Abstract
Successfully navigating a dynamic environment requires the efficient distribution of finite neural resources. Voluntary (endogenous) covert spatial attention selectively allocates those processing resources to goal–relevant locations in the visual scene in the absence of eye movements. However, the allocation of spatial attention is not always voluntary: abrupt onsets in the visual periphery automatically enhance processing of nearby stimuli (exogenous attention). In dynamic environments, exogenous events and internal goals likely compete to determine the distribution of attention, but how such competition is resolved is not well understood. To investigate how exogenous events interact with the concurrent allocation of voluntary attention, we used a speed–accuracy trade–off procedure (SAT). SAT conjointly measures the rate of information accrual and asymptotic discriminability, allowing us to measure how attentional interactions unfold over time during stimulus processing. We found that both types of attention sped information accrual and improved discriminability. However, focusing endogenous attention at the target location reduced the effects of exogenous cues on the rate of information accrual and rendered negligible their effects on asymptotic discriminability. We verified the robustness of these findings in four additional experiments that targeted specific, critical response delays. In conclusion, the speed and quality of visual processing depend conjointly on internally– and externally–driven attentional states, but it is possible to voluntarily diminish distraction by irrelevant events in the periphery.
Introduction
From moment to moment, the environment inundates our senses with a tremendous amount of information, far more than the brain can process and render for conscious awareness. To efficiently interact with a dynamic world, we must select for further sensory processing those things in the environment that are most relevant to our goals, while ignoring irrelevant stimuli that compete for access to limited resources. However, because we cannot always know what is immediately relevant, we need also the ability to track sudden changes that may require further action.
Covert visuospatial attention–the selective processing of visual information without eye movements–aids in this endeavor. Exogenous attention is involuntary, occurring rapidly and transiently (~80–120 ms) in response to sudden onsets in the visual periphery, whereas endogenous attention is voluntary, takes longer to be deployed (~300 ms), and can be sustained in a goal–driven fashion (Ling & Carrasco, 2006; Muller & Rabbitt, 1989; Nakayama & Mackeben, 1989). Numerous neurophysiological and neuroimaging studies have shown that exogenous and endogenous attention modulate activity in multiple brain areas, including early visual cortical areas (Busse, Katzner, & Treue, 2008; Corbetta, Patel, & Shulman, 2008; Hopfinger & West, 2006; Pestilli, Carrasco, Heeger, & Gardner, 2011; Reynolds & Heeger, 2009). Psychophysical studies have shown that both types of attention improve task performance and enhance subjective appearance (e.g., spatial resolution and apparent spatial frequency, contrast sensitivity and apparent contrast) at attended locations while impairing perception at unattended locations (for reviews, see: Carrasco, 2009, 2011; for an alternative view on appearance, see: Schneider & Komlos, 2008, Schneider, 2011, and for replies see Anton-Erxleben, Abrams, & Carrasco, 2010, 2011). Psychophysical studies using a speed–accuracy trade–off (SAT) analysis, which conjointly measures discriminability and speed of information processing (Reed, 1973; Wickelgren, 1977), have characterized visual (Carrasco, McElree, Denisova, & Giordano, 2003) and attentional (Carrasco, Giordano, & McElree, 2004, 2006; Carrasco & McElree, 2001; Dosher, Han, & Lu, 2004; Giordano, McElree, & Carrasco, 2009) processes. They have shown that exogenous and endogenous attention not only enhance perceptual sensitivity but also modulate the rate of information accrual, allowing discriminability to reach asymptote faster at attended relative to unattended locations. Exogenous and endogenous attention lead to similar behavioral consequences in most instances (Herrmann, Montaser-Kouhsari, Carrasco, & Heeger, 2010; Montagna, Pestilli, & Carrasco, 2009) but not all (Barbot, Landy, & Carrasco, 2011; Yeshurun, Montagna, & Carrasco, 2008). In short, covert spatial attention not only modulates how we process incoming stimuli, but actually changes they way in which we subjectively experience the world.
