Abstract
Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae are the common pathogens that colonize in the nasopharynx of children. Polymicrobial interactions are thought to play an important role in different sites throughout the human body. However, there are currently very few studies that investigate the interactions between S. aureus, S. pneumoniae, and H. influenzae in the nasopharynx. We retrospectively analyzed the adenoid tissue culture from 269 children who received adenoidectomy. S. aureus, S. pneumoniae, and H. influenzae constituted the major microorganisms which were cultured from these adenoidectomies, at 23.4%, 21.6%, and 18.2%, respectively. S. pneumoniae and H. influenzae were the most prevalent in the preschool-aged children (3 < age ≤ 6), whereas S. aureus was more prevalent in infants and toddlers (age ≤ 3) and school-aged children (age > 6). Bacterial interference was found between S. aureus and S. pneumoniae and between S. aureus and H. influenzae, whereas there was an association found between S. pneumoniae and H. influenzae. The synergism and antagonism among these three species are investigated in the following paper, with the possible mechanisms involved in these interactions also discussed.
Keywords: Adenoid;, Bacterial interactions;, Haemophilus influenzae;, Staphylococcus aureus;, Streptococcus, pneumoniae
1. Introduction
As the adenoid is located at the crossroads of the upper respiratory tract, adjacent to the middle ear, paranasal sinuses and oropharynx, chronic adenoiditis has been associated with the pathologies of the neighboring structures, such as otitis media and sinusitis [1]. The adenoid can serve as a bacterial reservoir that contributes to chronic otolaryngologic infections in children, infections such as otitis media and paranasal sinusitis [2]. The most common nasopharyngeal microbes that are found in children include S. aureus, S. pneumoniae, and H. influenzae [3]. S. pneumoniae is frequently concomitant with nasopharyngeal illnesses [4], while H. influenzae is a common pathogen of acute otitis media [5]. S. aureus is associated with skin or respiratory tract diseases such as chronic adenoiditis and rhinosinusitis [6, 7]. The emergence of methicillinresistant S. aureus (MRSA) has become an important public health problem, both as a rising community pathogen and with respect to its potential impact on strategies for antibiotic therapy [7].
More than one microorganism is frequently found in the nasopharynx and polymicrobial interactions definitely exist in the nasopharynx [3, 8-11]. Some bacterial species may co-exist more often with other species (synergistic interactions), while other species may compete with one another (antagonistic interactions). For an example of the latter: competitive interaction has been reported between S. pneumoniae and S. aureus [9, 11]. However, a detailed description regarding the interactions between S. aureus, S. pneumoniae, and H. influenzae in the nasopharynx of children is still limited.
The purpose of this study was to analyze the nasopharyngeal colonizations by the bacterial species S. aureus, S. pneumoniae, and H. influenzae in children receiving adenoidectomy. The interactions among the bacterial species were evaluated to see whether the colonization status of one species influences the colonization of the other two species.
2. Patients and methods
2.1. Patient selection
This study was carried out between January 2002 and December 2012 and comprised patients who were examined for otorhinolaryngologic infections, including chronic otitis media, otitis media with effusion, chronic rhinosinusitis, chronic adenoiditis, and chronic tonsillitis as well as those who were clinically diagnosed with upper respiratory problems. During this period, 276 participants were enrolled in this study and underwent routine adenoidectomy surgery and had a bacterial culture of their nasopharynx taken. A total of 269 patients whose ages ranged from 1 to 18 years old were analyzed. There were 102 girls (37.9%) and 167 boys (62.1%). The patients enrolled in this study had completed a self-administered questionnaire by their parents prior to being enrolled.
2.2. Ethics statement
This study was specifically approved by the Institutional Review Board of the China Medical University Hospital (approval number: DMR98-IRB-123, Taichung, Taiwan).
