Abstract
We examine how aging is impacted by various chemical challenges that organisms face and by the molecular mechanisms that have evolved to regulate lifespan in response to them. For example, environmental information, which is detected and processed through sensory systems, can modulate lifespan by providing information about the presence and quality of food as well as presence and density of conspecifics and predators. In addition, the diverse forms of molecular damage that result from constant exposure to damaging chemicals that are generated from the environment and from metabolism pose an informatic and energetic challenge for detoxification systems, which are important in ensuring longevity. Finally, systems of innate immunity are vital for recognizing and combating pathogens but are also seen as of increasing importance in causing the aging process. Integrating ideas of molecular mechanism with context derived from evolutionary considerations will lead to exciting new insights into the evolution of aging.
Keywords: Aging, chemical complexity, evolutionary conservation, sensory perception, innate immunity, xenobiotic metabolism
GENERAL INTRODUCTION
The evolution of the biology of aging
From the pioneering work of Medawar, Williams, and Hamilton in the 1950s until the early 1990s, evolutionary biology dominated the intellectual landscape surrounding analysis of not only why we age but how (Medawar 1952; Williams 1957; Hamilton 1966). Aging, a decline in condition with increasing age apparent as a reduction in survival and reproductive output, is apparently disadvantageous for the individual (Partridge and Barton 1993). Why then, does it exist at all? Is there some hidden advantage to aging? Evolutionary biologists tackled these questions early on and concluded that aging does not have a function and exists only because natural selection is less powerful late in life. Aging can then evolve through accumulation of mutations with deleterious effects at later ages or as the deleterious late-life consequence of traits that are beneficial in youth (Partridge and Barton 1993; Charlesworth 1994). More recent work has revealed that the force of natural selection does not always decline, and even increases, over some age classes (Baudisch 2005) and that kin selection (Bourke, this volume) and intergenerational transfers (Lee 2003) are important. Nonetheless, these two basic tenets, that aging is due to a declining force of natural selection and is not adaptive, still form the conceptual foundation of the biology of aging (Hughes and Reynolds 2005; Ackerman and Pletcher 2007).
A prediction of the traditional evolutionary models was that patterns of aging would be highly polygenic. Accumulation of random mutations with deleterious effects at older ages would mean that single-gene manipulations would, in principle, have minimal effect. A fix for the effects of one mutation would leave the deleterious effects of vast majority unaltered, and would hence have only a minor effect on the overall rate of aging (Rose 1991; Martin 2002). Even if trade-offs between youth and old age were important, survival and reproductive success are highly polygenic traits; any gene that did not affect one or the other would undergo mutational death. A second prediction was that the mechanisms that protected against aging would be lineage-specific, the rationale for this being that each species would have a particular lifestyle associated with a particular set of environmental and physiological impacts. There would hence be great diversity in the types of challenges that different kinds of organisms would experience. Aging was a trait that was not expected to show evolutionary conservation of mechanisms (Martin 2002).
Near the end of the 20th century the predictive foundation of the evolutionary biology of aging was severely shaken by the experiments of molecular geneticists using laboratory, model organisms. These established that alterations in the expression or function of single genes were sufficient to retard aging and increase lifespan, sometimes up to 20-fold (Klass 1983; Arantes-Oliveira et al. 2003; Kenyon 2005). More recently, particularly types of intervention have been shown to be effective in species ranging from budding yeast and the nematode worm to the laboratory mouse, establishing the existence of conserved mechanisms of aging across a broad range of taxa (Partridge and Gems 2002).
These findings came as a surprise and not only to evolutionary biologists. During aging, multiple forms of tissue-specific damage and pathology accumulate (Kirkwood et al. 1999). The process can also vary greatly between individuals (Kirkwood et al. 2005). This complexity and variability suggested that aging would be intractable to medical and experimental intervention. The discoveries that single gene mutations could extend lifespan by keeping organisms healthy and youthful for longer and that their effects showed evolutionary conservation has galvanized research into mechanisms of aging.
Chemical complexity and the diversity of aging
As alluded to earlier, aging is not selected for -- selection declines in strength with increasing age-- but it can be selected against, and an important challenge is to discover how slow aging is accomplished. The types of ecological circumstances that select for slow aging are well specified, and include low levels of extrinsic risk of death from disease, predation or accidents (Medawar 1952; Charlesworth 1994) and periods of resource depletion and overall variability and unpredictability of the environment (Orzack 1989). These factors have contributed to enormous biodiversity in patterns and rates of aging, as a consequence of evolved differences between taxa. Comparative analysis of the ecological and lifestyle determinants of slow aging (Holmes and Austad 1995; Austad 1997; Ricklefs et al. 2003) are insightful but have generally been less successful in identifying mechanisms, because of the presence of multiple, correlated, candidate traits. An additional, important limitation is the availability of genetic information on relevant species. This new field of ‘evo-gero’ (Partridge and Gems 2006), much like ‘evo-devo’, would greatly benefit from sequencing of the genomes of species with extraordinary lifespans (de Magalhaes et al. 2005).
While it is fascinating that a mouse lives three years and a parrot may live to 100 (a difference of 33-fold), it is arguably more stunning that individuals of identical genotype can vary enormously in their average life expectancy, by at least up to 80-fold (Gardner et al. 2006). This remarkable phenotypic plasticity in lifespan demonstrates that the same genome can give rise to very different patterns of aging, with the strong supposition that at least some of this naturally occurring plasticity represents adaptive responses to different environmental circumstances. Presumably, the manifestation of plasticity requires mechanisms that initiate and maintain differences in gene expression. Although work has begun, the details of this process are largely unknown (Jaenisch and Bird 2003).
The major theme of this review is that mechanisms that underlie variation in patterns of aging among and within species may often revolve around the issue of chemical complexity. By definition, living long increases the time for which the organism is exposed to myriad potentially damaging conditions. An important aspect of survival, therefore, must invoke mechanisms of somatic endurance, including protection against physical and biological challenges as well as systems to deal with exogenous or endogenous chemical toxicity. Chemicals are not all bad, however, and even potentially harmful molecules may serve as cues that may be used as sources of information. Both ensuring survival and producing appropriate patterns of phenotypic plasticity in response to environmental variation require that organisms gather information about their environment. This information often comes in the form of particular chemicals that are usually associated with particular states of the physical environment, food, conspecifics and other species, including predators and pathogens. This information can be used to guide mechanisms of phenotypic plasticity, including of life history and aging. Indeed, it is intriguing that many of the mutations that extend lifespan in model organisms impinge on stress resistance or affect signaling pathways that detect physical stress, nutrients or other organisms.
In addition to discovering how particular organisms resist aging, a second important challenge is to understand at what levels evolutionary conservation occurs, and why. Gross pathologies of aging can differ greatly in different organisms: humans can die from stroke and cancer, while nematodes and fruit flies do not. There are at least some differences at the molecular level too: for example, accumulation of extrachromosomal ribosomal DNA circles contributes to aging in budding yeast (Saccharomyces cerevisiae) (Sinclair and Guarente 1997), and extrachromosomal mitochondrial DNA circles (senDNAs) to aging in the filamentous fungus Podospora anserine (Osiewacz 2002); neither contribute to aging in mammals. Thus, at least some mechanisms of aging are private (lineage-specific) rather than public (evolutionarily conserved) (Martin et al. 1996). It is important to discover if any general principles are involved here. For instance, there could be little or no conservation of the specific forms of pathology that result from similar forms of molecular damage, or conserved sensing pathways could regulate different types of cellular biochemistry in different evolutionary lineages.
In this article we do not attempt to review the whole literature on the evolution of aging. Rather, we explore the idea that a primary factor modulating lifespan is how organisms deal with chemical complexity, both in their environment and internally. The way in which animals detect, decode and respond to a range of chemical factors they encounter may be critical for establishing somatic endurance and for specifying decisions that implement plasticity of the aging process. In the first section, we examine chemical sensing and adaptive plasticity of aging. Next, we explore the role of damage control systems, such as detoxification that ensure somatic endurance in the face of continuous chemical insult. Lastly we touch on the impact of well-controlled pathogen defense mechanisms on aging. It is becoming increasingly clear that the chemicals that confront individual species may be unique and diverse, but many aspects of the underlying response mechanisms may be evolutionarily conserved. Thus, we argue for serious consideration of ecological, environmental, and other chemical cues as potent regulators of potentially evolutionarily conserved molecular processes.
