Abstract
We analyzed by next-generation sequencing (NGS) 67 epilepsy genes in 19 patients with different types of either isolated or syndromic epileptic disorders and in 15 controls to investigate whether a quick and cheap molecular diagnosis could be provided. The average number of nonsynonymous and splice site mutations per subject was similar in the two cohorts indicating that, even with relatively small targeted platforms, finding the disease gene is not an univocal process. Our diagnostic yield was 47% with nine cases in which we identified a very likely causative mutation. In most of them no interpretation would have been possible in absence of detailed phenotype and familial information. Seven out of 19 patients had a phenotype suggesting the involvement of a specific gene. Disease-causing mutations were found in six of these cases. Among the remaining patients, we could find a probably causative mutation only in three. None of the genes affected in the latter cases had been suspected a priori. Our protocol requires 8–10 weeks including the investigation of the parents with a cost per patient comparable to sequencing of 1–2 medium-to-large-sized genes by conventional techniques. The platform we used, although providing much less information than whole-exome or whole-genome sequencing, has the advantage that can also be run on ‘benchtop' sequencers combining rapid turnaround times with higher manageability.
Introduction
Epilepsy is one of the most common neurological disorders in humans with a prevalence of 1% and a lifetime incidence of up to 3%.1 Epilepsies present with a broad range of clinical features and their genetic causes remain unknown in the vast majority of cases, although several genes have been identified in rare Mendelian forms, either heritable or sporadic. Finding the disease genes can be challenging, as the same epileptic phenotype may be associated with several genes. A molecular diagnosis of epilepsy is important especially in a pediatric setting in order to (1) establish the recurrence risk in following pregnancies, (2) stop the diagnostic odyssey that is frequently restless for undiagnosed epilepsies, and (3) provide, at least in some cases, specific therapies. Recently, genome-wide association studies revealed a few regions harboring high-ranking candidate genes, although these studies still necessitate further replication efforts.2 Genomic arrays had been more successfully, as they allowed to identify several possible pathogenic copy-number variants not present in controls in about 9% of the cases.3 Presently, high-throughput sequencing is becoming the most promising approach to improve molecular diagnosis of this condition,4, 5 although the interpretation of the results is far from being a standardized process.6 Indeed, next-generation sequencing (NGS) does not magically make diagnoses but typically provides a handful of possibilities requiring further studies on the function of each candidate gene. To overcome these problems, we composed a panel containing most epilepsy genes, covering several relevant phenotypes. With this NGS platform, we studied 19 index patients suffering from a range of seizures, either familial or sporadic. Although initially we performed a blind study trying to interpret the sequencing data without any knowledge of the clinical history, we then realized that no analysis was possible in absence of detailed phenotypes and familial information. This study allowed us to evaluate not only the diagnostic capability of this approach but also the cost and the time required to report the final result to the family.
Materials and methods
Patient cohort
The 19 index cases ranged from few days to 4 years of age at the time of the first clinical examination. Most of them have been then followed-up for several years. This cohort has been randomly selected from patients afferent to our epileptic center for children and adolescents. All available family members have been enrolled for segregation analysis. Informed consent was obtained from each family and clinical evaluation and genetic testing were carried out in accordance with the ethics approval granted (11017C-RC2011 IRCCS C. Mondino, Diagnosis and therapy of epileptic syndromes).
In Table 1, a brief report of each case is provided including clinical diagnosis and history, and presence of the epilepsy in relatives (family history), electroencephalograms, magnetic resonance findings, and administered antiepileptic drugs. In order to assess the diagnostic capability of our approach, we collected patients presenting with a wide range of epilepsy phenotypes: 11 are sporadic cases, whereas the remaining eight have a history of familial epilepsy. Patients have been subdivided in two groups (Table 1): the first one, subcohort A, was constituted by seven patients whose clinical features were either strongly or more loosely suggestive for a syndrome associated with a specific gene; the second group, subcohort B, included 12 subjects with very different types of epilepsy, presumably heterogeneous in their genetic basis and not suggestive of any or a single specific gene. In both subgroups other clinical features such as language impairment, psychomotor delay, or autism spectrum disorder were present in several patients. Magnetic resonance abnormalities were detected in some of them (Table 1). All cases had been previously analyzed by array comparative genomic hybridization and a few (6-A, 8-B(i), 8-B(ii)) by Sanger Sequencing for specific genes without any positive result.
Table 1. Patients' clinical data, subdivided in two subcohorts (A and B), including sex, age, AEDs administered, EEG, MRI results, and family history.
