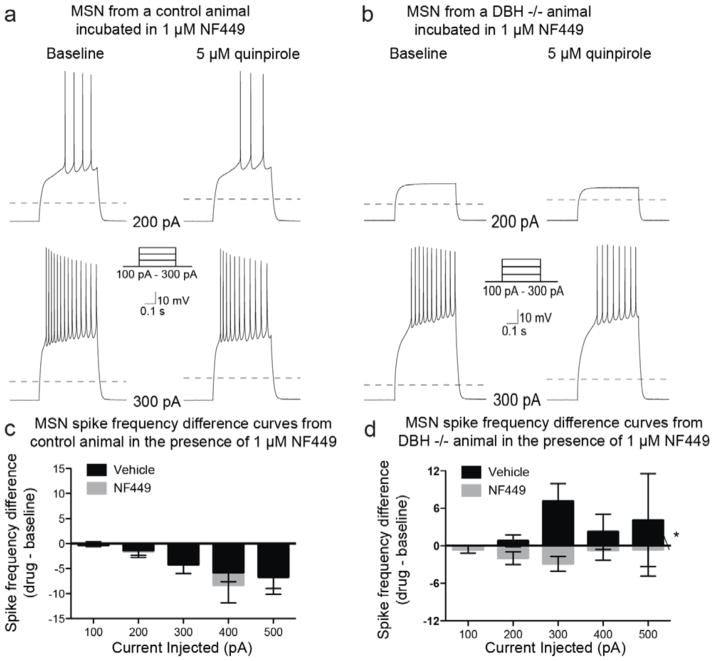
Application of the Gαs inhibitor, NF449, did not occlude the inhibitory effects of D2 activation in MSNs from control mice; no changes in spike frequency were observed when compared to quinpirole alone (Chi-square (df = 1) = 0.14, p = 0.71) (Fig. 6a, 6c). However, application of NF449 in Dbh −/− MSNs significantly reduced spike frequency compared to quinpirole alone (Chi-square (df = 1) = 14.99, p = 0.0001) (Fig. 6b, 6d). Combined, these results suggest that D2 receptors on NAc MSNs primarily couple to Gαi and suppress firing rate in control mice, but that D2-G protein coupling is altered and quinpirole-induced inhibition is lost in Dbh −/− NAc MSNs.
Figure 6. Gαs inhibition has no effect on quinpirole-mediated inhibition in control mice, but restores quinpirole-mediated inhibition of MSN spike frequency in Dbh −/− mice.
Example traces from MSNs recorded from a control (a) and a Dbh −/− (b) animal at baseline or following bath application of quinpirole (5 μM) with the Gαs inhibitor, NF449 (10 μM; slices pre-incubated in the Gαs antagonist for 1 h prior to recording, and then continuously exposed to NF449 throughout the recording process), while action potentials were evoked with a series of current steps. Dashed line indicates −70 mV. (c) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole – baseline) in the presence of vehicle (n = 46) or NF449 (n = 11) from cells recorded from control mice. (d) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole – baseline) in the presence of vehicle (n = 15) or NF449 (n = 22) from cells recorded from Dbh −/− mice. * indicates significant difference (p < 0.05) between vehicle and NF449 population data.
Discussion
Pharmacological and genetic DBH inhibition lead to behavioral hypersensitivity to dopaminergic drugs, including psychostimulant drugs of abuse. (Gaval-Cruz et al., 2012; Haile et al., 2003; Schank et al., 2006; Weinshenker et al., 2008; Weinshenker et al., 2002). We identified a decrease in βArr2 and an increase in ΔFosB in the NAc following chronic genetic or pharmacological DBH inhibition, and overexpression of βArr2 normalized cocaine responses in Dbh −/− mice. Slice electrophysiology experiments revealed that MSNs from control mice are inhibited by quinpirole in a Gαi-dependent manner, while the effects of quinpirole are altered and become sensitive to Gαs blockade in Dbh −/− NAc MSNs.