CHEMICAL SENSING AND THE PLASTICITY OF AGING
Chemicals as information
Ecologists and evolutionary biologists have long recognized the primary importance of chemical stimuli from the environment for influencing the phenotypes of individuals. Sensory perception of particular chemical cues can influence response to predation (Leonard et al. 1999), habitat selection (Morse 1991), mating (Brennan and Kendrick 2006), and the expression of costly defense mechanisms (Darst et al. 2006). Presence of predators can also induce dramatic physiological or developmental alterations (Voronezhskaya et al. 2004), such as the growth of protective helmets in various species of Daphnia in response to waterborne cues from predators (Pijanowska and Kloc 2004). Organisms can also respond to chemical cues that indicate the density or presence of conspecifics or competing species, a process that has been subjected to incisive evolutionary analysis in microorganisms (Keller and Genoud 1997; West et al. 2006). The function and potency of specific cues can also depend on the age of the organism (Zimmer et al. 2006).
Chemical cues from the environment can also influence the plasticity of aging, which can occur on different time scales. Broadly speaking, one form is largely developmental in nature and is often associated with the formation of different morphs within a given life-cycle. Social insects, for example, exhibit caste differences in lifespan (Chapuisat and Keller 2002), with the evolution of eusociality strongly associated with the evolution of increased lifespan in reproductive females (Keller and Genoud 1997). The nematode Strongyloides ratti can adopt a free-living form, which lives roughly 5 days, or a parasitic form, which can live up to 400 days (Gardner et al. 2006). Other forms of plasticity in aging are more malleable. The common laboratory nematode, Caenorhabditis elegans, has a well known alternate development stage called the dauer (Cassada and Russell 1975), which is reversible (discussed in detail in 2.3). In the fruit fly, Drosophila melanogaster, nutrient availability affects patterns of aging in a completely reversible manner (Mair et al. 2003).
Environmental Perception
Chemicals can provide information about environmental variables that can be used to inform life history decisions. For instance, sensing of the nutritional value of food may often involve the use of indirect chemical information. Nutritional content of food is usually considered in terms either of its contribution of critical components to biochemical reactions or of its overall energetic content. Many hypotheses concerning the mechanism of dietary restriction (DR; an environmental manipulation whereby reduced nutrient intake without malnutrition increases lifespan of a wide range of species (see sections 2.3, 2.4)) thus focus on the nutritional content of the food. These hypotheses postulate that increased longevity under DR results from changes in metabolic rate (Faulks et al. 2006), alterations in nutritional resource allocation (Shanley and Kirkwood 2000) and/or cellular responses to energy depletion (Lin et al. 2000). However, changes in metabolic rate are often not involved in the response to DR (Hulbert et al. 2004; Houthoofd et al. 2007). Furthermore many aspects of DR can be achieved by manipulating specific components of the diet (Mair et al. 2005) even with negligible change in overall energy content of the food (Zimmerman et al. 2003; Miller et al. 2005).
The focus on nutrients as energy has drawn attention away from the possibility that specific components of the food or aspects of the environment may provide powerful cues that alter biological processes. In their simplest manifestations, such as nutrient-directed chemotaxis, environmental perception and response have obvious advantages. After detecting the presence of food through olfactory or gustatory sensory systems, for example, most organisms will alter their behavior to isolate and converge on the source of the stimulus. Such basic information-processing capabilities are often considered critical for survival and are essentially present in nearly all living things (Keller and Surette 2006). The impact of relevant chemical cues might be mediated through traditional sensory systems, such as smell, taste or touch, or they may act at the cellular level through direct molecular interactions with target proteins.
In this section we consider the emerging literature on how specific chemical cues, which may indicate the overall status of the environment or the availability of nutrients, induce inclusive phenotypes such as physiology and aging. Work in laboratory model organisms indicates that activity of the external sensors of the perceptual system is sufficient to influence lifespan and a range of developmental and physiological processes. Furthermore, there is growing evidence for the importance of internal physiological and cellular processes, a phenomenon that we term molecular sensing, where specific chemical compounds derived from an organism’s environment bypass sensory systems and induce biological changes by directly interacting with target cellular proteins. In a few instances, the specific cues that trigger these responses and the downstream molecular pathways that implement them have been identified. Direct experimental evidence for an important role of environmental perception on aging is limited mostly to yeast, flies, and worms, but there is considerable indication that the pathways that implement response to environmental cues, if not the cues themselves, are evolutionarily conserved.
Canonical sensory systems
Genetically, one of the well-understood examples of environment-induced plasticity in development and lifespan is seen in C. elegans. At the first larval stage, the worm makes a developmental decision. Either it proceeds through normal development or it adopts an alternative third larval stage called dauer diapause. Normal larval development is usually complete within 3 days. Adult reproductive development follows and the most common adult form, a self-fertilizing hermaphrodite, lives approximately 14-20 days and produces roughly 250-300 eggs (Cassada and Russell 1975). Dauer animals undergo large-scale tissue remodeling and recycling of cellular components. They are non-feeding, remain sexually immature, and exhibit social behaviors not generally seen in hermaphrodite adults (Cassada and Russell 1975). Metabolism is shifted to favor fat and carbohydrate storage, and the dauer animal is stress resistant and can live for several months (Golden and Riddle 1982; Burnell et al. 2005).
The decision to enter dauer or proceed with normal development is largely determined by sensory integration of two cues: food availability and a dauer pheromone called the daumone (Golden and Riddle 1982). The daumone is constitutively secreted by the animals and consists of a pyranose sugar conjugated to a fatty acid (Jeong et al. 2005). Specific amphid neurons (ADF, ASI, ASG) likely sense the daumone (Bargmann 2006) and appropriate responses require G protein signaling and the genes gpa-2 and gpa-3 (Zwaal et al. 1997). Too high a population density will lead to increased local concentration of daumone and will force dauer development. Currently unidentified food cues are thought to be sensed by the same neurons and in this way increased food resources antagonize the daumone. Thus, the nematode senses environmental quality and induces adaptive metabolic decisions (Gerisch and Antebi 2004). In addition to their role in developmental decisions, the nematode neurosensory organs can modulate lifespan in normal adult worms. Mutations that disrupt the structure and/or function of sensory neurons result in increased longevity, as does laser ablation of the two amphid sheath cells, which support the amphid neurons (Apfeld and Kenyon 1999). Laser ablation of specific sensory neurons has variable effects; removal of specific gustatory and olfactory neurons can increase, decrease, or have no effect on lifespan. Thus, some sensory neurons produce signals that antagonize long life, while others promote it (Antebi 2004).
Evidence from similar work in D. melanogaster shows that the regulation of aging by canonical sensory input is evolutionarily conserved. When flies are confined to a regime of DR, exposure to food-based odorants reduces lifespan (Libert et al. 2007). This effect is absent when flies are fully-fed; suggesting that perception of nutrient availability may reverse a subset of the physiological alterations induced by DR and may limit its benefits. Loss of function of the odorant receptor, Or83b, which results in flies with severe olfactory defects, results in a striking longevity phenotype: over 50% increase in mean lifespan compared to wild-type animals. In addition to longevity, other general aspects of adult physiology are altered in flies with defective olfaction. While Or83b mutant flies have normal size and metabolic rate, they have increased triglyceride storage and are resistant to starvation and hyperoxia.
There are a few examples where specific sensory cues capable of stimulating alterations in adult physiology and lifespan have been conclusively identified. It is currently of some debate whether exposure of the adult C. elegans to daumone is sufficient to extend lifespan. Two reports measuring the effects of crude lipid extracts containing the dauer pheromone are inconsistent: one reporting no affect of the pheromone (Alcedo and Kenyon 2004) and the other reporting a significant increase in lifespan (Kawano et al. 2005). Further experiments using the purified daumone (Jeong et al. 2005) would help resolve the issue. Data that suggest specific compounds reduce lifespan should be interpreted with caution since toxins or other unpleasant molecules may shorten lifespan without accelerating the normal aging process. With this in mind, odorants from live yeast paste are sufficient to reduce lifespan when D. melanogaster are diet-restricted (Libert et al. 2007). In the ovoviviparous cockroach, Nauphoeta cinerea, specific components of the male pheromone may reduce longevity, although this effect may be confounded with mating activity (Moore et al. 2003).