In the lab, exogenous and endogenous attention are predominantly studied in isolation, but in everyday life, they compete with one another. Sudden changes in the environment may exogenously shift attention to one spatial location while endogenous attention attempts to selectively process another. How is this kind of competition resolved? Can the reflexive allocation of attention due to an exogenous onset be prevented when endogenous attention is focused at a different location? For example, when driving, can you ignore a flash of lightning while covertly monitoring cars on the other side of the road?
Psychophysical research on the interaction between exogenous and endogenous attention has produced inconsistent results. Some studies show that task–irrelevant distractors can be completely ignored if they occur outside the locus of endogenous attention (e.g., Theeuwes, 1991; Yantis & Jonides, 1990), whereas others (e.g., Berger, Henik, & Rafal, 2005; van der Lubbe & Postma, 2005) find significant effects of irrelevant exogenous cues despite top–down attempts to ignore them (for a review, see: Chica, Bartolomeo, & Lupianez, 2013). These behavioral studies are difficult to interpret because they used changes in response time (RT) to index attention, which can reflect changes in speed of processing, discriminability or decision criteria (Reed, 1973; Wickelgren, 1977). As a result, there is no consensus on the the behavioral consequences of this interaction.
Previous neurophysiological findings suggest that exogenous cues transiently modulate the processing of visual information, even with focused endogenous attention. EEG measurements in humans show that exogenous cues affect early, but not late, stages of visual processing at endogenously attended locations (Hopfinger & West, 2006), converging with single unit recordings in monkeys indicating that unpredictable onsets transiently interrupt the focus of endogenous attention (Busse et al., 2008). These studies shed light on the underlying neural processes that govern the interaction between exogenous and endogenous spatial attention. They do not, however, reveal the perceptual consequences arising from this interaction as visual processing unfolds through time.
In the present series of experiments we used a psychophysical SAT procedure to assess how endogenous and exogenous cues dynamically interact to modulate both visual discriminability and the rate of information accrual. The results demonstrate that the interaction depends on how much time is allowed for the perceptual decision and response. We verified the robustness of the key findings in a total of five experiments: the first used the full SAT procedure and the rest targeted specific, critical response delays and different stimulus arrangements.
Experiment 1
Method
Observers and psychophysical sessions
Eight observers (four female) participated in Experiment 1. All observers had normal or corrected to normal vision. Experimental procedures were approved by the University Committee on Activities Involving Human Subjects at New York University.
Protocol
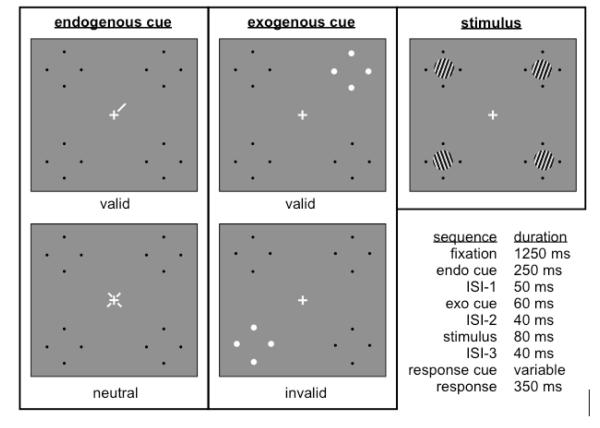
We manipulated spatial attention in a two–alternative, forced–choice orientation discrimination task in which the target (a sinusoidal luminance grating) was briefly presented with three distractor gratings [Figure 1; Supplementary Online Materials]. A centrally presented pre–cue indicated the location of the upcoming target (endogenous valid trials) or that the target could appear at any of the four locations (endogenous neutral trials). This was followed by a peripheral pre–cue next to the upcoming target (exogenous valid trials) or a distractor 180° (polar angle) away from the target (exogenous invalid trials). Valid and invalid exogenous trials were equally likely (i.e., uninformative about target location), and observers were told that the exogenous cues were uninformative. After the gratings disappeared, a central post–cue indicated which of them was the target. After a variable temporal delay, a response tone prompted observers to indicate, within a limited time window of 350 ms, whether the target had been tilted clockwise or counterclockwise of vertical. Endogenous cues (valid, neutral) and exogenous cues (valid, invalid) were crossed to yield 4 cueing conditions, each sampled at 7 response delays (40–1500 ms), randomly intermixed within each block. Different response delays allowed us to sample the full timecourse of processing, ranging from when discriminability (d’) was near chance to when it had reached asymptote.