Chronic DBH inhibition alters the abundance of DA receptor signaling proteins in the nucleus accumbens
Because DBH catalyzes the conversion of NE to DA in noradrenergic neurons, DBH inhibition decreases NE production, with a concomitant increase in tissue DA levels (Bourdelat-Parks et al., 2005; Goldstein, 1966; Musacchio et al., 1966). However, because NE provides direct and indirect excitatory drive onto midbrain DA neurons, basal and stimulant-evoked DA overflow is actually reduced in Dbh −/− mice (Schank et al., 2006; Weinshenker and Schroeder, 2007), producing a compensatory upregulation of D2 signaling and hypersensitivity to psychostimulants and quinpirole (Schank et al., 2006; Weinshenker et al., 2008; Weinshenker et al., 2002). Indeed, neurotoxic ablation of brain NE neurons, which reduces NE without an increase in tissue DA, confers a similar pattern of drug responses (Harro et al., 2000; Nowak et al., 2009; Weinshenker et al., 2008).
We originally reported increased high affinity-state D2 receptors in the striatum of Dbh −/− mice, which we speculated might underlie the behavioral hypersensitivity of the knockouts to psychostimulants (Schank et al., 2006). However, subsequent in vitro radioligand competition experiments failed to confirm these results (Skinbjerg et al., 2010) (our unpublished data). The discrepancy between studies measuring D2 affinity states may be due to some differences in the radioligands and approaches employed, but more concerning was our failure to observe a genotype difference in the abundance of high affinity-state D2 receptors in vivo using PET imaging (Skinbjerg et al., 2010).
Because of these issues and inconsistencies, we suspected that changes in downstream signaling molecules, rather than D2 receptor affinity state, were responsible for cocaine hypersensitivity following chronic NE deficiency. Both genetic and pharmacological DBH inhibition produced a decrease of βArr2 and an increase in ΔFosB in the NAc. ΔFosB is a transcription factor that is induced by chronic exposure to drugs or other environmental stimuli and enhances behavioral responses to cocaine (Kelz et al., 1999). We chose to pursue the contribution of βArr2 because it is upstream of ΔFosB in the DA receptor signaling pathway and because it had not been implicated in cocaine-induced behaviors; locomotor activity and conditioned place preference following cocaine administration are unchanged in βArr2 knockout mice (Bohn et al., 2003). We found that viral-mediated overexpression of βArr2 in the NAc suppressed the cocaine hypersensitivity in Dbh −/− mice. This effect was not due to a general suppression of motor activity because ambulatory behavior in a novel environment was unaffected by βArr2 overexpression, and cocaine-induced locomotion was normalized to, but not below, control levels. Because we examined used a CMV promoter to drive overexpression of βArr2, we cannot attribute its effects on cocaine-induced locomotion specifically to changes in D2 signaling. Future experiments using D1- and D2-specific promoters will be required to delineate the importance of βArr2 in direct versus indirect pathway MSNs. The discrepancies between the βArr2-mediated phenotypes in Dbh −/− and βArr2 knockout mice may be due to the complete lack of global βArr2 in the βArr2 knockout mice versus the partial reduction of βArr2 specifically in the NAc of Dbh −/− mice.
Aberrant cellular responses to quinpirole in nucleus accumbens medium spiny neurons of Dbh −/− mice
Because normal D2 autoreceptor function is preserved in Dbh −/− mice (Paladini et al., 2007), we focused our attention on potential changes in D2 receptor signaling in accumbal MSNs. Activation of D2 and other Gαi/o-coupled receptors typically inhibits evoked action potentials, reducing firing and spike frequency in MSNs. By contrast, Gαs-coupled receptors, such as D1, have a facilitatory effect on MSN responses (Hu and Wang, 1988; Surmeier et al., 2010; West and Grace, 2002). Our electrophysiological recordings from NAc neurons confirmed that the D2/3 agonist, quinpirole, suppressed evoked MSN firing in slices from control mice. By contrast, the inhibitory effects of quinpirole were abolished in Dbh −/− MSNs, and in fact there was a trend for quinpirole to be excitatory.
There are several potential explanations for this change in cellular response to D2/3 activation. For example, the involvement of βArr2 in receptor desensitization and endocytosis suggests a possible contribution of altered D2 trafficking and localization. In addition, because βArr2 recruits cAMP-degrading phosphodiesterase (PDE) to the membrane upon receptor binding (Kendall and Luttrell, 2009; Perry et al., 2002), reduction of βArr2 could dampen inhibition and promote excitation by potentiating cAMP abundance. The reduction in βArr2 could also affect neuronal firing by altering G protein-independent GSK3β/Akt signaling, although we did not detect any differences in these proteins in the NAc of Dbh −/− mice. It is also possible that the decrease in βArr2 and the altered quinpirole response are unrelated. Future experiments to determine whether loss of βArr2 directly causes aberrant D2/3 signaling will help identify the underlying mechanisms.
