Abstract
Injection of frogs with beta-adrenergic catecholamines for 1-24 hr produces marked subsensitivity of the erythrocyte membrane adenylate cyclase [ATP pyrophosphate-lyase (cyclizing); EC 4.6.1.1.] to in vitro stimulation by isoproterenol. The subsensitization is specific for catecholamine stimulation, since basal and fluoride-stimulated enzyme activity are unaffected. Maximum isoproterenol-stimulated adenylate cyclase activity declines by 75% in the isoproterenol-treated animals (P less than 0.001). The concentration of isoproterenol causing one-half maximal activation of adenylate cyclase, however, is unaltered. (-)[3H]Alprenolol, a potent competitive beta-adrenergic antagonist, was used to study directly the beta-adrenergic receptor binding sites in the erythrocyte membranes from control and subsensitized animals. A highly significant (P less than 0.005) 60% fall in the number of the beta-adrenergic receptor binding sites ("specific"(-)[3H]alprenolol binding sites) in the treated animals was found. The binding affinity of the sites was not markedly altered. These data suggest that beta-adrenergic catecholamines are able to regulate catecholamine sensitivity of tissues in vivo, by regulating the properties of the beta-adrenergic receptor binding sites.
Full text
PDF
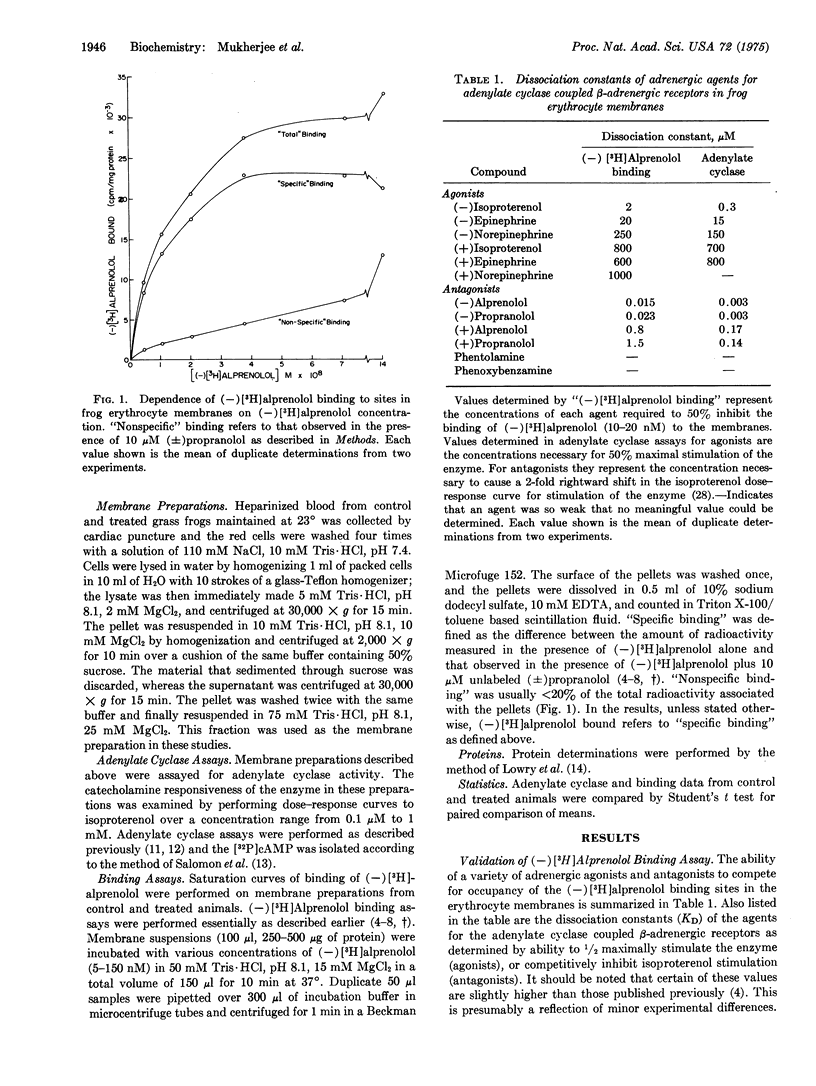
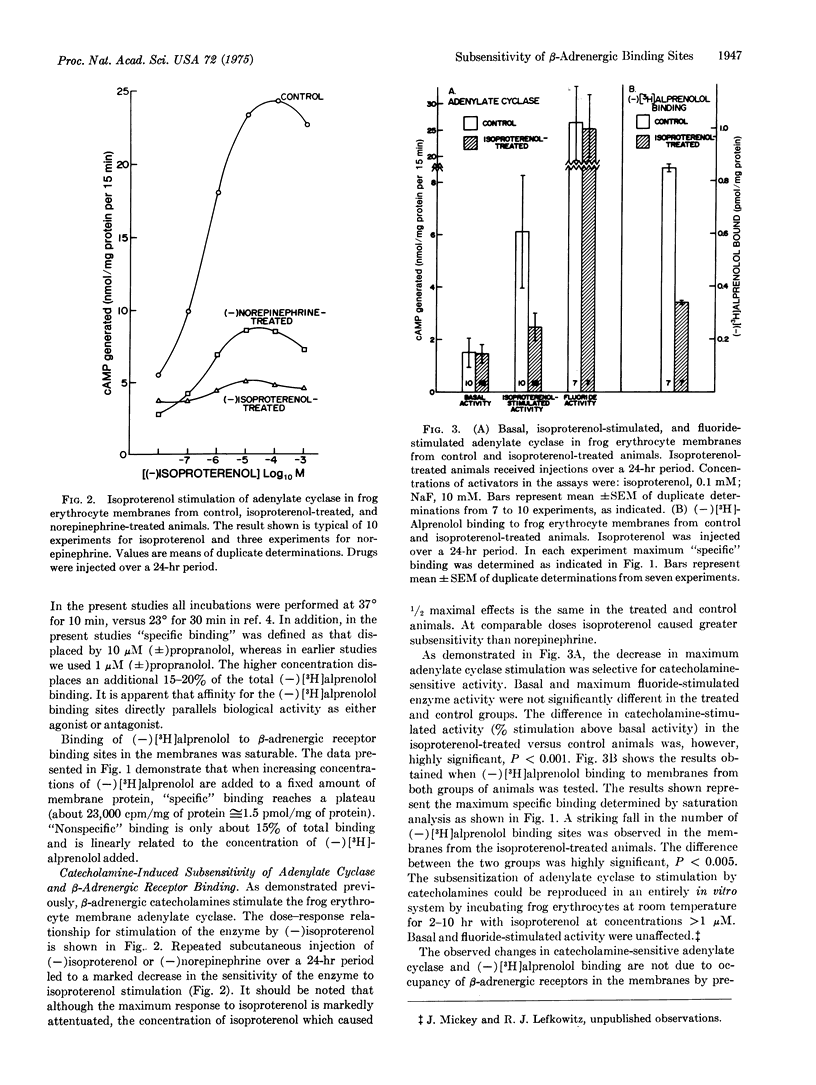
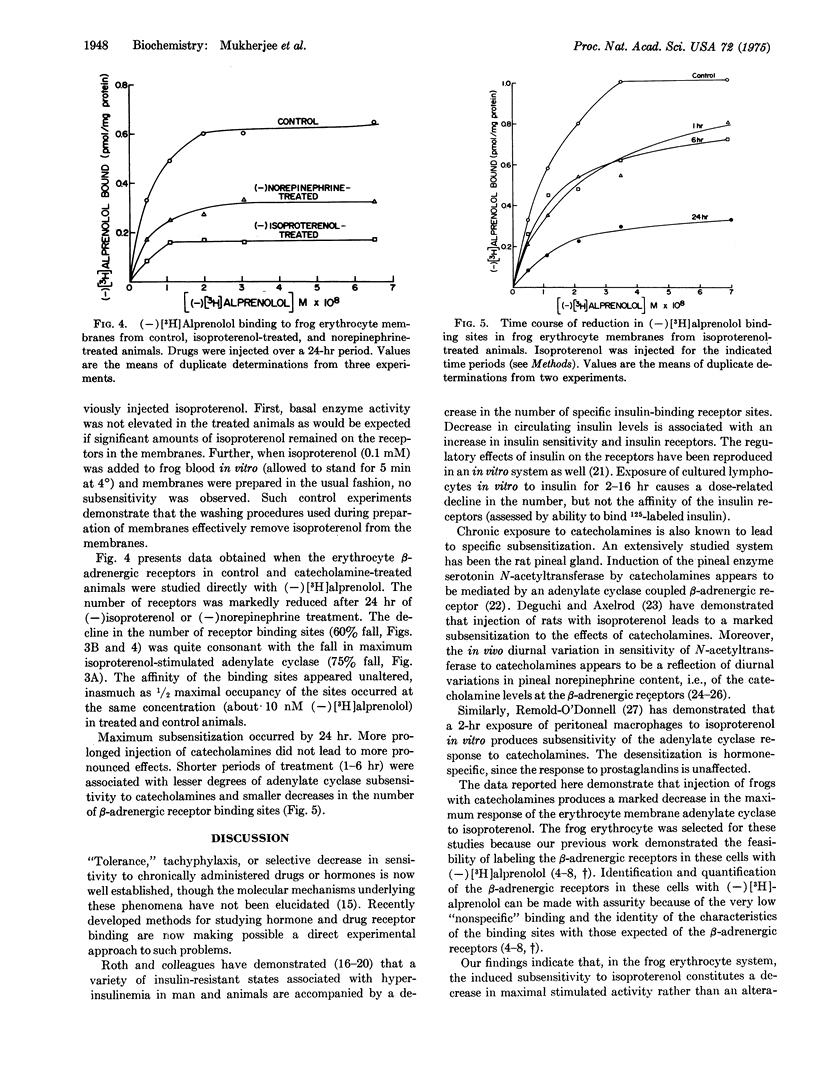

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W., Williams L. T., Lefkowitz R. J. Identification of cardiac beta-adrenergic receptors by (minus) [3H]alprenolol binding. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1564–1568. doi: 10.1073/pnas.72.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. A., Gorden P., Gavin J. R., 3rd, Lesniak M. A., Roth J. Insulin receptors in human circulating lymphocytes: application to the study of insulin resistance in man. J Clin Endocrinol Metab. 1973 Apr;36(4):627–633. doi: 10.1210/jcem-36-4-627. [DOI] [PubMed] [Google Scholar]
- Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974 Jun 28;184(4144):1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Aurbach G. D. A beta-adrenergic receptor of the turkey erythrocyte. I. Binding of catecholamine and relationship to adenylate cyclase activity. J Biol Chem. 1973 Aug 25;248(16):5577–5583. [PubMed] [Google Scholar]
- Brownstein M., Axelrod J. Pineal gland: 24-hour rhythm in norepinephrine turnover. Science. 1974 Apr 12;184(4133):163–165. doi: 10.1126/science.184.4133.163. [DOI] [PubMed] [Google Scholar]
- Caron M. G., Lefkowitz R. J. Temperature immutability of adenyl cyclase-coupled beta adrenergic receptors. Nature. 1974 May 17;249(454):258–260. doi: 10.1038/249258a0. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Induction and superinduction of serotonin N-acetyltransferase by adrenergic drugs and denervation in rat pineal organ. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2208–2211. doi: 10.1073/pnas.69.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Supersensitivity and subsensitivity of the beta-adrenergic receptor in pineal gland regulated by catecholamine transmitter. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2411–2414. doi: 10.1073/pnas.70.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. The pharmacological differentiation of adrenergic receptors. Ann N Y Acad Sci. 1967 Feb 10;139(3):553–570. doi: 10.1111/j.1749-6632.1967.tb41229.x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Kahn C. R., Neville D. M., Jr, Roth J., Garrison M. M., Bates R. W. Decreased binding of insulin to its receptors in rats with hormone induced insulin resistance. Biochem Biophys Res Commun. 