Figure 1. Protocol, Experiment 1.
Example cues for target in upper-right quadrant, stimulus, and trial sequence. ISI–interstimulus interval.
Data analysis
Data from individual observers were fit with an exponential function:
| (1) |
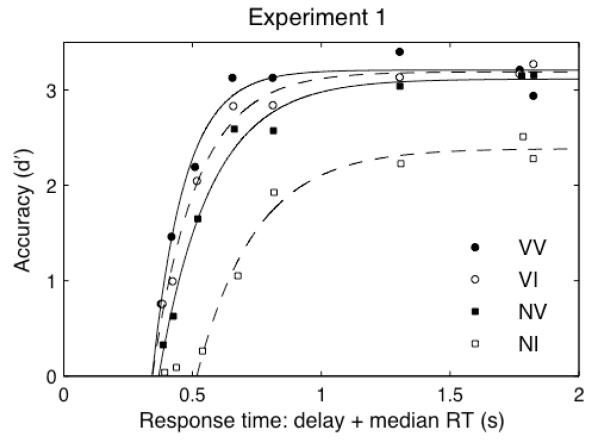
where t is time since stimulus onset. δ is the x–intercept (the time at which d’ departs from 0), λ is the asymptote (maximum d’), and β specifies the rate (how quickly performance rises from chance to asymptote). Parameters for each attention condition were estimated using nonlinear least–squares. For each attention condition, separately for each participant, we estimated two key parameters: asymptotic discriminability (λ) and processing time (δ+β−1), which is a composite measure that guards against potential trade–offs between the x–intercept and rate parameters. Processing time is the number of milliseconds required for d’ to reach ~67% of the asymptote. Exponential fits to data averaged across observers are shown in Figure 2.
Figure 2. SAT functions, Experiment 1.
Accuracy as a function of response time. Curves indicate best fitting exponential functions to averaged dataset (n=8). VV–endogenous valid, exogenous valid. VI–endogenous valid, exogenous invalid. NV–endogenous neutral, exogenous valid. NI–endogenous distributed, exogenous invalid. R2=0.973, 0.986, 0.988, 0.988, respectively.
Statistics
A within–participant, non–parametric randomization procedure (analogous to a paired t–test) was used to determine whether cueing effects on asymptotic discriminability and processing time were statistically significant. For example, the p–value for the effect of the exogenous cue on processing time in the endogenous valid condition [Figure 3A, left side] was determined in the following way. Null data sets were generated by randomly shuffling the labels of the exogenous attention condition across endogenous valid trials, separately for each response delay, and separately for each observer. d’ was recomputed at each response delay, and the timecourse data were re–fit, yielding new parameter estimates for each participant. Within– participant paired differences were determined, and the mean of these paired differences across participants was computed. Because the labels of the attention conditions were randomly shuffled, any observed effects will be due to chance. This procedure was repeated 1000 times, generating a null distribution of mean within–participant changes in processing time. The p– value reported is the proportion of null distribution values greater than or equal to the actual mean change in processing time (determined by taking the mean of the empirically observed paired differences across participants). Absolute values were used in the computation to make this a two–tailed test. The remaining comparisons for both asymptotic d’ and processing time were computed in the same manner.