| Case n-subcohort | Sex | Age (years) | Diagnosis | Clinical features | AEDsa | EEG | MRI | Family history |
|---|---|---|---|---|---|---|---|---|
| 1-A | F | 17 | Lennox–Gastaut syndrome in a subject with double cortex | Severe mental retardation. Tonic, atonic, and myoclonic seizures during sleep and awake | VPA+CLB+ETS | Lennox like | Double cortex | Questionable parental consanguinity |
| 2-A | F | 14 | Pyridoxine-dependent epilepsy | Neonatal seizures and epileptic status at 6 months of age responsive to pyridoxine. Normal IQ | LTG+B6 | Currently normal | Normal | Negative |
| 3-A | M | 14 | Pyridoxine-dependent epilepsy | Intractable seizures during the first year and epileptic status pyridoxine responsive. Normal IQ | B6 | Currently normal | Normal | Progressive motor and language disability (mother's paternal uncle) |
| 4-A | M | 4 | Dravet syndrome | Typical evolution of Dravet syndrome. Actually IQ 46 | VPA+TPM+CLB+STP | Typical of Dravet syndrome | Normal | Negative |
| 5-A | F | 0.8 | Benign familial neonatal seizures | Intractable tonic-clonic seizures during the first 25 days of life spontaneously remised | PB+TPM+MDL+Rivotril | Normal | Normal | Father with benign neonatal seizures |
| 6-A | M | 7 | Epileptic encephalopathy and pervasive developmental disorder | Neonatal seizures, followed by focal seizures. Severe mental retardation. Pervasive developmental disorder | VPA | Sharp and sharp-wave on central and frontal regions | Normal | Negative |
| 7-A | F | 7 | Febrile seizures plus | Febrile and afebrile GTC. Normal IQ | LEV | Large amplitude and diffuse sharp-wave abnormalities | Normal | Migraine in the maternal line |
| 8-B(i) | M | 6 | Myoclonic epilepsy | Epileptic negative myoclonus. Normal IQ | VPA+LTG | Spike-wave discharges | Not performed | Brother of 8-B(ii) |
| 8-B(ii) | M | 14 | Generalized epilepsy | One myoclonic seizure. Normal IQ. | LTG | Spike-slow wave discharges | Normal | Brother of 8-B(i) |
| 9-B | F | 3 | Febrile seizures | Numerous febrile seizures during the first year. Normal psychomotor development (GQ=116) | VPA | Normal | Not performed | Family history of febrile seizures |
| 10-B | M | 6 | Benign epilepsy of childhood with centrotemporal spikes (BECTS) | Few typical rolandic seizures. Borderline learning difficulties | None | Atypical BECTS | Normal | Family history of BECTS and mild intellectual disabilities |
| 11-B | M | 8 | Myoclonic epilepsy | Cognitive impairment from 4 years (GQ 90.4; IQ 53) | VPA+LTG | Currently normal | Not performed | Mother, maternal uncle, and grandfather with generalized epilepsy |
| 12-B | M | 16 | Febrile seizures plus | Febrile seizures from 2 to 6 years. Normal IQ | VPA, suspended in 2006 | Currently normal | Not performed | Family history of febrile afebrile seizures |
| 13-B | F | 5 | Febrile seizures | Febrile seizures, normal IQ | None | Normal | Not performed | Family history of febrile seizures (father) and FS and absences (mother) |
| 14-B | M | 12 | Febrile seizures plus | Febbrile seizures, then afebrile GTC. Normal IQ | VPA | Generalized abnormalities | Hippocampal asymmetry, incomplete rotation of hippocampal structure | Family history febrile and afebrile seizures (father and sister) |
| 15-B | M | 9 | Lennox–Gastaut syndrome | Severe cognitive impairment, myoclonic and tonic seizures or GTC Mental retardation | VPA+ETS+NTZ+HC+PB | Spikes and polyspikes tonic activity, slow waves during sleep and awake | Normal | Not reported |
| 16-B | M | 3 | Epileptic encephalopathy | GTC and partial seizures. Severe cognitive impairment | LTG+VPA+HC | Generalized sharps and sharp waves. Poor organization. | Normal | Consanguineous parents (first cousins) |
| 17-B | M | 4 | Focal epilepsy | Febrile seizures, status epilepticus. Normal psychomotor development. QS 90 at 2 years | VPA+TPM | Poor organization, slow, not epileptic abnormalities | Mesial temporal sclerosis and temporal lobe dysplasia left | Not reported |
| 18-B | M | 4 | Epileptic encephalopathy | Neonatal seizures, spasms, and hypsarrhythmia Severe cognitive impairment, hypotonia, strabismus, and motor stereotypies | VPA+LEV+NTZ | Poor organization, slow, slow waves, and polispikes during sleep | Cerebral atrophy | Not reported |
| 19-B | M | 5 | Epileptic encephalopathy and hypothyroidism | Severe cognitive impairment, spasms in flexion, and partial seizures, Hypothyroidism | VPA+TPM+LTG | Poor organization. Multifocal abnormalities | Modest diffuse cerebral atrophy | Not reported |
Abbreviations: AEDs, antiepileptic drugs; EEG, electroencephalogram; F, female; M, male; MRI, magnetic resonance imaging.
AEDs abbreviations: B6, pyridoxine; CBZ, carbamazepine; CLB, clobazam; ETS, ethosuximide; HC, hydrocortisone; LEV, levetiracetam; LTG, lamotrigine; MDL, midazolam; NTZ, nitrazepam; PB, phenobarbital; STP, stiripentol; TPM, topiramate; VPA, valproic acid.
Control cohort
Control subjects (nine females and six males), ranging from 18 to 35 years of age, were recruited among blood donors as controls for both this and a cardiovascular study. Besides, they were requested to answer a structured general medical questionnaire with specific emphasis on neurological and cardiac symptoms, control subjects had to answer two specific questions: (1) have you ever suffered from epilepsy or seizures, and (2) have you become acquainted with any seizure disorders or EEG abnormalities present in some of your family members? Only those who answered negatively were recruited.
Platform design
A custom-designed target enrichment library for 67 genes (see Supplementary Information) has been designed by using the Agilent eArray website (https://earray.chem.agilent.com/earray/). This library contains unique baits covering the exons, the UTRs, and the intron–exon junctions of the selected genes. The estimated base coverage of the library is 0.45 Mb (Supplementary Table 1S).
The selection was made on the basis of the following criteria: (1) genes associated with idiopathic epilepsy; (2) genes associated with syndromic epilepsy; (3) genes associated with epilepsy and cerebral malformations excluding holoprosencephaly; (4) genes that appeared to be the best candidates for epilepsy in microdeletion syndromes. Selected genes are reported in Supplementary Table 1.
Sample preparation
DNA (5 μg) extracted from peripheral blood by standard methods were diluted in 700 μl of nebulization buffer (Illumina, San Diego, CA, USA) and sheared using a nebulization technique (Invitrogen, Carlsbad, CA, USA), which breaks up DNA into pieces <500 bp, through the application of 60–70 psi (pound force per square inch) of purified air for 4 min. This process generates double-stranded DNA fragments containing 3′ or 5′ overhangs that were cleaned up using QIAquick spin columns (Qiagen, Hilden, Germany). A quality control step on the recovered DNA was then performed using Nanodrop 1000 to quantify the DNA by a 260-nm reading and Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) to check the size of the fragments.