2.3. Laboratory procedure and bacterial culture
Core tissues from adenoid specimens and pus swabs from patients’ noses were streaked across Tryptic soy agar (Becton- Dickinson, Franklin Lakes, NJ, USA) containing 5% sheep blood and incubated at 37°C for 18-24 h. Bacterial isolates were identified by a standard protocol using the BD PhoenixTM Automated Microbiology System (Becton-Dickinson) as described in our previous study [7].
2.4. Statistical analysis
The relationship of between-group comparisons was performed using a Chi-square test with Fisher’s exact test. The correlation of bacterial infections in two species was assessed by odd ratio (OR) analysis. Descriptive statistics were determined as the proportion for categorical variables with 95% confidence intervals (CI). Statistical analyses were carried out using the SPSS program (version 12.0; SPSS Inc., Chicago, IL, USA). A P value less than 0.01 was considered statistically significant.
3. Results
3.1. Demography of the enrolled patients
To analyze the association between the carriages of three bacterial species, the young children with otorhinolaryngologic infections who visited China Medical University Hospital were enrolled in this study. The bacterial colonizations of the nasopharynx from children receiving adenoidectomies were then identified by a traditional culture method. Of all 276 participants, 269 patients < 18 years old were enrolled in this analysis. We then stratified the patients into four age stages: stage 1: age ≤ 3; Stage 2: 3 < age ≤ 6; Stage 3: 6 < age ≤ 12; Stage 4: age > 12. As shown in Table 1, there were 15, 138, 106, and 10 children in stages 1, 2, 3, and 4, respectively. Within this analysis, no bacterial species was isolated in 37 patients. However, at least one bacterial species of microbial colonization was cultured in 232 patients.
Table 1.
The demography of the enrolled patients.
| Characteristic | No. (%) |
|---|---|
| Age (years)† | |
| age ≤ 3 | 15 (5.6) |
| 3 < age ≤ 6 | 138 (51.3) |
| 6 < age ≤ 12 | 106 (39.4) |
| 12 < age | 10 (3.7) |
| Gender¶ | |
| F | 102 (37.9) |
| M | 167 (62.1) |
| Bacteria present | |
| 0 | 37 (13.8) |
| 1 | 160 (59.5) |
| 2 | 56 (20.8) |
| ≥ 3 | 16 (6.0) |
†Age (years) of children at the time of adenoidectomy.
¶F, female; M, male.
3.2. The associations of bacterial colonizations in children receiving adenoidectomy
To further analyze the associations between the pathogens which colonized in children who were receiving adenoidectomies, three bacterial species’ (S. pneumoniae, S. aureus, and H. influenzae) colonization in the aforementioned stages were analyzed. As shown in Figure 1A, higher rates of S. aureus colonized in patients belonging to stages 1, 3, and 4, S. pneumoniae colonization was lower at the same stages. Consistently, higher S. aureus colonization in stages 1, 3, and 4 were inversely related to H. influenzae infection in patients in these stages (Figure 1B). The bacterial carriage of S. pneumoniae was negatively associated with H. influenzae in stages 1 and 4. However, higher rates of S. pneumoniae colonized in stages 2 and 3, with higher rates of H. influenzae infection in the same stages (Figure 1C).
Fig. 1.
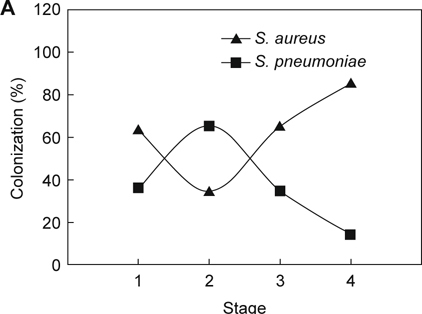

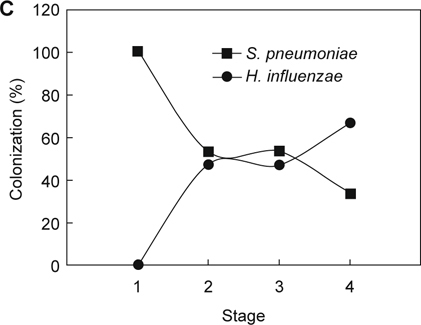
The age-related bacterial interactions in the nasopharynx of children receiving adenoidectomy. Patients who enrolled in this study were stratified into four age stages: Stage 1: age ≤ 3; Stage 2: 3 < age ≤ 6; Stage 3: 6 < age ≤ 12; Stage 4: age > 12. The colonization rates of each comparison between two bacterial species were determined and analyzed: (A) S. aureus vs. S. pneumoniae; (B) S. aureus vs. H. influenzae; (C) S. pneumoniae vs. H. influenzae.