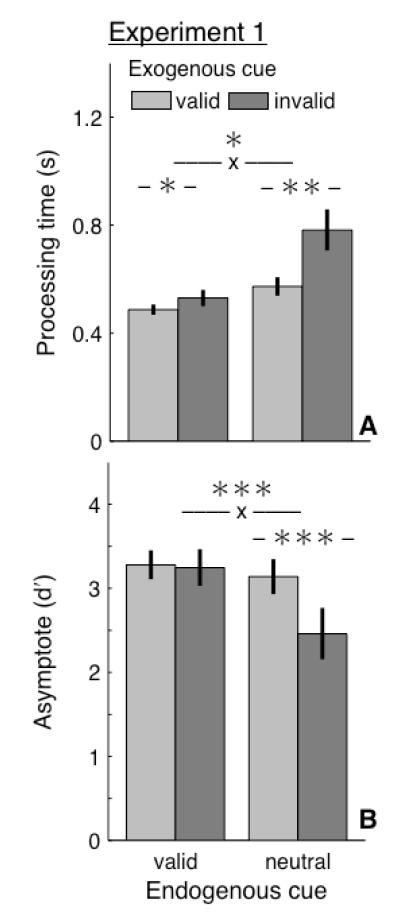
Figure 3. Results, Experiment 1.
(A) Mean processing time estimates (δ+β−1) from individual fits (n=8). –x– denotes interaction. Error bars, standard error of the mean across participants. (B) Mean asymptotic discriminability (λ) from individual fits. Same format as panel A. *, p<0.05. **, p<0.01. ***, p<0.001.
Results and Discussion
There was an interaction between the cue types: valid endogenous cues weakened the impact of exogenous cues [Figure 3A, B]. The degree to which exogenous cues modulated processing time and asymptotic discriminability (i.e., exogenous valid relative to invalid trials) was reduced in endogenous valid relative to neutral trials (processing time: P=0.011; asymptote: P<0.001, randomization procedure). These results demonstrate that endogenous attention can mitigate the degree to which exogenous onsets reflexively engage attention.
Moreover, the interaction between endogenous and exogenous cue types was time dependent. When the endogenous cue was neutral, exogenous cues modulated performance via changes in both the rate of information accrual and the asymptote of the SAT function: processing time was shorter (P=0.002) and asymptotic discriminability was higher (P<0.001) with valid relative to invalid exogenous cues, consistent with previous SAT studies (Carrasco et al., 2004, 2006; Carrasco & McElree, 2001; Dosher et al., 2004; Giordano et al., 2009). In contrast, when the endogenous cue was valid, exogenous cues modulated only the rate of information accrual: processing time was shorter with valid relative to invalid exogenous cues (P=0.029), but asymptotic discriminability was not significantly affected (P=0.778).
These data suggest that with enough time between the stimulus and the observer’s response to it, endogenous attention pre-allocated to the target location can completely overcome the exogenous effect of irrelevant onsets. It might also be the case, however, that accuracy was simply too high for the exogenous cues to have any impact on asymptotic d’ in the endogenous valid condition (although that could not explain the reduced effect on processing time). To test if such a performance ceiling might be confounding the interpretation of these results, we conducted two additional experiments, each with a more difficult orientation discrimination (less tilt, thereby decreasing overall performance, see Supplementary Online Materials) and with a single long response delay timed to put the response in the asymptotic range.
Experiments 2 and 3
Method
Observers and protocol
Six observers (two female) participated in Experiment 2. The task, stimuli, and attentional manipulations were identical to those used in Experiment 1, with the following exceptions: 1) a single response tone was used (1080 ms post stimulus offset) and participants had up to 5 seconds to make a response after the tone, 2) on exogenous invalid trials, the pre-cue was equally likely to appear near the location of any of the distractor gratings, and 3) this long response delay was used in the pre–test that determined the degree of tilt for each individual observer.
