Abstract
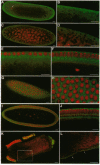
The role of cytoskeletal elements in the cellularization of syncytial Drosophila embryos is becoming evident; however, the distribution and role of organelles such as the Golgi complex, essential for membrane biogenesis, remain unknown. We have cloned a Golgi-membrane-associated polypeptide, beta-COP, from Drosophila. Immunocytochemical studies of syncytial Drosophila embryos with anti-Drosophila beta-COP antibody reveal that Golgi membranes are spatially segregated from the rapidly dividing nuclei. In early embryos, the Golgi membranes are located in the embryonic cortex and nuclei are confined to the core. This distribution of Golgi membranes may serve in preparation of the embryonic cortex for the accommodation of nuclei upon their eventual migration to the cortex and in biogenesis of the excessive plasma membrane needed for cellularization of syncytial embryos.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan V. J., Kreis T. E. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986 Dec;103(6 Pt 1):2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V. E., Alberts B. M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983 May;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Franzusoff A., Redding K., Crosby J., Fuller R. S., Schekman R. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J Cell Biol. 1991 Jan;112(1):27–37. doi: 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullilove S. L., Jacobson A. G. Nuclear elongation and cytokinesis in Drosophila montana. Dev Biol. 1971 Dec;26(4):560–577. doi: 10.1016/0012-1606(71)90141-2. [DOI] [PubMed] [Google Scholar]
- Kamakaka R. T., Tyree C. M., Kadonaga J. T. Accurate and efficient RNA polymerase II transcription with a soluble nuclear fraction derived from Drosophila embryos. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1024–1028. doi: 10.1073/pnas.88.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. M., Berger E. G., Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989 Aug;109(2):463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. M., Pryde J. G., Berger E. G., Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J Cell Biol. 1987 Apr;104(4):865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. M., Warren G. Fragmentation and partitioning of the Golgi apparatus during mitosis in HeLa cells. EMBO J. 1987 Nov;6(11):3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald A. P., Goralski T. J., Caulton J. H. In vitro activation of Drosophila eggs. Dev Biol. 1983 Aug;98(2):437–445. doi: 10.1016/0012-1606(83)90373-1. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Sedat J. Localization of antigenic determinants in whole Drosophila embryos. Dev Biol. 1983 Sep;99(1):261–264. doi: 10.1016/0012-1606(83)90275-0. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprins A., Duden R., Kreis T. E., Geuze H. J., Slot J. W. Beta-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993 Apr;121(1):49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics. 1989 Feb;121(2):333–352. doi: 10.1093/genetics/121.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Clermont Y., Hermo L. Three-dimensional structure of the Golgi apparatus. Methods Cell Biol. 1981;23:155–166. doi: 10.1016/s0091-679x(08)61497-1. [DOI] [PubMed] [Google Scholar]
- Redding K., Holcomb C., Fuller R. S. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J Cell Biol. 1991 May;113(3):527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski A. A., Singer S. J. Associations of elements of the Golgi apparatus with microtubules. J Cell Biol. 1984 Sep;99(3):1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Smiley S., Wong M. L., Alberts B. M. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992 Aug;115(4):923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]