According to the Agilent SureSelectXT protocol, sheared DNA overhangs were end-repaired and then purified using the magnetic bead-based Agencourt AMPure XP purification system (Beckman Coulter Genomics, Brea, CA, USA). Then we performed the adding of ‘A' bases to the 3′ end of the DNA fragments and the ligation of indexing-specific paired-end adapters.
After a few cycles of PCR amplification, 500 ng of DNA from the resulting libraries were hybridized to the bait set using the SureSelectXT MP Capture Library Kit (Design no. 5190-0312931—Agilent) at 65 °C for 24 h. Hybrids capture was performed according to the manufacturer's protocol with Streptavidin-coated Dynal magnetic beads (Invitrogen). Captured samples were further purified through Agencourt AMPure XP beads and subjected to a PCR-based amplification reaction to add index tags (each is a sequence of six bases in length allowing to identify samples after pooling), accordingly to the Agilent SureSelectXT protocol. For each step of library preparation, all samples were quantified on a Bioanalyzer 2100 (Agilent). We performed a multiplexed run on the Illumina Genome Analyzer IIx, where nine multiple samples were sequenced in a single lane of a flow cell; number of samples to be pooled has been calculated on the basis of the enriched target's size, according to Agilent's instructions. The sample libraries from nine individuals were denatured with NaOH and loaded on a single lane of a Illumina Flowcell v4 where DNA clusters were generated through a one-step workflow (according to Illumina protocol) on the Cluster Station using TruSeq PE Cluster Kit v5 (Illumina).
One percent volume of a PhiX control library (Illumina) was used as internal control and loaded in each lane of the flowcell.
The capture was considered successful if at least 99% of our target regions were covered by more than eight reads of high quality (that is a Phred-scaled mapping quality score of at least 20 for each).
Annotation and interpretation of data
Sequences for each sample were generates by Illumina software CASAVA v1.8.1. Reads were filtered by quality relying on the standard Illumina quality filter test. Reads were aligned to the most recent version of human genome (GRCh37/hg19) using BWA software package v0.6.07 and filtered out for PCR duplicates by Samtools v0.1.18.8
Reads were realigned around inferred indels and their base qualities were recalibrated taking into account the context of alignment by the Genome Analysis Toolkit (GATK) v1.6 suite.9
SNPs and short indels were called using GATK UnifiedGenotyper module and the resulting variants were filtered using GATK Variant Filtration module and specific Perl scripts, as such variants were probably owing to alignment errors and, in general, they cannot be considered reliable variants. Several filtering constraints were also applied, such as minimum variant quality (50, Phred scaled), a minimum of five reads supporting variant, number of ambiguous mapped reads overlapping variant, neighborhood of each reliable indel or homopolymer excluding those single-nucleotide variations that overlap within.
Variants annotation was performed on the resulting data set by in-house genomic database application. Prediction tracks for each annotated variant were generated by automatic remote calling procedures to Mutation Taster10 and Polyphen2 (version 2.2.2).11
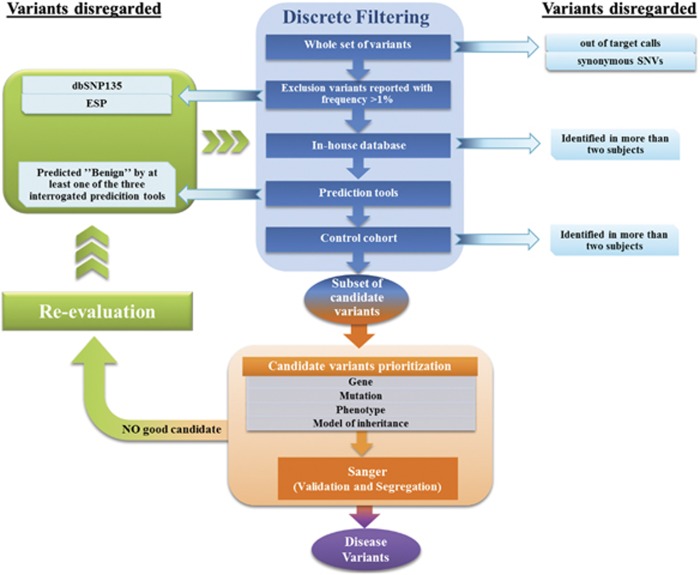
In order to identify potential causative mutations, we applied the so-called discrete filtering approach.12 We first excluded synonymous out of target and UTR-overlapping variants. We then excluded the variants present in dbSNP135 and Exome Sequencing Project Databases (ESP) with a frequency higher than 1%. Moreover, we discarded variants reported in our in-house database (66 whole exomes) that were identified in at least two individuals without epilepsy or other neurological disorders.
We then took into account only variants predicted to alter the protein structure or function by at least one of the three prediction tools we used (Mutation Taster, SIFT, Polyphen2) as well as variants for which all prediction tools failed (see Supplementary Table 2S). At the end, we excluded all variants occurring in at least three cases and/or at least two subjects of the control cohort.
We prioritized the candidate alterations on the base of the expression and function of the altered gene, the type of mutation and its effect at protein level, presence of the variant in the Human Gene Mutation Database 2012.3 (HGMD) or in the literature, the metabolic pathway involved, and obviously the clinical features of the patient/family. A manual inspection of the variants eliminated by the prediction tools filtering step allowed us to reconsider them on the base of possible correlations with the patients' phenotype. For example, this permitted reconsidering a variant of ALDH7A1, which was then ascertained as causative (see Results, case 2). The entire protocol of data analyses is illustrated in the flowchart reported in Figure 1.
Figure 1.
Flow chart representing the strategy adopted to analyze sequencing data. Discrete filtering, prioritization, and re-evaluation steps are highlighted in blue, orange and green, respectively.