We then analyzed the correlation of bacterial infections in two species using logistic regression analysis. As shown in Table 2, S. aureus, S. pneumoniae, and H. influenzae constitute major microorganisms cultured from these adenoidectomies, at 23.4%, 21.6% to 18.2%, respectively. S. aureus colonization was significantly inversely associated with S. pneumoniae colonization and vice versa (OR = 0.31; 95% CI = 0.13-0.77, P = 0.008). Additionally, a negatively associated relationship was observed between S. aureus and H. influenzae (OR = 0.17; 95% CI = 0.05-0.58, P = 0.002). Although the bacterial carriages of S. pneumoniae was inversely associated with H. influenzae in stages 1 and 4 (Figure 1C), there was no significance (P = 0.325).
Table 2.
Inverse association of bacterial colonization in the nasopharynx of children.
| S. aureus | S. pneumoniae | H. influenzae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | No. (%) | OR† (95% CI) | P value¶ | No. (%) | OR (95% CI) | P value | No. (%) | OR (95% CI) | P value | ||
| S. aureus |
Positive Negative |
63 206 |
|||||||||
| S. pneumoniae |
Positive Negative |
58 211 |
6 (10.3) 57 (27.0) |
0.31 (0.13-0.77 ) | 0.008 | - | |||||
| H. influenzae |
Positive Negative |
49 220 |
3 (4.8) 46 (22.3) |
0.17 (0.05-0.58 ) | 0.002 |
8 (13.8) 41 (19.4) |
0.66 (0.29-1.51) | 0.325 | - |
†OR, odd ratio.
¶P value was determined from logistic regression model. A significant difference is indicated by a number in bold.
4. Discussion
In this study, we investigated the colonization of the nasopharynx in children receiving adenoidectomies using a traditional culture method. Our data showed that S. aureus, S. pneumoniae, and H. influenzae constitute major microorganisms cultured from the adenoidectomies at 23.4%, 21.6% to 18.2%, respectively (Table 2). These findings are similar to the previous study that S. pneumoniae, H. influenzae, Moraxella catarrhalis, and S. aureus are common nasopharyngeal colonizations found in children [3], though M. catarrhalis was not frequently isolated in our study. More than one microorganism was found in 26.8% of children receiving adenoidectomies, whereas no bacterium was cultured in 13.8% of the adenoid specimens (Table 1). The identification rate of microorganisms in this study may be under estimated. With the advances in microbial techniques such as real-time quantitative polymerase chain reaction (qPCR) technique, in future studies there may be more diverse microorganisms identified [12].
Our study showed that S. pneumoniae and H. influenzae were most prevalent in stage 2 (preschool period) (3 < age ≤ 6), whereas S. aureus was more prevalent in stage 1 (infant and toddler stage) (age ≤ 3) and stage 3&4 (age > 6). This result demonstrated that the prevalence of bacterial species may be varied in different age groups. Host factors including age may be important in the nasopharyngeal reservoir. Dynamic changes in nasopharyngeal microflora have been described [13]. Healthy children were generally colonized with relatively non-pathogenic microbes in their nasopharynx. S. aureus was frequently found in the infant period, with its carriage decreasing with a person’s age [14]. Conversely, S. pneumoniae and H. influenzae were not frequent isolates from the infant period [14] until the pre-school period [13]. However, carriage of potential respiratory pathogens such as S. pneumoniae and H. influenzae increased when purulent nasopharyngitis occurred [13]. In addition to age, other factors may influence the dynamic alterations of microbes in the nasopharynx. These factors include immunity, sibling number, crowding, season, use of antibiotics, acute respiratory tract infection, vaccine application, and passive smoking exposure [5, 15, 16].