1973 Aug 6;53(3):852–857. doi: 10.1016/0006-291x(73)90171-x. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Gorden P., Freychet P., Roth J. Insulin receptor defect in insulin resistance: studies in the obese-hyperglycimic mouse. Biochem Biophys Res Commun. 1972 Jul 11;48(1):135–142. doi: 10.1016/0006-291x(72)90354-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mukherjee C., Coverstone M., Caron M. G. Stereospecific (3H)(minus)-alprenolol binding sites, beta-adrenergic receptors and adenylate cyclase. Biochem Biophys Res Commun. 1974 Sep 23;60(2):703–709. doi: 10.1016/0006-291x(74)90297-6. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J. Stimulation of catecholamine-sensitive adenylate cyclase by 5'-guanylyl-imidodiphosphate. J Biol Chem. 1974 Oct 10;249(19):6119–6124. [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Axelrod J. Pineal beta-adrenergic receptor: diurnal variation in sensitivity. Science. 1974 Jun 7;184(4141):1091–1092. doi: 10.1126/science.184.4141.1091. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Erlichman J., Rosen S. M. The structure-activity relationships of adrenergic compounds that act on the adenyl cyclase of the frog erythrocyte. Mol Pharmacol. 1970 Sep;6(5):524–531. [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Goldfine I. D., Roth J., Kahn C. R. Thymic lymphocytes in obese (ob-ob) mice. A mirror of the insulin receptor defect in liver and fat. J Biol Chem. 1974 Jul 10;249(13):4127–4131. [PubMed] [Google Scholar]
- Waud D. R. Pharmacological receptors. Pharmacol Rev. 1968 Jun;20(2):49–88. [PubMed] [Google Scholar]