The downstream mechanisms that are triggered by canonical sensory perception to modulate lifespan are largely unknown. In C. elegans, daumone may suppress daf-7/TGF-β expression, insulin-like peptide (daf-28) and the production of the lipophilic hormone dafachronic acid, which together act to prescribe the dauer phenotype and may influence dauer lifespan (Motola et al. 2006; Rottiers and Antebi 2007). Dauer pheromone results in increased fat storage, a phenotype that is common in manipulations that extend lifespan by down-regulation of insulin/IGF-1 signaling (IIS) (Tatar et al. 2003). Indeed, in adult C. elegans, sensory neurons produce insulin-like peptides. Since these peptides may act as receptor agonists or antagonists (Pierce et al. 2001), direct modulation of the insulin pathway is an attractive hypothesis for the longevity effects (Antebi 2004). Complicating a simple interpretation, however, is the observation that olfactory neurons regulate aging somewhat differently than gustatory neurons. While gustatory neurons appear to affect lifespan by perturbing IIS, lifespan-extension produced by ablating olfactory neurons is less dependent on IIS and appears to act in a pathway that involves the reproductive system (Alcedo and Kenyon 2004). Reduced olfactory function in D. melanogaster is correlated with increased triglyceride storage suggesting that, as in C. elegans, the effect may be regulated at least partly through IIS (Libert et al. 2007). There is, however, currently no genetic evidence linking IIS with modulation of aging by olfaction in flies.
Molecular sensors
It has also been suggested that DR exerts its effects in yeast, worms, and flies by activating the protein deacetylase Sir2. Overexpression of Sir2 extends lifespan in several species (Longo and Kennedy 2006). Because Sir2 is an NAD-dependent histone deacetylase, it has been postulated that it may serve as a molecular sensor of the metabolic state of the cell. This has led to the hypothesis that overexpression of Sir2 mimics a diet-restriction condition (Lin et al. 2000). Consistent with this interpretation, specific strains of yeast cells and fly lines that lack Sir2 protein fail to response to diet, and the longevity of Sir2 over-expressing animals is not further extended by reduced nutrient availability (Lin et al. 2000). It should be noted, however, that the molecular and physiological basis of the effects of Sir2 and its role in dietary restriction are controversial (Kennedy et al. 2005).
Pharmocological activators of Sir2 have been identified (Howitz et al. 2003) and several of these compounds are plant-derived polyphenols, which show a diversity of structures, ranging from rather simple molecules (monomers and oligomers) to polymers (Cheynier 2005). In some studies these small molecules increased the lifespan of several species (Howitz et al. 2003; Wood et al. 2004). The most well-known of these compounds, Resveratrol, ameliorated liver pathology and increased lifespan in mice fed a high fat diet (Baur et al. 2006). Resveratrol also seems to impact many aspects of mammalian health (Ates et al. 2006; Rahman et al. 2006), although the mechanism is still unclear. Many of the polyphenols that influence aging and physiology are produced by plants in times of stress. Their longevity-promoting effects may therefore be a type of molecular sensory perception and like dietary restriction, they may provide an accurate assessment of environmental condition (Lamming et al. 2004).
Several other types of molecular sensors have been implicated in modulation of longevity. The energy status of the cell is monitored by the AMP-activated protein kinase (AMPK) system and this pathway promotes longevity in nematode worms (Apfeld et al. 2004). Chemicals that induce oxidative stress are sensed by MAPK signaling modules and activation of this pathway increases lifespan in both flies (Wang et al. 2005) and worms (Oh et al. 2005). Health benefits to livestock fed antibiotics may represent a neurohormonal response triggered by alterations in gut flora that are recognized by the immune response (Yun et al. 2006).
As alluded to earlier, manipulation of IIS slows down aging in evolutionarily diverse organisms, as does alteration of signaling in the TOR Pathways (Vellai et al. 2003; Kapahi et al. 2004; Kaeberlein et al. 2005). These pathways are both involved in cellular sensing of nutrients, but it is at present not clear whether this role is important in relation to the effect of the pathways on aging, or whether instead these functions are attributable to different mechanisms. Both pathways have been implicated in the response to DR (Clancy et al. 2002; Kapahi et al. 2004; Kaeberlein et al. 2005; Bonkowski et al. 2006), but the upstream signaling mechanisms by which the pathways extend lifespan and interact with nutrients and other chemicals await elucidation. The deep evolutionary conservation of the effects of these pathways on aging suggests that variation in their expression could explain some of the biodiversity and phenotypic plasticity in lifespan.
Evolutionary perspectives
Pathways that are important in sensory regulation of lifespan in simple model systems have counterparts conserved in mammals (Partridge and Gems 2002; Tatar et al. 2003). Indeed, we postulate that the regulatory pathways are public mechanisms of aging and are shared by many different organisms. The specific chemical compounds that initiate regulation are probably environment- and species-specific and thus involve private mechanisms of aging. This view predicts that the large number of genes that have been shown to have small effects on aging (Murphy et al. 2003) may be subject to coordinated regulation. Such coordination may underlie plastic responses to the environment and form an evolutionarily ancient aspect of aging in all organisms (Keller and Surette 2006). Experimental manipulations that slow the aging process may, therefore, at least in part, tap into evolved mechanisms of detecting and responding to informative chemicals. If perception mediates an adaptive response to alterations in environmental conditions, then our hypothesis predicts that manipulation of these regulatory systems should, in some cases, have adverse fitness consequences. For example, a DR mimetic, despite increasing lifespan, might ultimately be maladaptive if resources are abundant. In C. elegans, mutations in genes encoding components of the insulin signaling pathway significantly increase worm lifespan in non-competitive conditions but result in severely reduced individual fitness in the competitive laboratory environments (Jenkins et al. 2004; Van Voorhies et al. 2005). Life-span extending mutations are often pleiotropic and have effects that are environment-dependent (Partridge et al. 2005). Genetic manipulations and experimental conditions that target sensory cues may actually trigger inappropriate life-history decisions.
Might such sensory systems be a useful target for therapeutic manipulations in humans? There are indications this may be the case. Humans in westernized societies are in general not short of food and are not subject to many of the challenges that are encountered in nature. One result is the epidemic of obesity, a major risk factor for many aging-related diseases. Different life history decisions from those that evolved in the past might therefore be associated with benefits to health. The cephalic phase response, a group of physiological changes elicited by the sight, smell or taste of food, involve pre-absorptive release of enzymes and hormones elicited by neural activation, as opposed to nutrient-induced stimulation (Teff 2000). The primary aspect of this response is the short term release of pancreatic polypeptides, insulin, and glucagon and it is generally viewed as a mechanism to optimize nutrient digestion, absorption and metabolism (Yun et al. 2006). Such endocrine responses are reminiscent of those induced in mice by DR or by genetic mutations that increase lifespan (Mattes 1997; Lindemann 2001). Notably, the cephalic phase insulin response is significantly greater in women that are considered “restrained” eaters (Crystal and Teff 2006) suggesting that, similar to Drosophila, the physiological impact of nutrient perception depends on current environmental conditions. Olive oil, a well-established but poorly understood cardioprotective agent, has potent anti-inflammatory properties that may arise in part from signaling effects (Beauchamp et al. 2005) and the taste of certain pharmaceutical compounds correlates with their pharmacological activity (Fischer et al. 1965).
Some basic, evolutionary questions remain outstanding. For instance, increase in lifespan in response to DR may represent a case of genuine homology of mechanisms but it could, instead, be a case of evolutionary convergence. We therefore do not yet know what role may be played in humans by mechanisms of DR in animals. Much of the work described above has been done with laboratory model organisms. These are far from a random sample of biodiversity, because particular life history traits, such as high fecundity, lead to ease of laboratory culture. Furthermore, these animals are in general kept in relatively pathogen-free environments and scarcely have to move to obtain their food. Although such studies will be challenging, it is important that this work in the laboratory is related to the ecology and evolution of these organisms. The role of DR in natural conditions is not clear, and its status as an evolved response to natural conditions of food shortage has recently been questioned (Miller et al. 2002). To answer the last question, it will be necessary to move out from the laboratory into natural conditions.