Given that NF023 abolished quinpirole-induced inhibition in MSNs from control mice, but had no effect on quinpirole response in Dbh −/− MSNs, while NF449 had no effect on quinpirole-induced inhibition in control MSNs, but suppressed firing in the presence of quinpirole in Dbh −/− mice, it is possible that at least some D2 receptors are coupled to Gαs instead of Gαi in Dbh −/− MSNs. In vivo Gαi-to-Gαs switching has been reported for μ-opioid receptors and CB1 cannabinoid receptors following chronic agonist exposure (Paquette et al., 2007; Wang et al., 2005), and a reduction in βArr2 promotes Gαs-to-Gαi switching of β-adrenergic receptors (Baillie et al., 2003). However, these data are associated with several limitations and must be interpreted with caution. Quinpirole is an agonist of both D2 and D3 DA receptors, and both subtypes are present in the NAc. D3 receptors and D2-D3 heterodimers receptors have been reported to couple to Gαq, and thus promote neuronal excitation. However, increased D3- Gαq or D2/D3- Gαq heterodimer signaling is unlikely to underlie quinpirole-induced excitation in Dbh −/− MSNs for two reasons. First, Dbh −/− mice are hypersensitive to quinpirole but not the preferential D2/D3 heterodimer agonist SKF83959 (our unpublished data). thus the Gαs-to-Gαi switch could be solely or preferentially affecting one of these two subtypes. An increase in firing following quinpirole as that seen in Dbh −/− mice could also be explained if these mice had a greater proportion of D2-D3 Gq-coupled heteromers. However, systemic administration of the preferential D2-D3 heteromer agonist SKF83959 does not result in differences in locomotion between Dbh −/− and Dbh +/− mice. NF023 and NF449 are not totally selective for Gαi and Gαs; for example, both compounds are also X and P2Y receptor antagonists (Braun et al., 2001; Lambrecht, 1996). This is nevertheless unlikely given our results since P2X, as a ligand-gated ion channel, would have a direct effect on membrane potential and firing rate, which we did not observe. NF023 and NF449 also had different effects in our different groups of animals, which is inconsistent with these drugs acting as purinergic antagonists. Finally, our methods did not allow us to distinguish between D1 and D2 MSNs, which precludes assigning direct effects of quinpirole and could also account for the high variability in some of our experiments, particularly the ones involving Dbh −/− recordings. In addition, it will be important to test alterations in D2-G protein associations using other techniques such as co-immunoprecipitation. Unfortunately, D2 antibodies of sufficient quality and specificity for this approach are not currently available.
Clinical implications
The consequences of chronic reduction in DBH function may be relevant to drug addiction. Non-selective DBH inhibitors, like disulfiram, have shown promise in human laboratory studies and clinical trials for the treatment of stimulant dependence (Gaval-Cruz and Weinshenker, 2009), and a large phase II trial of nepicastat for cocaine addiction is ongoing (http://clinicaltrials.gov/show/NCT01704196). Genetic or chronic pharmacological reduction of DBH activity enhances some interoceptive properties of cocaine, particularly its aversive effects, such as paranoia and anxiety (Gaval-Cruz and Weinshenker, 2009; Hameedi et al., 1995; Kalayasiri et al., 2007; McCance-Katz et al., 1998a, b; Mutschler et al., 2009; Schank et al., 2006; Sofuoglu et al., 2008)_ENREF_46, suggesting that the clinical efficacy of DBH inhibitors is related to an increase in cocaine aversion. Interestingly, optogenetic inhibition of D2 MSNs drives, while excitation of D2 MSNs inhibits, compulsive cocaine seeking in mice (Bock et al., 2013). Thus, under normal conditions, cocaine may facilitate its own use by promoting DA signaling via the D2 receptor and suppressing D2 MSN activity; however, our data suggest that, under conditions of chronic DBH inhibition, cocaine may actually limit its own use because D2-mediated inhibition is absent, leading to increased cell excitability.