The final subset of mutations was confirmed by Sanger sequencing followed by segregation analysis in each family. Only a close collaboration with the specialist allowed us to find a specific genotype–phenotype correlation discarding those variants that were either pathogenic in recessive state in families where the condition segregated in a dominant manner or those that correlated to a neurological phenotype totally different from that of the patient. This point was taken with extreme caution as it could not be excluded, a priori, that a novel mutation might cause a totally different phenotype with respect to the ones known to be associated with the same gene.13 Some of the remaining alterations, even if predicted damaging by at least one tool, have been discarded when they did not segregate with the epileptic phenotype in familial cases (ie, MAGI2 in case 5-A that was inherited by the healthy mother, whereas KCNQ2 was considered causative because it was inherited by the affected father).
We applied the same filtering strategies to the control cohort in order to perform a statistical test to assess whether the number of deleterious variants in cases was significantly higher than in controls.
Results
Targeted massive parallel sequencing of patient and control cohorts produced for each subject about 180 Mb of sequence, which yielded an average coverage of about 400 × at each targeted base. On average, 96% of target bases exceeded the 15 × coverage threshold required for confident analysis, defined as 99% power to detect a variant.
We compared the number of variants per subject between patients and controls using the non-parametric statistical hypothesis test of WMW (see Supplementary Information). We did not find any significant difference between the two cohorts after filtering (P-value=0.4928). This might be owing to the limited number of subjects analyzed by this platform. In Table 2, we listed all the variants remaining after the filtering and prioritization processes (ranging from one to three per subject). In bold we highlighted those variants considered as having the main effect on the patient's phenotype.
Table 2. Selected variants after the filtering protocol. In bold are reported those variants that fulfill the criteria for disease-causing mutations: affected genes already associated with patient's phenotype, exhibit complete segregation with the disease, and are absent in healthy controls.
| Case | UCSC gene | Genotype | Regiona | Inheritance | gDNA level (GRCh37) | cDNAb | Protein | Mutation taster | Polyphen2 | SIFT |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-A | SHANK3 | het | Exon 8 | pat | Chr22: g.51121780C>T | NM_001080420.1:c.898C>T | p.(Arg300Cys) | NP | Probably damaging | |
| DCX | het | Exon 3 | dn | ChrX: g.110653572G>A | NM_000555.3:c.298C>T | p.(Arg100*) | Disease causing | NP | ||
| 2-A | ALDH7A1 | het | Exon 6 | pat | Chr5: g.125912837T>C | NM_001201377.1:c.500A>G | p.(Asn195Ser) | Disease causing | Probably damaging | Deleterious |
| ALDH7A1 | het | Intron | mat | Chr5: g.125885616C>T | NM_001182.2:c.1405+5G>A | — | Polymorphism | NP | NP | |
| 3-A | GPR98 | het | Exon 85 | mat | Chr5: g.90281186T>C | NM_032119.3:c.17999T>C | p.(Val6000Ala) | Disease causing | NP | Neutral |
| 4-A | SCN1A | het | Splice site | dn | Chr2: g.166904280T>C | NM_006920.4:c.1029-2A>G | — | Disease causing | NP | NP |
| POLG | het | Exon 17 | pat | Chr15: g.89864482C>T | NM_001126131.1:c.2608G>A | p.(Val870Ile) | Disease causing | Probably damaging | Neutral | |
| 5-A | MAGI2 | het | Exon 16 | mat | Chr7: g.77789528G>A | NM_012301.3:c.2659C>T | p.(Arg887Cys) | Disease causing | Probably damaging | Deleterious |
| KCNQ2 | het | Exon 5 | pat | Chr20: g.62073806del | NM_172107.2:c.769del | p.(Glu257Argfs*16) | Disease causing | NP | NP | |
| 6-A | GPR98 | het | Exon 33 | pat | Chr5: g.89990155C>T | NM_032119.3:c.7582C>T | p.(Pro2528Ser) | Disease causing | Benign | Neutral |
| TBC1D24 | het | Exon 2 | pat | Chr16: g.2546790G>A | NM_020705.2:c.641G>A | p.(Arg214His) | Disease causing | Probably damaging | Neutral | |
| KCNQ2 | het | Exon 6 | dn | Chr20: g.62070955G>A | NM_172107.2:c.923C>T | p.(Pro308Leu) | Disease causing | Probably damaging | Deleterious | |
| 7-A | SCN1A | het | Exon 1 | dn | Chr2: g.166929960C>T | NM_006920.4:c.172G>A | p.(Gly58Arg) | Disease causing | Possibly damaging | Deleterious |
| SCN1B | het | Exon 2 | pat | Chr19: g.35523542G>A | NM_199037.3:c.151G>A | p.(Ala51Thr) | Disease causing | Possibly damaging | Deleterious | |
| 8-B | No candidate mutations | |||||||||
| 9-B | RELN | het | Exon 24 | mat | Chr7: g.103244917G>A | NM_005045.3:c.3022C>T | p.(Arg1008Cys) | Disease causing | Possibly damaging | Deleterious |
| GABRG2 | het | Exon 4 | pat | Chr5: g.161524667dup | NM_198904.2:c.351dup | p.(Ala118Cysfs*6) | Disease causing | NP | ||
| 10-B | RELN | het | Exon 11 | pat | Chr7: g.103322621G>T | NM_005045.3:c.1231C>A | p.(Leu411Ile) (rs144978163) | Disease causing | Benign | Neutral |
| GRIN2A | het | Exon 11 | mat | Chr16: g.9892164C>A | NM_001134407.1:c.2326G>T | p.(Asp776Tyr) | Disease causing | Probably damaging | Deleterious | |
| 11-B | No candidate mutations | |||||||||
| 12-B | No candidate mutations | |||||||||
| 13-B | SCN2A | het | Exon 11 | mat | Chr2: g.166172087G>A | NM_001040142.1:c.1490G>A | p.(Ser497Asn) | Disease causing | Benign | Neutral |
| 14-B | No candidate mutations | |||||||||
| 15-B | No candidate mutations | |||||||||
| 16-B | GPR98 | het | Exon 19 | pat | Chr5: g.89948228C>G | NM_032119.3:c.3482C>G | p.(Ser1161Cys) | Disease causing | Possibly damaging | Probably damaging |
| GRIN2A | het | Exon 8 | pat | Chr16: g.9927982C>T | NM_001134407.1:c.1757G>A | p.(Arg586Lys) | Disease causing | Probably damaging | Neutral | |
| 17-B | No candidate mutations | |||||||||
| 18-B | No candidate mutations | |||||||||
| 19-B | SCN9A | het | Exon 21 | mat | Chr2: g.167089942G>C | NM_002977.3:c.3799C>G | p.(Leu1267Val) | Disease causing | Probably damaging | Deleterious |
Abbreviations: dn, de novo; het, heterozygous; hom, homozygous; mat, maternal; pat, paternal; NP, not predicted.