CHEMICAL INSULTS AND AGING
Aging and molecular damage
Aging is characterized by loss of functional capacity and is presumably caused by some constellation of the multiple forms of damage and pathology that accumulate with age. The biochemical routes to the generation of molecular damage are diverse. The need to maintain homeostasis in the face of physical, chemical and biological challenges has driven the evolution of an array of gene families and pathways that protect against and repair damage. Directly relating specific losses of function to specific forms of damage has proved elusive and there is currently no real consensus on whether specific types of molecular damage are of predominant importance. Nor is it clear if the biochemical processes that generate damage and the types of damage that they produce show evolutionary conservation, on which rests the utility of model organisms and evolved differences in lifespan for understanding the biochemistry of human aging. We need to understand how deep into the causal chain leading to aging any evolutionary conservation extends. This provides fertile ground for evolutionary biologists to undertake comparative analysis, work on mechanisms of evolved phenotypic plasticity and evolutionary genomic analysis. The tools are at hand to make progress with this important topic. Recent findings are starting to point to the critical importance of diverse toxins, potentially of both exogenous and endogenous origin, in causing the aging process and hence to the possible key role of detoxification systems in defense against aging.
The free radical theory
The main candidate mechanism for generation of aging-related damage, predominant for half a century, is the free radical theory (Harman 1956). This points to the key role of damage by reactive oxygen species (ROS) (Martin et al. 1996; Sohal and Weindruch 1996 Jul 5). ROS, (e.g. superoxide, hydroxyl radicals and hydrogen peroxide) are produced as a by-product of normal cellular metabolism, mainly from mitochondria. The degree of oxidative stress and, hence, oxidative damage to macromolecules, is determined by the balance between ROS production and a finely tuned antioxidant defense system, including enzymatic (e.g. superoxide dismutase, catalase), and non-enzymatic (e.g. ubiquinol, Vitamin E, ascorbate and glutathione) scavengers together with a variety of molecular repair processes. Oxidative damage is a universal accompaniment to aging (Sohal and Weindruch 1996 Jul 5). Furthermore, both generation of ROS from isolated mitochondria and rate of accumulation of oxidative damage during aging are lower in long lived species and in animals subjected to DR (Ruiz et al. 2005; Sanz et al. 2006). Endogenous antioxidant defenses are, in general, similar or lower in DR animals and long-lived species and also in long-lived ant (Parker et al. 2004) and honey bee queens (Corona et al. 2005) suggesting that long-lived forms may be subject to lower levels of oxidative challenge.
Despite these suggestive correlations, direct, experimental evidence for the free radical theory is scant and at times contradictory (Gems and McElwee 2005). For instance, in C. elegans, catalytic antioxidants fed to the worm and in Drosophila over-expression of the genes for cytoplasmic (Cu/Zn-SOD) and mitochondrial (MnSOD) superoxide dismutases have both been reported to extend lifespan. However, the work on catalytic antioxidants was not repeatable in another lab (Keaney et al. 2004) and the degree of extension of lifespan from over-expression of antioxidant defenses in Drosophila is greater in short-lived strains, suggesting that it might extend lifespan by rescuing pathology in strains that have become inbred or adapted to the laboratory environment (Orr and Sohal 2003; Spencer et al. 2003).
There are many reasons why the kinds of negative results just described could occur yet the free radical theory in fact be correct. For instance, manipulations that alter oxidative stress could also interfere with cellular signaling processes that require strict control of the redox state. The almost universal correlative evidence in favor of the importance of free radicals would make it surprising if they are eventually found genuinely to have no role in aging. However, other processes may be as, or more, important, and recent work in comparative genomics has started to identify what these may be.
Endobiotics, xenobiotics and aging
The genome revolution has opened up a quite different and potentially much more powerful approach to identifying candidate mechanisms of aging, which does not involve exploring the pre-conceptions of the experimenter. Genome-wide surveys of changes in gene expression associated with slowing of the aging process can potentially be conducted using unbiased procedures, allowing the data to point to the mechanisms at work. This approach has so far proved particularly powerful for analysis of the mechanisms by which mutations in single genes extend lifespan, but it also has great potential in the context of evolved differences and phenotypic plasticity in the rate of aging.
Analysis of single gene mutations has produced clear evidence for an evolutionarily conserved role of signaling pathways in the determination of lifespan. The best-studied case is an endocrine system that resembles the mammalian insulin and insulin-like growth factor 1 systems: the insulin/IGF-like signaling (IIS) pathway. This pathway evolved early in animal phylogeny and may, indeed, have played a crucial role in the evolution of the metazoa (Skorokhod et al. 1999). It has diverse roles, in regulation of growth and body size, blood sugar and metabolism, stress resistance and fecundity. Its role in aging was originally identified in C. elegans (Klass 1983; Lin et al. 1997) and it was subsequently found to play a similar role in Drosophila and mice; reviewed in (Piper et al. 2005).
Several of the genes in C. elegans that encode components of the IIS pathway first came to light because mutations in them caused the worm to form dauer larvae in the absence of any crowding or food shortage. Because dauer larvae are very long-lived the extended lifespan of the IIS mutant adult worms was suggested to act through the same biochemical processes (Kenyon et al. 1993). A comparison of RNA-expression profiles of dauer larvae and of long-lived IIS mutant adults confirmed this possibility; there was a highly significant overlap between the genes that were up- or down-regulated upon entry into the dauer larva and during extension of lifespan by IIS mutations (McElwee et al. 2004). Particular categories of genes, identified objectively using the functional annotation of the genome, were statistically over-or under-represented in the genes that were differentially regulated in both dauers and long-lived adults. Reassuringly, genes already known to be essential for extension of lifespan by IIS, such as small heat shock proteins, were identified by this procedure. In addition, cytochrome P450s, short chain dehydrogenase/reductases and UDP-glucuronosyltransferases were significantly up-regulated. In mammals these three enzyme classes act in concert to dispose of toxic endobiotic or xenobiotic compounds. In the long-lived adult worms, glutathione-S-transferases were up-regulated, and these are also linked to detoxification. The toxic compounds that these gene categories target are therefore implicated as a cause of aging (McElwee 2007).
These findings led to the ‘Green Theory’ of aging (Gems and McElwee 2005) which suggests that cells constantly have to deal with mainly lipophilic, metabolic waste products and xenobiotics. These are chemically highly diverse, which both poses an informatic challenge for the cell and hence potentially explains the size of the gene families involved and the broad spectrum of substrates of each gene product. The detoxification and removal of this toxic waste is energetically expensive, because it has to be not only solubilized and detoxified, but the resulting products have to be excreted. This contrasts markedly with the machinery for removing ROS such as superoxide and hydrogen peroxide from the cell, where the detoxification process is rapid, specific and does not require energy, and where the detoxification products do not require excretion.
An evolutionarily conserved biochemistry of aging?
RNA expression-profiling of long-lived mutant mice provided some evidence that this role of cellular detoxification processes in slowing aging may be evolutionarily conserved. Ames dwarf and Little mice are both long-lived, as a result of mutations in single genes, encoding a transcription factor involved in pituitary development and the growth hormone releasing hormone receptor, respectively. Interestingly, both have reduced circulating levels of Igf-1, suggesting that their extended lifespan may be related to altered IIS. RNA expression profiling of the livers of these mice showed that, amongst other changes, there was a marked elevated of expression levels of gene classes involved in endobiotic and xenobiotic metabolism, paralleling the findings with C. elegans (Amador-Noguez et al. 2004). Subsequent work has demonstrated that this altered pattern of gene expression has the expected physiological consequence; Little mice are resistant to the effects of a variety of xenobiotic compounds (Amador-Noguez and Darlington 2007).
These results suggest that these cellular detoxification mechanisms could be a public mechanism for resisting the aging process, and a recent three-way species comparison of RNA expression in long-lived IIS mutant C. elegans and Drosophila with that of the Ames and Little mouse has both provided further support for this idea, and illuminated the issue of the level at which this system shown evolutionary conservation. Genes involved in cellular detoxification were up-regulated in long-lived mutant IIS representatives of all 3 species. However, this pattern was apparent only at the level of the gene families involved. There was no tendency towards common expression changes in genes that qualified as orthologues in the different species on the basis of their sequence similarity (McElwee 2007). For this system longevity assurance mechanisms through which altered IIS extend lifespan thus appear to be lineage-specific at the gene level (private), but conserved at the process level (or semi-public).