Five observers (three female) participated in Experiment 3. The task, stimuli, and attentional manipulations were identical to those used in Experiment 2, with the following exception: there were only two possible target gratings, each located to the left or right of fixation at the same eccentricity used in Experiments 1 and 2.
Results and Discussion
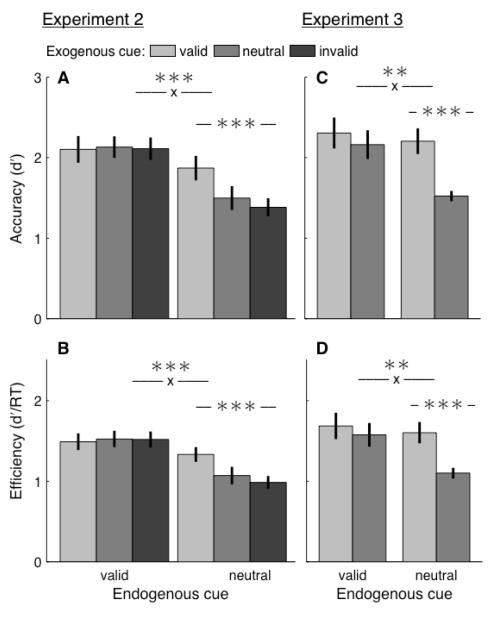
The results of both Experiments 2 and 3 matched those at long delays in Experiment 1 [Figure 4A,C]. Exogenous cues significantly modulated accuracy when the endogenous cue was neutral (P<0.001; Experiments 2 and 3) but not when it was valid (Experiment 2: P=0.93; Experiment 3: P=0.299). Further, the exogenous cueing effects significantly differed across endogenous conditions (Experiment 2: P<0.001; Experiment 3: P=0.005). We also verified that “inverse efficiency”, a combined metric of accuracy and reaction time (e.g., Kimchi & Peterson, 2008), produced the same results [Figure 4B,D]. There was no evidence for a change in efficiency in exogenous valid relative to invalid trials when the endogenous cue was valid (Experiment 2: P=0.708; Experiment 3: P=0.284). However, the exogenous cues did significantly modulate efficiency when the endogenous cue was neutral (P<0.001; Experiments 2 and 3), and the magnitude of these two effects were significantly different from each other (Experiment 2: P<0.001; Experiment 3: P=0.005).
Figure 4. Results, Experiments 2 and 3.
(A) Mean accuracy in Experiment 2 (n=6). –x– denotes interaction. Error bars, standard error of the mean across participants. (B) Mean efficiency in Experiment 2. Same format as panel A. (C) Mean accuracy in Experiment 3 (n=5). Same format as panel A. (D) Mean efficiency in Experiment 3. Same format as panel C. *, p<0.05. **, p<0.01. ***, p<0.001.
Taken together, these results rule out the possibility that a performance ceiling might be confounding the interpretation of the asymptotic results in Experiment 1. Additionally, the findings from Experiment 3 verify that these effects are robust not only to the spatial location of the potential targets but also to the number of accompanying distractors.
The processing time results from Experiment 1 suggest that exogenous cues should modulate task performance at pre–asymptotic levels, even when endogenous attention has been pre–allocated to the target location. In two final experiments, we test this prediction by using a single, earlier tone timed to force responses while processing is still below asymptote.
Experiments 4 and 5
Method
Observers and protocol
Five observers (three female) participated in Experiment 4. The task, stimuli, and attentional manipulations were identical to those used in Experiment 3, with the following exception: a single response tone was used (600 ms post stimulus onset) and participants were required to make a response within 520 ms.
Ten observers (six female) participated in Experiment 5. The task, stimuli, and attentional manipulations were identical to those used in Experiment 4, with the following exceptions: 1) the exogenous cue consisted of a single white dot that appeared directly above the target/distractor on valid/invalid trials, and 2) the two potential target locations were demarcated by four small corner brackets.