Our study showed that S. aureus was inversely associated with S. pneumoniae and H. influenzae. This finding is consistent with several previous studies about the negative association of S. aureus with S. pneumoniae in the nasopharynx [10, 17-19]. Adaptive immunity has been proposed because such interference between S. aureus and S. pneumoniae was not shown in HIV-infected children [10, 17]. Free radicals may be another possible mechanism to explain this bacterial interference as the hydrogen peroxide produced by S. pneumoniae could elicit bactericidal activity toward S. aureus and prevent its colonization [19]. The interference between S. aureus and H. influenzae has also been shown [20]. Additionally, the different susceptibility in biofilm formation to environment such as hyaluronic acid has been proposed [21].
Contrary to the interference phenomenon between S. aureus and the other two species, an association was found between S. pneumoniae and H. influenzae, although the interaction was not significant. This result was similar to the previous epidemiologic observations [9, 10, 22, 23]. H. influenzae has been shown to promote the biofilm formation in S. pneumoniae [24]. However, similar free radical formation was also shown in vitro that the formation of hydrogen peroxide from the S. pneumoniae could inhibit the growth of H. influenza [25]. Another epidemiological observation showed an interference phenomenon between S. pneumoniae and H. influenzae, but the association could shift from negative to positive when M. catarrhalis appeared in the interaction [3]. These studies showed the complicated phenomenon in the microenvironment between bacterial synergism and antagonism.
This study presents the microbiological dynamics and the microbial interactions in the nasopharynx of children receiving adenoidectomies. A more complete understanding of how bacteria interact with each other may be important in future designs of preventive or therapeutic strategies. This may be important in the era of new vaccine or antimicrobial development, in which the influence of one specific bacterium may have a positive or negative impact on other species. Our study confirmed the interference between S. aureus and both S. pneumoniae and H. influenzae, and a possible association between S. pneumoniae and H. influenzae. The potential implications of targeting these interactions may serve as a route towards control of bacterial infections.
5. Conclusions
In this study, polymicrobial interactions were studied in the nasopharynxes of children who received adenoidectomies. Bacterial interference was found between S. aureus and S. pneumoniae and between S. aureus and H. influenzae, whereas, an association was found between S. pneumoniae and H. influenzae. These findings lead to the appreciation that many infections are polybacteria in nature, and that interactions between different microorganisms may contribute to disease progression and clinical outcomes.
Acknowledgements
The authors thank Dr. Ming-Chei Maa for her valuable suggestions and editorial assistance. We also thank Biostatistics Center at China Medical University for the data analysis. This work was funded by the Ministry of Science and Technology (102-2314- B-039-023-MY2, 103-2633-B-039-001, and 103-2815-C-039- 062-B), Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University (CMU102-ASIA-21 and DMR103- 024), and the Tomorrow Medicine Foundation.
Declaration of interest
The authors declare no conflicts of interest for this work.
Contributor Information
Hao-Xiang Chen, Email: d6355@mail.cmuh.org.tw.
Chih-Ho Lai, Email: d6355@mail.cmuh.org.tw.
Chia-Der Lin, Email: d6355@mail.cmuh.org.tw.