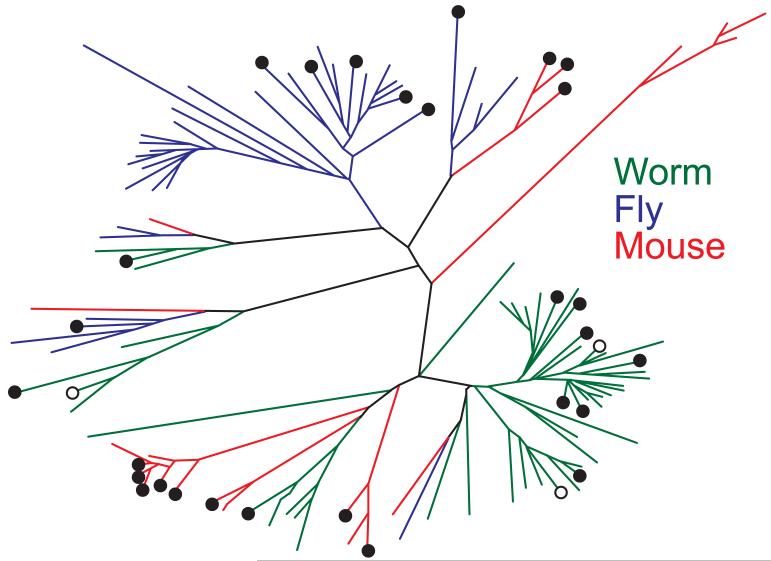
The reason for the evolutionary conservation at the level of gene families but not of genes is explicable from the way that the large gene families involved in these detoxification processes evolved (Figure 1). They tend to undergo lineage-specific expansion, indicating that their diversification occurred after the divergence of C. elegans, Drosophila and the mouse from each other. This is typical of proteins whose function entails recognizing diverse chemical moieties in a changing chemical environment. Such proteins include chemoreceptors and antigen recognition proteins of the innate and acquired immune systems, as well as those involved in cellular detoxification (Lespinet et al. 2002; Thomas et al. 2005). Other processes involved in longevity-assurance may show more conventional gene-to-gene orthology. For instance, the 3-species comparison also identified down-regulation of protein synthesis as consistently associated with extension of lifespan.
Figure 1.
Phylogenetic tree of the GST gene families from worms, flies, and mice. Genes from each species are color-coded, and significantly (q < 0.1) differentially expressed genes in long-lived IIS mutant C. elegans, D. melanogaster and mice are shown by closed (up-regulated) or open (down-regulated) circles. There is little gene-to-gene orthology between species, because the extant genes are largely the product of lineage-specific expansions. The GSTs in each species therefore usually have a more recent common ancestor than the common ancestor of C. elegans. D. melanogaster and mouse.
It thus appears that the mechanisms of aging against which longevity-assurance mechanisms act are to some extent public and also to some extent lineage specific (private). The fact that the gene families involved in detoxification undergo lineage-specific expansion suggests in itself that the biochemical challenges faced by the system change during evolution. Detoxification genes are known to be of ecological importance and are, for instance, specifically induced in association with different hosts in cactophilic Drosophila (Matzkin et al. 2006) as well as in response to drugs and insecticides (Willoughby et al. 2006). In addition, differences in diet and metabolism could also result in the generation of a different spectrum of endobiotics in different evolutionary lineages.
CHEMICALS AS DEFENSES
Ancient systems of pathogen recognition and response
Recognizing pathogenic invaders and responding accordingly is the primary function of immunity. The highly complex mammalian immune response has its roots in unicellular organisms that possess enzyme systems to protect against viral infections. More complex mechanisms evolved in different eukaryotic lineages, such as insects, fish, and reptiles. Immunity in these organisms depends primarily on innate (natural) immunity, where receptors recognize patterns of molecular structures on the surface of microorganisms that are absent from eukaryotic cells. Outcomes of this recognition include induction of genes that encode antimicrobial peptides and initiation of an inflammatory response (Renshaw et al. 2002). An additional function of innate immunity is to recruit cells involved in adaptive immunity, which has appeared more recently, in the ancestors of cartilaginous fishes (Hoffmann and Reichhart 2002). Adaptive immune systems use a large repertoire of antigen-recognizing receptors to establish an immunological memory, which provides better protection from fast-evolving pathogens and a more rapid response to subsequent rechallenge from particular infectious agents.
Although most immunological research over the last 20 years has been focused on adaptive immunity, the role of innate immunity in aging has become a subject of considerable attention. There are at least two reasons for this. First, there is an increasing realization that the innate immune response triggers and orients the adaptive response (Fearon and Locksley 1996) and that age-dependent changes in traditional components of innate immunity may lie at the root of increased susceptibility to infections in old individuals (Plowden et al. 2004). Second, genetic mechanisms of innate immunity are highly conserved across species (Hoffmann and Reichhart 2002) and remarkable progress in understanding recognition and signaling pathways involved in the innate response has come from work in model systems, such as D. melanogaster, which also serve as important models for the study of aging (Hoffmann and Reichhart 2002).
In insects and many invertebrates, innate immunity is mediated by generic molecules, including peptidoglycan recognition proteins (PGRP), which have evolved to recognize the chemical structure of the pathogens, and anti-microbial peptides, which are effective at eliminating them (Hoffmann and Reichhart 2002). Cellular mechanisms are also present and involve phagocytosis and encapsulation of foreign structures or organisms by hemocytes that circulate in the blood. Recognition of invading pathogens and activation of an immune response in these organisms are homologous to the innate immune defense systems of vertebrates. The most characterized aspect of insect innate immunity surrounds signaling cascades in two pathways, the Imd/Relish pathway and the Toll/Dif pathway (Hultmark 2003), which are evolutionarily conserved and are homologous to mammalian Toll-like receptor and tumor necrosis factor receptor signaling pathways, respectively (Kaneko and Silverman 2005). The two pathways differ in their specificity to pathogen-associated molecular patterns; fungal and gram (+) bacteria infections primarily result in stimulation of the Toll signaling pathway while the Imd pathway is stimulated by infections of gram (−) bacteria (Hoffmann and Reichhart 2002)..
Aging and the innate immune response
The mammalian innate immune system mounts inflammatory responses, and age-related changes in these responses have been postulated to be a causative factor for aging (Franceschi and Bonafe 2003). An inflammatory state has also been reported in old D. melanogaster (Pletcher et al. 2005) suggesting that the causes and consequences of age-dependent inflammation may be evolutionarily conserved. Under normal circumstances, inflammation is triggered by the innate system as an initial step of defense (Nguyen et al. 2002). Chronic inflammatory status with aging is believed to be caused by continuous exposure to molecules with a chemical structure that triggers the response (Franceschi and Bonafe 2003). A constant inflammatory state exhausts the innate system, activates various stress responses, and leads to accumulation of cellular and molecular damage. Chronic inflammation is considered to be a driving force for age-related pathologies including neurodegenerative diseases (Wilson et al. 2002), atherosclerosis (Mach 2005), diabetes (Craft 2006) and cancer (Schwartsburd 2004).
Many factors may play a role in aging-related changes in immune function. In the honey bee, Apis mellifora, reduced immune function is associated with a change in social behavior (Amdam et al. 2005). At roughly 23 days of age, worker bees transition from colony tasks, such as nursing, to more active duties, including foraging. At this time, there is a severe decline in immunity, including apoptotic loss of hemocytes, which are responsible for producing antimicrobial peptides and other immunity molecules. The loss of hemocytes is likely caused by increases in juvenile hormone titer. When older worker bees are forced to resume colony maintenance, juvenile hormone levels are reduced, vitellogenin levels are increased, and immune function is restored. These data suggest that organisms may exhibit adaptive plasticity of immunoscenescence (Amdam et al. 2005).