Bold entries indicate deleterious mutations. In addition, the associated amino-acid substitutions are located in evolutionarily highly conserved residues and are predicted to functionally affect the encoded protein. Results of segregation analyses by Sanger sequencing are reported too.
Exon numbering refers to the NCBI transcript number reported inb.
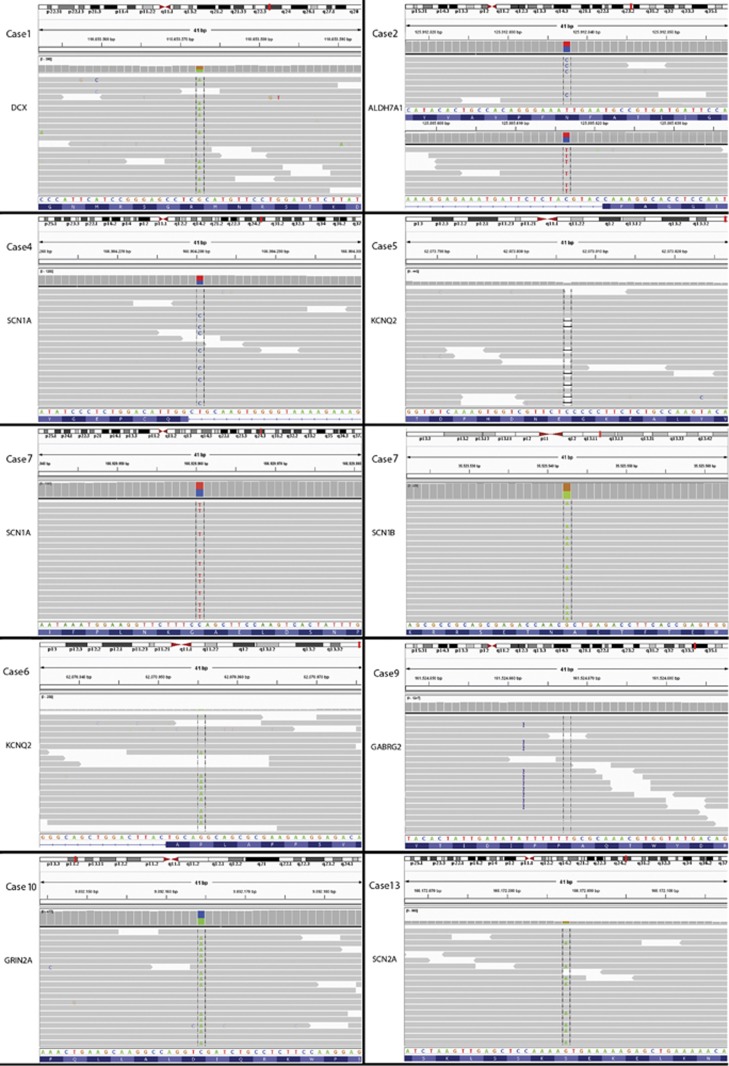
By this approach we were able to identify candidate SNVs, very likely causative of the epileptic phenotype, in nine out of 19 patients, six of which belong to subcohort A and three belonging to subcohort B (Figure 2). In the remaining 10 cases, we could not find any potential causative alterations even after a careful re-evaluation by manual inspection of variants filtered out. Causative mutations were validated by Sanger sequencing (data not shown). All variants thereafter reported have been submitted to Leiden Open Variation Database 3.0 (Supplementary Table 3S).
Figure 2.
Alignments loaded on IGV 2.1(Integrative Genomics Viewer) for every causative mutation reported in bold in Table 2. Chromosomal view has the covered 41 bp delimitated in red, followed by genomic coordinates, relative coverage for single base pair, and alignments covering 101 bp in average. At the bottom of every image the reference sequence and the corresponding amino-acid sequence are visible.
Subcohort A
Case 1-A, a female, showed a de novo transition in exon 3 of the DCX gene (NM_000555.3) leading to a nonsense mutation. The reading frame was interrupted by a premature stop codon possibly leading to nonsense-mediated decay (NMD) of the mRNA as suggested by the prediction tools (Polyphen2, Mutation Taster, and SIFT). Leger et al14 has reported the same mutation. A paternally inherited SHANK3 mutation, considered probably damaging by Polyphen2, was also present.
Case 2-A showed two mutations both in ALDH7A1 gene, one inherited from the father and the other one from the mother. The paternal allele had a transition reported in ESP (MAF 0.0077%) and HGMD (CM087549).15 The maternal allele had a mutation occurring in intron 16, 5 bp upstream to the previous exon. This mutation was present in ESP (MAF 0.0231%) in heterozygosis and was also reported in HGMD (CS091873).16 The intronic variant was predicted to alter the splicing by skipping of exon 16.