Acknowledgments
We thank Dainippon-Sumitomo Pharmaceuticals Inc. (Osaka, Japan) for providing the DOPS needed to maintain our Dbh mouse colony, Synosia Therapeutics for providing the nepicastat, and C. Strauss for helpful editing of the manuscript. This work was supported by the National Institute of Drug Abuse, National Institute of Mental Health, and National Institute of Neurological Disorders and Stroke (NIDA grants DA017963 and DA027535 to DW, DA25040 and DA015040 to MGC, and DA030530 to CP, NIMH grant MH079276 to CP, and NINDS grant NS060658 to CAP). MGC, RBG, DJP, DEB, RCM, RAH, DJ, and CAP declare no conflict of interest. DW is co-inventor on a patent concerning the use of selective DBH inhibitors for the treatment of cocaine dependence (US-2013-0274303-A1; “Methods and Compositions for Treatment of Drug Addiction”).
Footnotes
Authorship Contributions. MGC, RBG, CAP, and DW participated in the research design, MGC, DJP, and RCM conducted the western blot experiments, MGC and DJP conducted the behavioral experiments, RBG conducted the electrophysiology experiments, DK conducted the statistical analysis of the electrophysiology experiments, RAH and DEB contributed analytic tools, and MGC, RBG, CAP, and DW wrote the manuscript.
References
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature neuroscience. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology. 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- Braun K, Rettinger J, Ganso M, Kassack M, Hildebrandt C, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. NF449: a subnanomolar potency antagonist at recombinant rat P2X1 receptors. Naunyn-Schmiedeberg’s archives of pharmacology. 2001;364:285–290. doi: 10.1007/s002100100463. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Molecular psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Del’guidice T, Lemasson M, Beaulieu JM. Role of Beta-arrestin 2 downstream of dopamine receptors in the Basal Ganglia. Frontiers in neuroanatomy. 2011;5:58. doi: 10.3389/fnana.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Boehm S, Beindl W, Nickel P, Ijzerman AP, Hohenegger M, Nanoff C. Suramin analogues as subtype-selective G protein inhibitors. Molecular pharmacology. 1996;49:602–611. [PubMed] [Google Scholar]
- Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D. Chronic Inhibition of Dopamine beta-Hydroxylase Facilitates Behavioral Responses to Cocaine in Mice. PloS one. 2012;7:e50583. doi: 10.1371/journal.pone.0050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol Interv. 2009;9:175–187. doi: 10.1124/mi.9.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. Inhibition of norepinephrine biosynthesis at the dopamine-beta-hydroxylation stage. Pharmacological reviews. 1966;18:77–82. [PubMed] [Google Scholar]
- Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA. Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biological psychiatry. 2003;54:915–921. doi: 10.1016/s0006-3223(03)00241-5. [DOI] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biological psychiatry. 1995;37:560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- Harro J, Merikula A, Lepiku M, Modiri AR, Rinken A, Oreland L. Lesioning of locus coeruleus projections by DSP-4 neurotoxin treatment: effect on amphetamine-induced hyperlocomotion and dopamine D2 receptor binding in rats. Pharmacology & toxicology. 2000;86:197–202. doi: 10.1034/j.1600-0773.2000.d01-35.x. [DOI] [PubMed] [Google Scholar]
- Hohenegger M, Waldhoer M, Beindl W, Boing B, Kreimeyer A, Nickel P, Nanoff C, Freissmuth M. Gsalpha-selective G protein antagonists. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:346–351. doi: 10.1073/pnas.95.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Wang RY. Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. Dopamine beta-hydroxylase gene (DbetaH) -1021C-->T influences self-reported paranoia during cocaine self-administration. Biological psychiatry. 2007;61:1310–1313. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Shandilya M, Kundu S. Structural insight of dopamine beta-hydroxylase, a drug target for complex traits, and functional significance of exonic single nucleotide polymorphisms. PloS one. 2011;6:e26509. doi: 10.1371/journal.pone.0026509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kendall RT, Luttrell LM. Diversity in arrestin function. Cellular and molecular life sciences : CMLS. 2009;66:2953–2973. doi: 10.1007/s00018-009-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht G. Design and pharmacology of selective P2-purinoceptor antagonists. Journal of autonomic pharmacology. 