Changes in the activity of the innate immune system is associated with aging-related diseases in humans. Expression of interleukin-6 (IL-6), a pro-inflammatory cytokine, increases with age (Franceschi and Bonafe 2003), and expression level is associated with disability and mortality (Ferrucci et al. 1999). Consistent with the idea that immune activation limits lifespan, the level of IL-6 is low in centenarians (Franceschi and Bonafe 2003). Constitutive activation of NFκB, a key mediator of inflammation, is associated with a variety of cancers (Haefner 2006). Also, in mammals, activation of NFκB related transcription factors leads to up-regulation of anti-apoptotic pathways (Zamorano et al. 2001). It has been suggested that aging in general and deterioration of the immune system in particular are consequences of a pro-inflammatory response that was mainly selected for resistance to infections in youth (Franceschi et al. 2000; van den Biggelaar et al. 2004; Vasto et al. 2006). Age-related decline in the efficiency of the immune response and system-wide inflammatory processes are attenuated by DR (Morgan et al. 2007). In rodents, DR decreases the age-associated susceptibility to infectious diseases. It also improves the maintenance and production of circulating immune cells, preserves receptor repertoire diversity, and reduces the production of inflammatory cytokines (Messaoudi et al. 2006).
Aging and the costs of chemical defenses
Despite the importance of the innate immune system in promoting pathogen resistance, activation of the response can have adverse consequences, particularly for aging and aging-related disease (Fedorka et al. 2004; Libert et al. 2006). Maintenance and the initiation of the immune response are costly and are associated with trade-offs (Demas et al. 1997). Ubiquitous activation of immune pathways throughout larval stages in Drosophila produces developmental abnormalities, including activation of the prophenoloxidase cascade (Takehana et al. 2002) and short-lived adults (DeVeale et al. 2004). Adult-specific, chronic activation of these same pathways results in an inflammation-like state and reduced lifespan (Libert et al. 2006). Immune activation in bumble-bee, Bombus terrestris results in shorter lifespan under starvation conditions (Moret and Schmid-Hempel 2000). Several lines of evidence show that testosterone is associated with suppression of the immune response as a cost of the development of secondary sexual traits (Alonso-Alvarez et al. 2007)
One of the emerging links between innate chemical defense mechanisms and aging focuses on the adaptive use of those traditional enemies of longevity, ROS. In addition to antimicrobial proteins produced by the innate immune response, organisms also ward off pathogens by producing microbicidal bursts of ROS. Indeed, cells need to generate massive amounts of ROS to kill the invading pathogens. Although they are effective weapons, the disproportionate production of ROS can oxidize and damage cellular macromolecules and jeopardize host cellular metabolism and integrity. In Drosophila, the NADPH oxidase-dependent oxidative burst is critical for surviving natural infection (Ha et al. 2005a), but it can also severely shorten lifespan if not effectively controlled (Ha et al. 2005b). There are other examples where free radicals serve as important immune signaling molecules. Superoxide anion or low micromolar concentrations of hydrogen peroxide increase the production of the T-cell growth factor, IL-2, in activated T cells (Roth and Droge 1987). NF-κB is activated by hydrogen peroxide during the launching of an immune response (Schreck et al. 1991).
From flies to humans, signaling networks associated both with aging and with immunity are evolutionarily conserved. Effector molecules of these networks are emerging as correlates of aging-related disease in humans and as key regulators of longevity in invertebrate systems. A critical path for future research leads toward dissecting the mechanistic links between these processes and the extent to which those links are evolutionarily conserved. Using laboratory model systems to dissect the molecular interactions between these pathways in combination with ecological studies on related species provides a critical direction, and an examination of the role of adaptive production of reactive oxygen species may be a first step.
CONCLUSIONS AND FUTURE ISSUES
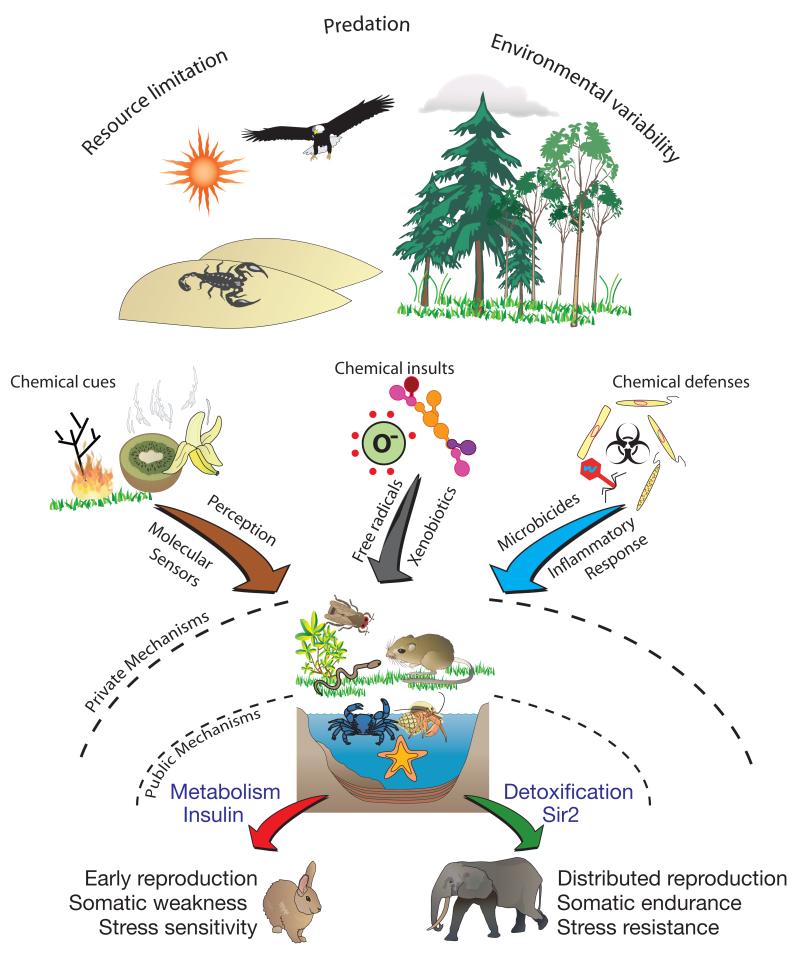
There is growing evidence that organism aging and physiology are strongly modulated by mechanisms that detect and decode environmental chemical cues, initiate defenses to endogenous and exogenous chemicals, and mediate physiological decisions in response to chemical insults (Figure 2). The particular chemical information and insults encountered are likely to be strongly influenced by environment and life style, and hence to show little evolutionary conservation. However, the systems that decode and respond to them represent mechanisms that are, at least in part, public. If we understood more about the ecology of laboratory model organisms, we could learn which aspects of biology of their aging processes are likely to be lineage-specific. For instance, recent work suggested that inhibition of protein translation extends lifespan in C. elegans. Could this be a private mechanism for dealing with the presence of antibiotics, which often interact with ribosomes, in the soil environment that C. elegans inhabits in nature?
Figure 2.
Chemical complexity and aging: Ecological factors such as variability, resource scarcity, and predation provide selection pressures for alternative life histories and survival mechanisms. To cope with environmental challenges, organisms have evolved ways to handle various aspects of chemical complexity, and these systems play a significant role in aging. Through perceptual systems, chemicals may act as cues to trigger physiological decisions and alternative life histories. To resist aging, organisms need to protect themselves from chemical insults, such as molecular damage from free radicals and endobiotic toxins. Mechanisms of natural immunity are also critical for survival in nature, and mechanisms that detect and respond to pathogens can impact lifespan. Nearly all species are exposed to some form of these challenges, but the exact nature of the threats is largely species-specific. The downstream processes that specify and enact life-history decisions (such as Sir2 and Insulin signaling), however, may be public mechanisms. Adapted and extended from Ackerman and Pletcher (2007).
We suggest that other, currently unknown, environmental conditions may affect aging through the filter of sensory perception. Recent work in several model systems have begun to characterize mechanisms of thermosensation (Rosenzweig et al. 2005), and there is reason to believe that such mechanisms are conserved in mammals. Is it possible that the age-old relationship between temperature and longevity in poikilotherms, which has long been thought to result from a direct affect of temperature on metabolic reactions, is due, at least in part, to molecular sensing leading to an adaptive temperature response?
Evolutionary biologists were the first to pose rigorous, scientific questions concerning how and why organisms age. The simple, yet effective, models that resulted from this early work form the foundation for all of the current research in this area. However, the power of molecular biology has begun to unravel the detailed processes through which organisms respond to environmental conditions and enact systems that slow aging. In so doing, many of the traditional views about aging, that it was species-specific and that it would prove resistant to genetic or pharmacological intervention, were overthrown. This has, in our opinion, resulted in a relative stagnation in the advance of evolutionary research into aging, with most work still struggling to distinguish hypotheses of mutational accumulation from those of antagonistic pleiotropy (Ackerman and Pletcher 2007). To maintain its importance in the future, evolutionary biology must work together with molecular biology to address the role of conserved mechanisms of aging. In the end, a synergistic approach that combines ecological and evolutionarily defined cues and chemicals with molecular, genetic manipulations may provide unique insight into the evolution of patterns of “normal” aging and may help in prescribing manipulations by which lifespan may be extended.