Case 3-A had a maternally inherited missense mutation at GPR98 gene that was predicted as damaging by one prediction tool.
Case 4-A, showed a de novo splice site mutation in the SCN1A gene. A heterozygous missense mutation of POLG, was also detected both in the patient and in his normal father. This alteration was given as damaging by prediction tools.
Case 5-A showed a 1-bp deletion, in the KCNQ2 gene creating a frame shift with a premature stop 15 codons downstream. The mutation was inherited from the father who suffered from the same type of epilepsy. A missense mutation in MAGI2, predicted as damaging and inherited from her normal mother was also present.
Case 6-A showed a de novo missense substitution in the KCNQ2 gene. Other two heterozygous missense mutations were detected in GPR98 and TBC1D24, both inherited from the normal father.
Case 7-A had two missense mutations at SCN1A and SCN1B, both predicted as damaging. SCN1A mutation was de novo, whereas SNC1B alteration was inherited from the father.
Subcohort B
Case 9-B showed a 1-bp exonic duplication in the GABRG2 gene. This duplication creates a frame shift starting at codon Ala118 with the new reading frame ending in a stop five codons downstream. The mRNA was predicted as target for NMD by Mutation Taster. The same duplication was found in the father who suffered from the same condition. A mutation of RELN, predicted as damaging and inherited from her normal mother was also detected.
Case 10-B had a heterozygous missense mutation in the GRIN2A gene inherited from his mother who showed an overlapping phenotype. This transversion occurred in a highly conserved nucleotide. A missense mutation in the RELN gene, predicted disease-causing by Mutation Taster, resulted to be inherited from the normal father.
Case 13-B showed a maternally inherited missense transition in the SCN2A gene, affecting a highly conserved nucleotide and predicted to be damaging by SIFT and Mutation Taster but not by Polyphen.
Case 16-B had two missense mutations at GPR98 and GRIN2A, both predicted to be disease causing by at least one prediction tool. Both GRIN2A and GPR98 mutations were inherited from the normal father.
Case 19-B had a predicted damaging missense mutation at SCN9A, inherited from the mother.
Cases 8-B, 11-B, 12-B, 14-B, 15-B, 17-B, and 18-B did not show any possible causative mutation.
Control cohort variants
The examination of the 22 variants found in controls after discrete filtering revealed that only 4 of these, all heterozygous, were potentially disease causing.
In particular, we found a missense substitution p.(Gly216Ala) (c.647G>C) in CHRNA4 gene (NM_000744) in one case and a heterozygous missense mutation p.(Gly1602Ser) (c.4804G>A) in FLNA gene (NM_001110556) in a female subject. A third control subject had a mutation of UBE3A (NM_000462) (c.1735G>A, p.(Val579Met)) and a second mutation of SCN1B (NM_199037) (c.178C>T, p.(Arg60Cys)).
Discussion
We used a NGS-based approach to test 67 epilepsy genes in 19 patients with different types of epilepsy. Patients had been stratified in two groups according to their neurological phenotypes. In the group A, including seven patients whose clinical features were rather suggestive for a specific syndrome, we detected a likely causative mutation in six (cases 1-A, 2-A, 4-A, 5-A, 6-A, 7-A). In the group B, including 12 patients with a phenotype not distinctive for any specific gene, we have been able to find a plausible causative mutations only in three (cases 9-B, 10-B, 13-B), whereas the remaining cases were either negative (seven cases) or had mutations whose role was unclear (cases 16-B and 19-B). These results were not unexpected and emphasize the restriction of this approach is a lack of knowledge about the functional role of most variants, resulting in a large number of variants of uncertain significance.17 For this reason, the diagnostic yield of 47% (9/19) is quite high. The 12 cases who had at least one mutation are discussed below.
Patient 1-A had a typical clinical picture of Lennox–Gastaut syndrome and magnetic resonance imaging showed a very large double cortex overlapping with the subcortical band heterotopia syndrome. The de novo truncating mutation of DCX fits very well with her double cortex.14 The mutation of SHANK3 was inherited from her normal father. SHANK3 alterations are causative of Phelan-McDermid syndrome18 and are characterized by complete penetrance. Thus, we assumed that this missense mutation was likely benign.
Patient 2-A showed neonatal seizures and multifocal epileptiform discharges at EEG, which became normalized with pyridoxine. Presently the patient is 14 years old and the treatment with pyridoxine allowed a complete control of seizures with a normal psychomotor development. She had a compound heterozygous mutation for ALDH7A1. The intronic mutation would have been lost if we had disregarded all SNPs reported in public databases, whereas we have taken into consideration all the SNPs with a MAF<1%. Actually, both mutations have already been described in patients with pyridoxine-dependent epilepsy.15, 16 The finding that her epileptic crisis ceased after pyridoxine treatment indeed demonstrated the causality of the ALDH7A1 mutations.
Patient 3-A, who was also affected by pyridoxine-dependent epilepsy, had normal IQ No mutations were detected in the candidate ALDH7A1 gene, whereas a missense mutation was present in GPR98 inherited from the normal mother. Alterations of GPR98 have been associated with familial febrile seizures and autosomal recessive or digenic dominant Usher syndrome. However, the clinical phenotype of the patient was completely different from these conditions. Our findings suggest that pyridoxine-dependent seizure does not only rely on ALDH7A1 mutations.19
Patient 4-A had a clinical diagnosis of Dravet syndrome, so the de novo splice site mutation of SCN1A fitted well with his phenotype.20, 21, 22 The heterozygous mutation in POLG was considered not associated with his condition because it was also present in the healthy father, whereas dominant POLG mutations are associated with adult onset progressive external ophthalmoplegia.23
Patient 5-A had neonatal generalized tonic-clonic seizures, occurring on the second day of life, not responsive to any therapy. Seizures ceased spontaneously at the 25th day of life. Her father, who was found to carry the same mutation, had the same neonatal condition also ending at the 25th day of life. The frameshift mutation at KCNQ2 well correlated with the phenotype.24
The mutation in MAGI2, inherited from the healthy mother, was not considered disease causing, although it was predicted as probably damaging. Haploinsufficiency for MAGI2 has been associated with hypsarrhythmia,25 a condition different from the one we observed in this family.