Results and Discussion
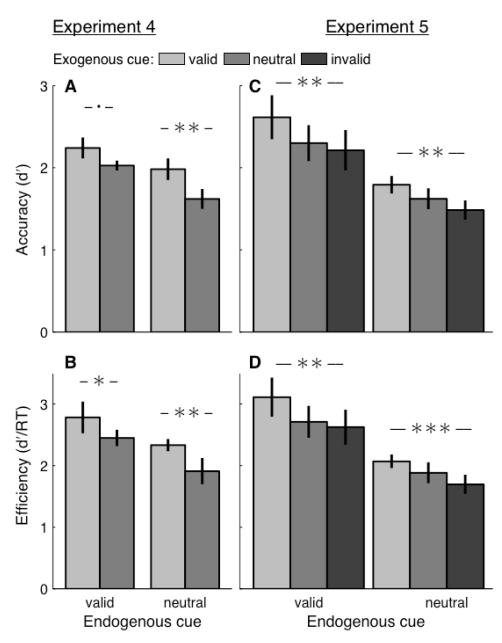
The results supported the prediction that follows from the data at early response times in Experiment 1. Exogenous cues significantly modulated accuracy when the endogenous cue was neutral (Experiment 4: P=0.003; Experiment 5: P=0.001) and when it was valid (Experiment 5: P=0.001, with a trend of P=0.088 in Experiment 4) [Figure 5A,C]. The exogenous cueing effects did not significantly differ across endogenous conditions in either experiment (Experiment 4: P=0.413; Experiment 5: P=0.594).
Figure 5. Results, Experiments 4 and 5.
(A) Mean accuracy in Experiment 4 (n=5). Error bars, standard error of the mean across participants. (B) Mean efficiency in Experiment 4. Same format as panel A. (C) Mean accuracy in Experiment 5 (n=10). Same format as panel A. (D) Mean efficiency in Experiment 5. Same format as panel C. •, p<0.1. *, p<0.05. **, p<0.01. ***, p<0.001.
Given the importance of processing time on these effects, the efficiency results are particularly critical here. Using the combined measure of accuracy and reaction time, we again support the predictions that follow from Experiment 1 [Figure 5B,D]. Exogenous cues significantly modulated efficiency when the endogenous cue was neutral (Experiment 4: P=0.004; Experiment 5: P<0.001) and when it was valid (Experiment 4: P=0.038; Experiment 5: P=0.002). As with accuracy alone, the endogenous cue did not significantly modulate the exogenous cueing effects in either experiment (Experiment 4: P=0.686; Experiment 5: P=0.593).
Taken together, the results from Experiments 4 and 5 provide further evidence for the hypothesis that the interaction of endogenous and exogenous cues changes as visual processing advances through time. By simply requiring responses earlier in time, we show that exogenous cues can modulate task performance, even with a valid endogenous cue. Additionally, results from Experiment 5 confirm that the observed exogenous effects are robust to variations in the form of the exogenous cue.
General Discussion
In five experiments, we provide converging evidence for three main findings on the interaction of endogenous and exogenous spatial attention. (1) Focused endogenous attention can mitigate the impact of task–irrelevant exogenous onsets. In Experiment 1, we show that a valid endogenous cue can diminish the degree to which exogenous onsets modulate both the rate of information accrual and asymptotic discriminability in an SAT task. In Experiments 2 and 3, we confirm that the asymptotic result is robust to changes in task difficulty, number of distractors, and location of targets. (2) Focused endogenous attention can render negligible the exogenous impact of task–irrelevant onsets altogether once task performance has reached asymptotic levels. When paired with a valid endogenous cue, we found no evidence for an exogenous cueing effect on the asymptotic discriminability parameter of the SAT function (Experiment 1) or on accuracy in two tasks with a forced response delay period of ~1000 ms (Experiments 2 and 3). (3) Focused endogenous attention cannot completely overcome the impact of task–irrelevant onsets (i.e., exogenous cues) when stimulus processing is still below asymptote. Even when paired with a valid endogenous cue, exogenous cues significantly modulated the SAT function’s rate of information accrual (Experiment 1) and performance in two tasks with a forced response delay period of 600 ms (Experiments 4 and 5). Experiments 4 and 5 confirmed that this result is robust to changes in the number of distractors, the spatial location of targets, and visual characteristics of the exogenous cue. In sum, the five experiments indicate that the interaction between endogenous and exogenous attention is a dynamic one that changes as visual processing unfolds.