Summary Point List.
-
1-
Until the 1990s work on the biology of aging was dominated by evolutionary analysis, which led to the predictions that mechanisms of aging were likely to be highly polygenic and lineage-specific. This view has been challenged by the discovery of mutations in single genes that extend healthy lifespan in evolutionarily diverse model organisms.
-
2-
The pathways discovered to modulate aging in the laboratory are often involved in various forms of sensing, particularly of chemicals. Chemical cues can indicate the overall status of the environment, the availability of nutrients or the presence of other organisms, and they can be detected and decoded through canonical sensory systems, such as smell and taste, or through molecules that function to sense the cellular milieu. Single gene mutations that extend lifespan may therefore tap into evolved mechanisms to ensure longevity and match organismal life history decisions to the environment.
-
3-
Aging is caused by accumulation of damage, and recent work indicates that detoxification of xenobiotic and endobiotic toxins may play an important role in combating aging. These toxins pose an informatic challenge to detoxification systems, and the gene families involved often undergo lineage-specific expansions.
-
4-
Mechanisms that detect environmental cues, and the cues themselves are likely to be private (species-specific) mechanisms of aging, while the downstream mechanisms that coordinate adaptive responses to these cues may be public.
-
5-
The innate immune system mounts inflammatory responses, and age-related changes in these responses have been postulated to be a causative factor for aging. Age-related immune activation has been reported in several species, suggesting an intimate link between immune function and the causes and consequences of aging.
-
6-
An emerging area of interest is the link between aging and the adaptive use of ROS to protect against pathogenic organisms.
-
7-
Environmental variables, such as temperature, that are traditionally considered to act directly on ectothermic organisms by altering their rate of metabolism, could instead be sensed and responded to with evolved phenotypic plasticity.
-
8-
Integrating ideas of molecular mechanism with context derived from evolutionary considerations will lead to exciting new insights into the evolution of aging.
Acronym
- DR
dietary restriction
- IIS
insulin/Igf1-like signaling
- ROS
reactive oxygen species
- PGRP
peptidoglycan recognition protein
- NFκB
nuclear factor κ B
- AMPK
AMP-activated protein kinase
LITERATURE CITED
- Ackerman M, Pletcher SD, editors. Oxford University Press; Oxford: 2007. Evolutionary biology as a foundation for studying aging and aging-related disease. [Google Scholar]
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proceedings. 2007;274:819–825. doi: 10.1098/rspb.2006.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Noguez D, Darlington G. Alterations in xenobiotic metabolism in the long-lived mice. Aging cell. 2007 doi: 10.1111/j.1474-9726.2007.00300.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Aase AL, Seehuus SC, Kim Fondrk M, Norberg K, Hartfelder K. Social reversal of immunosenescence in honey bee workers. Experimental gerontology. 2005;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi A. Long life: a matter of taste (and smell) Neuron. 2004;41:1–3. doi: 10.1016/s0896-6273(03)00839-0. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes & development. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2006 doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- Austad SN. Small Nonhuman Primates as Potential Models of Human Aging. Ilar J. 1997;38:142–147. doi: 10.1093/ilar.38.3.142. [DOI] [PubMed] [Google Scholar]
- Bargmann C. T. C. e. R. Community, editor. Wormbook. Wormbook. 2006 Oct 25; 2006. C. elegans. doi/10.1895/wormbook.1.123.1. [Google Scholar]
- Baudisch A. Hamilton’s indicators of the force of selection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8263–8268. doi: 10.1073/pnas.0502155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, Lee CH, et al. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philosophical transactions of the Royal Society of London. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell AM, Houthoofd K, O’Hanlon K, Vanfleteren JR. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Experimental gerontology. 2005;40:850–856. doi: 10.1016/j.exger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Developmental biology. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Keller L. Division of labour influences the rate of ageing in weaver ant workers. Proceedings. 2002;269:909–913. doi: 10.1098/rspb.2002.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Cambridge Univ. Press; Cambridge: 1994. Evolution in Age-Structured Populations. [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. The American journal of clinical nutrition. 2005;81:223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech Ageing Dev. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer disease and associated disorders. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- Crystal SR, Teff KL. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiology & behavior. 2006;89:213–220. doi: 10.1016/j.physbeh.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Darst CR, Cummings ME, Cannatella DC. A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5852–5857. doi: 10.1073/pnas.0600625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JP, Costa J, Toussaint O. HAGR: the Human Ageing Genomic Resources. Nucleic Acids Res. 2005;33:D537–543. doi: 10.1093/nar/gki017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. The American journal of physiology. 1997;273:R1631–1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- DeVeale B, Brummel T, Seroude L. Immunity and aging: the enemy within? Aging Cell. 2004;3:195–208. doi: 10.1111/j.1474-9728.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. The journals of gerontology. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Zuk M, Mousseau TA. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution; international journal of organic evolution. 2004;58:2478–2485. doi: 10.1111/j.0014-3820.2004.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, et al. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Fischer R, Griffin F, Archer RC, Zinsmeister SC, Jastram PS. Weber ratio in gustatory chemoreception; an indicator of systemic (drug) reactivity. Nature. 1965;207:1049–1053. doi: 10.1038/2071049a0. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochemical Society transactions. 2003;31:457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gardner MP, Gems D, Viney ME. Extraordinary plasticity in aging in Strongyloides ratti implies a gene-regulatory mechanism of lifespan evolution. Aging cell. 2006;5:315–323. doi: 10.1111/j.1474-9726.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- Gems D, McElwee JJ. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mech Ageing Dev. 2005;126:381–387. doi: 10.1016/j.mad.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005a;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, et al. An antioxidant system required for host protection against gut infection in Drosophila. Developmental cell. 2005b;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Haefner B. Targeting NF-kappaB in anticancer adjunctive chemotherapy. Cancer Treat Res. 2006;130:219–245. doi: 10.1007/0-387-26283-0_10. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senesence by natural selection. Journal of theoretical biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. Journals of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- Holmes DJ, Austad SN. Birds as animal models for the comparative biology of aging: a prospectus. The journals of gerontology. 1995;50:B59–66. doi: 10.1093/gerona/50a.2.b59. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Gems D, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. Interdisciplinary topics in gerontology. 2007;35:98–114. doi: 10.1159/000096558. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annu Rev Entomol. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp Gerontol. 2004;39:1137–1143. doi: 10.1016/j.exger.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Drosophila immunity: paths and patterns. Curr Opin Immunol. 2003;15:12–19. doi: 10.1016/s0952-7915(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jenkins NL, McColl G, Lithgow GJ. Fitness cost of extended lifespan in Caenorhabditis elegans. Proceedings. 2004;271:2523–2526. doi: 10.1098/rspb.2004.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Silverman N. Bacterial recognition and signalling by the Drosophila IMD pathway. Cellular microbiology. 2005;7:461–469. doi: 10.1111/j.1462-5822.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Kataoka N, Abe S, Ohtani M, Honda Y, Honda S, Kimura Y. Lifespan extending activity of substances secreted by the nematode Caenorhabditis elegans that include the dauer-inducing pheromone. Bioscience, biotechnology, and biochemistry. 2005;69:2479–2481. doi: 10.1271/bbb.69.2479. [DOI] [PubMed] [Google Scholar]
- Keaney M, Matthijssens F, Sharpe M, Vanfleteren J, Gems D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic Biol Med. 2004;37:239–250. doi: 10.1016/j.freeradbiomed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Keller L, Surette MG. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Smith ED, Kaeberlein M. The enigmatic role of Sir2 in aging. Cell. 2005;123:548–550. doi: 10.1016/j.cell.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, et al. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech Ageing Dev. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Martin GM, Partridge L. Evolution, senescence and health in old age. In: Stearns SC, editor. Evolution in Health and Disease. Oxford University Press; 1999. [Google Scholar]