Patient 6-A showed neonatal seizures since his 3rd day of life that ceased 1 month later. He then suffered from sporadic seizures episodes persisting until now (8 years old). He also presented a severe pervasive developmental disorder. The family history was negative. He had a de novo missense mutation of KCNQ2. This type of mutation has been reported in several patients with early onset epileptic encephalopathy24, 26, 27 in contrast to frameshift or nonsense mutations that are more frequently found among patients with BFNS.
Patient 7-A was affected by generalized epilepsy with febrile seizures plus (GEFSP) and had normal IQ. Our investigations revealed a de novo missense mutation of SCN1A and a missense mutation of SCN1B inherited by the father. The two mutations could explain her phenotype as GEFSP is extremely heterogeneous, and both SCN1A and SCN1B are among the genes associated with this condition.28
Patient 9-B, during her first year of life suffered from numerous febrile seizures that diminished later on. She had a frameshift mutation at GABRG2 present also in her father who suffered from febrile seizure until 5 years of age. In fact, mutations of GABRG2, either missense or truncating, cause a spectrum of seizure disorders, ranging from early-onset isolated febrile seizures to GEFSP, type 3, which represents the most severe phenotype.29, 30, 31 The type of febrile seizures present in this patient and in her father fits well with the milder phenotype. The heterozygous mutation of RELN has not been considered as causal of her phenotype because only recessive mutations of this gene are associated with a pathogenic condition characterized by lissencephaly32 not present in our patient who has a normal psychomotor development.
Patient 10-B showed a typical clinical and EEG's picture of benign childhood epilepsy with centrotemporal spikes. We detected a missense mutation at GRIN2A that was also present in his mother and aunt (the sister of the mother) showing the same clinical and EEG's picture with remission of seizures in adolescence and borderline cognitive level. Our patient did not have overt seizures until the age of 7 years when rolandic epilepsy appeared. A series of mutations of this gene have been described in subjects/families with a phenotype overlapping that of our patient including learning disabilities.33, 34
Patient 13-B had a missense mutation of SCN2A inherited from her mother. Mutations of this gene are associated with noteworthy clinical variability ranging from familial benign seizures to generalized epilepsy with febrile seizures or epileptic encephalopathy.35 The patient presented only febrile seizures, whereas her mother had benign generalized epilepsy with absences.
Patient 16-B had an epileptic encephalopathy with severe cognitive impairment. The two missense mutations highlighted in GPR98 and GRIN2A did not seem related to his phenotype. As he was born from healthy consanguineous parents, an autosomal recessive condition has to be taken into consideration.
Finally, patient 19-B had a missense mutation of SCN9A predicted as damaging. However, mutations of this gene are usually associated with febrile seizure, GEFSP, and Dravet syndrome,36 whereas the patient's phenotype was suggestive of an epileptic encephalopathy strongly resembling West syndrome with hypsarrhythmia, spasm, and psychomotor regression. Both his parents were healthy.
Our targeted platform was thought with the aim to provide a quick and cheap molecular diagnosis to most patients with an epileptic disorder. When we built the platform, we thought we could identify the causative mutation independently from any clinical information. In this sense we required the specialist to select the cases in a totally random way and without giving us any information about their phenotype and family history. The only request was to exclude cases with holoprosencephaly for which we have a dedicated NGS platform. Actually, the finding of multiple candidate mutations made clear that the culprit gene could be highlighted only by knowing in detail both the patient's phenotype and the family history. Moreover, predicted damaging mutations had been detected in the healthy controls as well. Eventually, in 9 of 19 patients we identified a very likely causative mutation (Table 2) with most of them detected in cohort A including patients whose phenotype indeed suggested the involvement of a specific gene. Among the 12 patients owing to cohort B, affected by different types of epilepsy not suggestive for any or a single specific gene, we could find the most likely causative mutation only in three and in all of them (cases 9-B, 10-B, 13-B) the alteration could be hardly suspected a priori.
The absence of any mutation in seven patients (cases 8-B, 11-B, 12-B, 14-B, 15-B, 17-B, 18-B) indicated that alterations in many other genes not present in our platform are associated with epilepsy, stressing the high genetic heterogeneity of this disorder.
The analysis of the control cohort revealed four potentially damaging mutations in three healthy individuals. None of these variants were previously reported in HGMD. One female subject had a FLNA mutation predicted to be damaging. We could not define whether this was a benign variant rather than a really damaging mutation with incomplete penetrance as reported for females with mutation of this gene and cardiac valvular dysplasia (OMIM #314400).37 The interpretation of the CHRNA4 mutation was also difficult as alterations of this gene can cause either nocturnal frontal lobe epilepsy type 1, although with incomplete penetrance, or nightmares and other sleep disorders that are often undiagnosed.38 Variants in SCN1B and UBE3A were identified in the same subject. As in the case of FLNA, the SCN1B mutation could be either neutral or pathological with incomplete penetrance,28 whereas the UBE3A mutation could be either a benign variant or disease causing but inherited from the father.39 To conclude, we were unable to interpret some of the genetic lesions we met in the control cohort, further stressing that our knowledge of genetic variants is presently limited, increasing the risk of false-positive and false-negative information.40 If these lesions were indeed pathogenic, we should consider the hypothesis that common disease traits such as epilepsy are the result of different genetic components. In fact, the observation of deleterious mutations in 237 ion-channel genes with the same prevalence in individuals with epilepsy and control subjects suggested that, at least for these genes, the personal risk assessment in epilepsy depends more on the combination of the variants rather than specific deleterious variants.41 The lack of enrichment of protein-disrupting ion-channel mutations in individuals with epilepsy has been confirmed by Heinzen et al.42 These authors also demonstrated that single epilepsy-susceptibile variants identified by exome sequencing in patients with idiopathic generalized epilepsy (juvenile myoclonic epilepsy and absence epilepsy), although rare, were possibly real risk factors, each of them accounting for only a small fraction of individuals with epilepsy. This burden of data makes evident the complex architecture of epilepsy with genetic heterogeneity much higher than expected. It is conceivable that in the near future the collection of clinical history, EEG, and imaging will be combined with the analysis of NGS-dedicated platforms. The negative cases will be analyzed for whole exome if not for whole genome. The advantages of this approach are evident both in the immediate (consulting for risk of recurrence) and in the long run when specifically targeted treatments will be adopted.