The SAT approach has significantly advanced our understanding of a variety of cognitive processes, ranging from visual perception and attention (Carrasco et al., 2004, 2006; Carrasco & McElree, 2001; Carrasco et al., 2003; Dosher et al., 2004; Giordano et al., 2009) to memory (McElree, 1996; McElree & Dosher, 1989) to psycholinguistics (McElree, Murphy, & Ochoa, 2006). The present findings illustrate the advantage of characterizing the interaction of endogenous and exogenous attention with an SAT procedure. By taking into account not only changes in perceptual discriminability but also modulations in the temporal dynamics of visual processing, we were able to uncover a temporal component to this interaction and to characterize its impact on perception. We complemented the SAT approach by conducting four experiments with a single response tone, occurring at critical points of the SAT function. Acquiring behavioral responses at different times during processing leads to different outcomes, thus potentially accounting for some of the inconsistencies in previous RT studies (e.g., Berger et al., 2005; Theeuwes, 1991; van der Lubbe & Postma, 2005; Yantis & Jonides, 1990). Furthermore, because response tones controlled when perceptual judgements were made, we can rule out the possibility that decisional biases explain our results, thus overcoming the interpretability issues that affect RT–based studies (Wickelgren, 1977).
To conclude, we revealed that the perceptual consequences of the interaction between endogenous and exogenous spatial attention are temporally contingent. Exogenous attention reflexively modulates task performance during information accrual, both when endogenous attention has been pre–allocated to the target location and when it is distributed across the visual scene. With enough time, however, a focused attention system can render negligible the exogenous effect of irrelevant onsets.
Supplementary Material
Acknowledgments
This research was supported by NIH grant R01-EY019693 to DH and MC and by T32 EY007136 to NYU. We thank members of the Carrasco Lab for helpful comments.
References
- Anton-Erxleben K, Abrams J, Carrasco M. Evaluating comparative and equality judgments in contrast perception: attention alters appearance. Journal of Vision. 2010;10(11):6, 1–22. doi: 10.1167/10.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K, Abrams J, Carrasco M. Equality judgments cannot distinguish between attention effects on appearance and criterion: a reply to Schneider (2011) Journal of Vision. 2011;11(13):8, 1–8. doi: 10.1167/11.13.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Landy MS, Carrasco M. Exogenous attention enhances 2nd-order contrast sensitivity. Vision Research. 2011;51(9):1086–1098. doi: 10.1016/j.visres.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Henik A, Rafal R. Competition between endogenous and exogenous orienting of visual attention. Journal of Experimental Psychology: General. 2005;134(2):207–221. doi: 10.1037/0096-3445.134.2.207. [DOI] [PubMed] [Google Scholar]
- Busse L, Katzner S, Treue S. Temporal dynamics of neuronal modulation during exogenous and endogenous shifts of visual attention in macaque area MT. Proceedings of the National Academy of Sciences of the USA. 2008;105(42):16380–16385. doi: 10.1073/pnas.0707369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Attention: Psychophysical approaches. In: Bayne T, Cleeremans A, Wilken P, editors. The Oxford Companion to Consciousness. Oxford University Press; 2009. [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision Research. 2011;51(13):1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM, McElree B. Temporal performance fields: visual and attentional factors. Vision Research. 2004;44(12):1351–1365. doi: 10.1016/j.visres.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM, McElree B. Attention speeds processing across eccentricity: feature and conjunction searches. Vision Research. 2006;46(13):2028–2040. doi: 10.1016/j.visres.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, McElree B. Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences of the USA. 2001;98(9):5363–5367. doi: 10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, McElree B, Denisova K, Giordano AM. Speed of visual processing increases with eccentricity. Nature Neuroscience. 2003;6(7):699–670. doi: 10.1038/nn1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P, Lupianez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behavioral Brain Research. 2013;237:107–123. doi: 10.1016/j.bbr.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Han S, Lu ZL. Parallel processing in visual search asymmetry. Journal of Experimental Psychology: Human Perception and Performance. 2004;30(1):3–27. doi: 10.1037/0096-1523.30.1.3. [DOI] [PubMed] [Google Scholar]