1996;16:341–344. doi: 10.1111/j.1474-8673.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Maj J, Przegalinski E, Wielosz M. Disulfiram and the drug-induced effects on motility. The Journal of pharmacy and pharmacology. 1968;20:247–248. doi: 10.1111/j.2042-7158.1968.tb09735.x. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Depoy L, Weinshenker D. Dopamine beta-Hydroxylase Inhibitors Enhance the Discriminative Stimulus Effects of Cocaine in Rats. The Journal of pharmacology and experimental therapeutics. 2013 doi: 10.1124/jpet.113.207746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biological psychiatry. 1998a;43:540–543. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug and alcohol dependence. 1998b;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D. The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biological psychiatry. 2006;60:1046–1052. doi: 10.1016/j.biopsych.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Musacchio JM, Goldstein M, Anagnoste B, Poch G, Kopin IJ. Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. The Journal of pharmacology and experimental therapeutics. 1966;152:56–61. [PubMed] [Google Scholar]
- Mutschler J, Diehl A, Kiefer F. Pronounced paranoia as a result of cocaine-disulfiram interaction: case report and mode of action. Journal of clinical psychopharmacology. 2009;29:99–101. doi: 10.1097/JCP.0b013e3181934451. [DOI] [PubMed] [Google Scholar]
- Nowak P, Nitka D, Kwiecinski A, Josko J, Drab J, Pojda-Wilczek D, Kasperski J, Kostrzewa RM, Brus R. Neonatal co-lesion by DSP-4 and 5,7-DHT produces adulthood behavioral sensitization to dopamine D(2) receptor agonists. Pharmacological reports : PR. 2009;61:311–318. doi: 10.1016/s1734-1140(09)70037-4. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Beckstead MJ, Weinshenker D. Electrophysiological properties of catecholaminergic neurons in the norepinephrine-deficient mouse. Neuroscience. 2007;144:1067–1074. doi: 10.1016/j.neuroscience.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette JJ, Wang HY, Bakshi K, Olmstead MC. Cannabinoid-induced tolerance is associated with a CB1 receptor G protein coupling switch that is prevented by ultra-low dose rimonabant. Behavioural pharmacology. 2007;18:767–776. doi: 10.1097/FBP.0b013e3282f15890. [DOI] [PubMed] [Google Scholar]
- Perez MF, White FJ, Hu XT. Dopamine D(2) receptor modulation of K(+) channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. Journal of neurophysiology. 2006;96:2217–2228. doi: 10.1152/jn.00254.2006. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Alisha Epps S, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Seneca N, Liow JS, Hong J, Weinshenker D, Pike VW, Halldin C, Sibley DR, Innis RB. Dopamine beta-hydroxylase-deficient mice have normal densities of D(2) dopamine receptors in the high-affinity state based on in vivo PET imaging and in vitro radioligand binding. Synapse. 2010;64:699–703. doi: 10.1002/syn.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Waters A, Sewell A, Hill K, Kosten T. Disulfiram enhances subjective effects of dextroamphetamine in humans. Pharmacology, biochemistry, and behavior. 2008;90:394–398. doi: 10.1016/j.pbb.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, Walker K, Martinez G, Eglen RM, Whiting RL, Hegde SS. Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. British journal of pharmacology. 1997;121:1803–1809. doi: 10.1038/sj.bjp.0701315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. Progress in brain research. 1993;99:309–324. doi: 10.1016/s0079-6123(08)61354-0. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Progress in brain research. 2010;183:149–167. doi: 10.1016/S0079-6123(10)83008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. Journal of neurochemistry. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Ferrucci M, Busceti CL, Biagioni F, Lazzeri G, Liles LC, Lenzi P, Pasquali L, Murri L, Paparelli A, Fornai F. Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. Journal of neurochemistry. 2008;105:471–483. doi: 10.1111/j.1471-4159.2007.05145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM. Serum dopamine beta-hydroxylase. Pharmacological reviews. 1978;30:133–166. [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S, Tanaka E, Hattori G, Maeda H, Higashi H. Direct and indirect actions of dopamine on the membrane potential in medium spiny neurons of the mouse neostriatum. Journal of neurophysiology. 2002;87:1234–1243. doi: 10.1152/jn.00514.2001. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Current opinion in neurobiology. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]