- Klass MR. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev. 1983;22:279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Molecular microbiology. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Lee RD. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard GH, Bertness MD, Yund PO. Crab predation, waterborne cues, and indicible defenses in the blue mussel, Mytilus edulis. Ecology. 1999;80:1–14. [Google Scholar]
- Lespinet O, Wolf YI, Koonin EV, Aravind L. The role of lineage-specific gene family expansion in the evolution of eukaryotes. Genome research. 2002;12:1048–1059. doi: 10.1101/gr.174302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Van Voorhies WA, Roman G, Pletcher S. Regulation of Drosophila lifespan by olfaction and food-derived odors. Science. 2007 doi: 10.1126/science.1136610. In Press. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Mach F. Inflammation is a crucial feature of atherosclerosis and a potential target to reduce cardiovascular events. Handbook of experimental pharmacology. 2005:697–722. doi: 10.1007/3-540-27661-0_26. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of lifespan by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Keynote: mechanisms of senescence--complificationists versus simplificationists. Mech Ageing Dev. 2002;123:65–73. doi: 10.1016/s0047-6374(01)00335-9. discussion 75-69. [DOI] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nature genetics. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Mattes RD. Physiologic responses to sensory stimulation by food: nutritional implications. Journal of the American Dietetic Association. 1997;97:406–413. doi: 10.1016/S0002-8223(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Matzkin LM, Watts TD, Bitler BG, Machado CA, Markow TA. Functional genomics of cactus host shifts in Drosophila mojavensis. Mol Ecol. 2006;15:4635–4643. doi: 10.1111/j.1365-294X.2006.03102.x. [DOI] [PubMed] [Google Scholar]
- McElwee JJ. Genome Biology. 2007 doi: 10.1186/gb-2004-5-8-337. ??? In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. The Journal of biological chemistry. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An unsolved problem in biology. H. K. Lewis; London: 1952. [Google Scholar]
- Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Experimental biology and medicine. 2002;227:500–508. doi: 10.1177/153537020222700715. (Maywood, N.J. [DOI] [PubMed] [Google Scholar]
- Moore AJ, Gowaty PA, Moore PJ. Females avoid manipulative males and live longer. J Evol Biol. 2003;16:523–530. doi: 10.1046/j.1420-9101.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdisciplinary topics in gerontology. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- Morse ANC. Hoe do planktonic larvae know where to settle? Am. Sci. 1991;79:154–167. [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nature reviews. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Experimental gerontology. 2003;38:227–230. doi: 10.1016/s0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- Orzack SH, a ST. Population dynamics in variable environments. VII The demography and evolution of iteroparity. The American Naturalist. 1989;133:901–923. [Google Scholar]
- Osiewacz HD. Aging in fungi: role of mitochondria in Podospora anserina. Mech Ageing Dev. 2002;123:755–764. doi: 10.1016/s0047-6374(01)00421-3. [DOI] [PubMed] [Google Scholar]
- Parker JD, Parker KM, Sohal BH, Sohal RS, Keller L. Decreased expression of Cu-Zn superoxide dismutase 1 in ants with extreme lifespan. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3486–3489. doi: 10.1073/pnas.0400222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Barton NH. Optimality, mutation and the evolution of ageing. Nature. 1993;362:305–311. doi: 10.1038/362305a0. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–175. doi: 10.1038/nrg753. 11972154. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Beyond the evolutionary theory of ageing, from functional genomics to evo-gero. Trends in ecology & evolution. 2006;21:334–340. doi: 10.1016/j.tree.2006.02.008. (Personal edition) [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijanowska J, Kloc M. Daphnia response to predation threat involves heat-shock proteins and the actin and tubulin cytoskeleton. Genesis. 2004;38:81–86. doi: 10.1002/gene.20000. [DOI] [PubMed] [Google Scholar]
- Piper MDW, Selman C, McElwee JJ, Partridge L. Models of insulin signalling and longevity. Drug Discovery To-day: Disease Models. 2005;2:249–256. [Google Scholar]
- Pletcher SD, Libert S, Skorupa D. Flies and their golden apples: the effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Res Rev. 2005;4:451–480. doi: 10.1016/j.arr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006 doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Scheuerlein A, Cohen A. Age-related patterns of fertility in captive populations of birds and mammals. Experimental gerontology. 2003;38:741–745. doi: 10.1016/s0531-5565(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Rose MR. Evolutionary Biology of Aging. Oxford University Press; Oxford: 1991. [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes & development. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Droge W. Regulation of T-cell activation and T-cell growth factor (TCGF) production by hydrogen peroxide. Cellular immunology. 1987;108:417–424. doi: 10.1016/0008-8749(87)90224-3. [DOI] [PubMed] [Google Scholar]
- Rottiers V, Antebi A. Regulation of C. elegans aging by a bile acid. Proceedings of the National Academy of Sciences of the United States of America. 2007 doi: 10.1073/pnas.0700847104. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MC, Ayala V, Portero-Otin M, Requena JR, Barja G, Pamplona R. Protein methionine content and MDA-lysine adducts are inversely related to maximum life span in the heart of mammals. Mech Ageing Dev. 2005;126:1106–1114. doi: 10.1016/j.mad.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. Faseb J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. The EMBO journal. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartsburd PM. Age-promoted creation of a pro-cancer microenvironment by inflammation: pathogenesis of dyscoordinated feedback control. Mech Ageing Dev. 2004;125:581–590. doi: 10.1016/j.mad.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TB. Calorie restriction and aging: a life-history analysis. Evolution; international journal of organic evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Skorokhod A, Gamulin V, Gundacker D, Kavsan V, Muller IM, Muller WE. Origin of insulin receptor-like tyrosine kinases in marine sponges. The Biological bulletin. 1999;197:198–206. doi: 10.2307/1542615. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996 Jul 5;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CC, Howell CE, Wright AR, Promislow DE. Testing an ‘aging gene’ in long-lived drosophila strains: increased longevity depends on sex and genetic background. Aging cell. 2003;2:123–130. doi: 10.1046/j.1474-9728.2003.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana A, Katsuyama T, Yano T, Oshima Y, Takada H, Aigaki T, Kurata S. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc Natl Acad Sci U S A. 2002;99:13705–13710. doi: 10.1073/pnas.212301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34:206–213. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Kelley JL, Robertson HM, Ly K, Swanson WJ. Adaptive evolution in the SRZ chemoreceptor families of Caenorhabditis elegans and Caenorhabditis briggsae. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4476–4481. doi: 10.1073/pnas.0406469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Biggelaar AH, Huizinga TW, de Craen AJ, Gussekloo J, Heijmans BT, Frolich M, Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Experimental gerontology. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Van Voorhies WA, Fuchs J, Thomas S. The longevity of Caenorhabditis elegans in soil. Biology letters. 2005;1:247–249. doi: 10.1098/rsbl.2004.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Malavolta M, Pawelec G. Age and immunity. Immun Ageing. 2006;3:2. doi: 10.1186/1742-4933-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Voronezhskaya EE, Khabarova MY, Nezlin LP. Apical sensory neurones mediate developmental retardation induced by conspecific environmental stimuli in freshwater pulmonate snails. Development. 2004;131:3671–3680. doi: 10.1242/dev.01237. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK Extends Life Span and Limits Growth by Antagonizing Cellular and Organism-Wide Responses to Insulin Signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Willoughby L, Chung H, Lumb C, Robin C, Batterham P, Daborn PJ. A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides, caffeine and phenobarbital. Insect Biochem Mol Biol. 2006;36:934–942. doi: 10.1016/j.ibmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yun AJ, Lee PY, Doux JD. Are we eating more than we think? Illegitimate signaling and xenohormesis as participants in the pathogenesis of obesity. Medical hypotheses. 2006;67:36–40. doi: 10.1016/j.mehy.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Zamorano J, Mora AL, Boothby M, Keegan AD. NF-kappa B activation plays an important role in the IL-4-induced protection from apoptosis. International immunology. 2001;13:1479–1487. doi: 10.1093/intimm/13.12.1479. [DOI] [PubMed] [Google Scholar]
- Zimmer RK, Schar DW, Ferrer RP, Krug PJ, Kats LB, Michel WC. The Scent of Danger: Tetrodotoxin (Ttx) as an Olfactory Cue of Predation Risk. Ecological Monographs. 2006;76:585–600. [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Experimental gerontology. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]