- Giordano AM, McElree B, Carrasco M. On the automaticity and flexibility of covert attention: a speed-accuracy trade-off analysis. Journal of Vision. 2009;9(3):30, 31–10. doi: 10.1167/9.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: attention affects performance by contrast or response gain. Nature Neuroscience. 2010;13(12):1554–1559. doi: 10.1038/nn.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, West VM. Interactions between endogenous and exogenous attention on cortical visual processing. Neuroimage. 2006;31(2):774–789. doi: 10.1016/j.neuroimage.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Kimchi R, Peterson MA. Figure-ground segmentation can occur without attention. Psychological Science. 2008;19(7):660–668. doi: 10.1111/j.1467-9280.2008.02140.x. [DOI] [PubMed] [Google Scholar]
- Ling S, Carrasco M. When sustained attention impairs perception. Nature Neuroscience. 2006;9(10):1243–1245. doi: 10.1038/nn1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElree B. Accessing short-term memory with semantic and phonological information: A time-course analysis. Memory & Cognition. 1996;24:173–187. doi: 10.3758/bf03200879. [DOI] [PubMed] [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: Time course of recognition. Journal of Experimental Psychology: General. 1989;18:346–373. [Google Scholar]
- McElree B, Murphy GL, Ochoa T. Time course of retrieving conceptual information: a speed-accuracy trade-off study. Psychonomic Bulletin & Review. 2006;13(5):848–853. doi: 10.3758/bf03194008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna B, Pestilli F, Carrasco M. Attention trades off spatial acuity. Vision Research. 2009;49(7):735–745. doi: 10.1016/j.visres.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15(2):315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Research. 1989;29(11):1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M, Heeger DJ, Gardner JL. Attentional enhancement via selection and pooling of early sensory responses in human visual cortex. Neuron. 2011;72(5):832–846. doi: 10.1016/j.neuron.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AV. Speed-accuracy trade-off in recognition memory. Science. 1973;181(4099):574–576. doi: 10.1126/science.181.4099.574. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61(2):168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA. Attention alters decision criteria but not appearance: a reanalysis of Anton-Erxleben, Abrams, and Carrasco (2010) Journal of Vision. 2011;11(13):7, 1–8. doi: 10.1167/11.13.7. [DOI] [PubMed] [Google Scholar]
- Schneider KA, Komlos M. Attention biases decisions but does not alter appearance. Journal of Vision. 2008;8(15):3, 1–10. doi: 10.1167/8.15.3. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: the effect of visual onsets and offsets. Perception and Psychophysics. 1991;49(1):83–90. doi: 10.3758/bf03211619. [DOI] [PubMed] [Google Scholar]
- van der Lubbe RH, Postma A. Interruption from irrelevant auditory and visual onsets even when attention is in a focused state. Experimental Brain Research. 2005;164(4):464–471. doi: 10.1007/s00221-005-2267-0. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Speed-accuracy tradeoff and information processing dynamics. Acta Psychologica. 1977;41(1):67–85. [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. Journal of Experimental Psychology: Human Perception and Performance. 1990;16(1):121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Montagna B, Carrasco M. On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Research. 2008;48(1):80–95. doi: 10.1016/j.visres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.