References
- 1.van Cauwenberge PB, Bellussi L, Maw AR, Paradise JL, Solow B. The adenoid as a key factor in upper airway infections. Int J Pediatr Otorhinolaryngol 1995; 32 Suppl: S71–80. [DOI] [PubMed]
- 2.Nistico L, Kreft R, Gieseke A, Coticchia JM, Burrows A, Khampang P. Adenoid reservoir for pathogenic biofilm bacteria. J Clin Microbiol. 2011;49:1411–20. doi: 10.1128/JCM.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–91. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009; 30: 189–209. [DOI] [PubMed]
- 5.Garcia-Rodriguez JA, Fresnadillo Martinez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 2002; 50 Suppl S2: 59–73. [DOI] [PubMed]
- 6.Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:34–8. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 7.Lin CD, Tsai MH, Lin CW, Ho MW, Wang CY, Tsou YA. Association of adenoid hyperplasia and bacterial biofilm formation in children with adenoiditis in Taiwan. Eur Arch Otorhinolaryngol. 2012;269:503–11. doi: 10.1007/s00405-011-1704-x. [DOI] [PubMed] [Google Scholar]
- 8.Dunne EM, Smith-Vaughan HC, Robins-Browne RM, Mulholland EK, Satzke C. Nasopharyngeal microbial interactions in the era of pneumococcal conjugate vaccination. Vaccine. 2013;31:2333–42. doi: 10.1016/j.vaccine.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby P, Watson K, Bowman J, Taylor A, Riley TV, Smith DW. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine. 2007;25:2458–64. doi: 10.1016/j.vaccine.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhi SA, Adrian P, Kuwanda L, Cutland C, Albrich WC, Klugman KP. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae-and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization-in HIV-Infected and HIV-uninfected children. J Infect Dis. 2007;196:1662–6. doi: 10.1086/522164. [DOI] [PubMed] [Google Scholar]
- 11.Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. JAMA. 2004;292:716–20. doi: 10.1001/jama.292.6.716. [DOI] [PubMed] [Google Scholar]
- 12.Chien YW, Vidal JE, Grijalva CG, Bozio C, Edwards KM, Williams JV. Density interactions among Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in the nasopharynx of young Peruvian children. Pediatr Infect Dis J. 2013;32:72–7. doi: 10.1097/INF.0b013e318270d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook I. Microbial dynamics of purulent nasopharyngitis in children. Int J Pediatr Otorhinolaryngol. 2003;67:1047–53. doi: 10.1016/S0165-5876(03)00203-9. [DOI] [PubMed] [Google Scholar]
- 14.Harrison LM, Morris JA, Telford DR, Brown SM, Jones K. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol. 1999;25:19–28. doi: 10.1111/j.1574-695X.1999.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 15.Torun MM, Namal N, Demirci M, Bahar H. Nasopharyngeal carriage and antibiotic resistance of Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis in healthy school children in Turkey. Indian J Med Microbiol. 2009;27:86–8. [PubMed] [Google Scholar]
- 16.Peacock SJ, Justice A, Griffiths D, de Silva GD, Kantzanou MN, Crook D, Sleeman K, Day NP. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol. 2003;41:5718–25. doi: 10.1128/JCM.41.12.5718-5725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintero B, Araque M, van der Gaast-de Jongh C, Escalona F, Correa M, Morillo-Puente S. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. Eur J Clin Microbiol Infect Dis. 2011;30:7–19. doi: 10.1007/s10096-010-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rumke HC. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–2. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 19.Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis E, Yates A, Levin BR. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiol. 2010;10:5–9. doi: 10.1186/1471-2180-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drago L, Cappelletti L, De Vecchi E, Pignataro L, Torretta S, Mattina R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS. 2014;122:1013–9. doi: 10.1111/apm.12254. [DOI] [PubMed] [Google Scholar]
- 22.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27:59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jourdain S, Smeesters PR, Denis O, Dramaix M, Sputael V, Malaviolle X. Differences in nasopharyngeal bacterial carriage in preschool children from different socio-economic origins. Clin Microbiol Infect. 2011;17:907–14. doi: 10.1111/j.1469-0691.2010.03410.x. [DOI] [PubMed] [Google Scholar]
- 24.Weimer KE, Armbruster CE, Juneau RA, Hong W, Pang B, Swords WE. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis. 2010;202:1068–75. doi: 10.1086/656046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pericone CD, Overweg K, Hermans PW, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun. 2000;68:3990–7. doi: 10.1128/IAI.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