We have been able to conclude the analysis of nine patients (the number of patients we pool in a single lane), including the enlargement of the investigation to parents, in 8–10 weeks with a cost per patient comparable to sequencing 1–2 medium-to-large-sized genes by conventional techniques, a result overlapping that reported by Lemke et al.4 Our results suggest that using a single platform to sequence all or most of the epilepsy genes may increase the diagnostic yield. This is particularly true in absence of clinical signs suggestive for involvement of a specific gene like in three patients of our cohort. Obviously novel mutations require that their causative role is further confirmed by segregation or functional analyses. On the contrary, smaller platforms containing a limited number of genes may reduce the efficacy of the NGS-based approach, as epilepsy is extremely heterogeneous both under the genetic and phenotypic point of view. Our platform, as well as the one already described by Lemke et al,4 has the advantage that can also be run on ‘benchtop' sequencers, which forego high yields in exchange for low capital costs, small physical footprints, and more rapid turnaround times, making them far more attractive to smaller biomedical laboratories.43
Acknowledgments
We are grateful to the patients and families for their participation in the study. This work was supported by the Ricerca Corrente RC11017C (Diagnosis and therapy of epileptic syndromes), Fondazione IRCCS C Mondino, Pavia, and the Telethon Foundation (project GGP10121C).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Web resources
1. NIH Undiagnosed Diseases Program: http://rarediseases.info.nih.gov/Resources.aspx?PageID=31
2. dbSNP135: http://www.ncbi.nlm.nih.gov/projects/SNP/
3. 1000 Genomes Project Database: http://www.1000genomes.org/data
4. Exome Sequencing Project Database: https://esp.gs.washington.edu/drupal/
5. HGMD 2012.3: http://www.biobase-international.com/product/hgmd
6. Mutation Taster: http://www.mutationtaster.org
7. Polyphen2: http://genetics.bwh.harvard.edu/pph2/
8. SIFT: http://SIFT.jcvi.org
9. LOVD: http://www.lovd.nl/3.0/home
Supplementary Material
References
- Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- EPICURE Consortium, EMINet Consortium. Steffens M, et al. Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum Mol Genet. 2012;21:5359–5372. doi: 10.1093/hmg/dds373. [DOI] [PubMed] [Google Scholar]
- Mefford HC, Muhle H, Ostertag P, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke JR, Riesch E, Scheurenbrand T, et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53:1387–1398. doi: 10.1111/j.1528-1167.2012.03516.x. [DOI] [PubMed] [Google Scholar]
- Epilepsy Phenome/Genome Project. EPICURE Consortium. Allen AS, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen Taber KA, Dickinson BD, Wilson M. The promise and challenges of next-generation genome sequencing for clinical care. JAMA Intern Med. 2013;174:275–280. doi: 10.1001/jamainternmed.2013.12048. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad MJ, Ng SB, Bigham AW, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- Webb TR, Parfitt DA, Gardner JC, et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23) Hum Mol Genet. 2012;21:3647–3654. doi: 10.1093/hmg/dds194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger PL, Souville I, Boddaert N, et al. The location of DCX mutations predicts malformation severity in X-linked lissencephaly. Neurogenetics. 2008;9:277–285. doi: 10.1007/s10048-008-0141-5. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Chen Y, Hahn S, Glass IA, Gospe SM., Jr Prevalence of ALDH7A1 mutations in 18 North American pyridoxine-dependent seizure (PDS) patients. Epilepsia. 2009;50:1167–1175. doi: 10.1111/j.1528-1167.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- Striano P, Battaglia S, Giordano L, et al. Two novel ALDH7A1 (antiquitin) splicing mutations associated with pyridoxine-dependent seizures. Epilepsia. 2009;50:933–936. doi: 10.1111/j.1528-1167.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- Jacob HJ. Next-generation sequencing for clinical diagnostics. N Engl J Med. 2013;369:1557–1558. doi: 10.1056/NEJMe1310846. [DOI] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Beri S, et al. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet. 2011;7:e1002173. doi: 10.1371/journal.pgen.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Cook J, Gospe SM., Jr Epilepsy due to 20q13.33 subtelomere deletion masquerading as pyridoxine-dependent epilepsy. Am J Med Genet A. 2012;158A:3190–3195. doi: 10.1002/ajmg.a.35633. [DOI] [PubMed] [Google Scholar]
- Depienne C, Trouillard O, Saint-Martin C, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46:183–191. doi: 10.1136/jmg.2008.062323. [DOI] [PubMed] [Google Scholar]
- Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010;51:1650–1658. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102. [PubMed] [Google Scholar]
- Kiechl S, Horvath R, Luoma P, et al. Two families with autosomal dominant progressive external ophthalmoplegia. J Neurol Neurosurg Psychiatry. 2004;75:1125–1128. doi: 10.1136/jnnp.2003.025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- Marshall CR, Young EJ, Pani AM, et al. Infantile spasms is associated with deletion of the MAGI2 gene on chromosome 7q11.23-q21.11. Am J Hum Genet. 2008;83:106–111. doi: 10.1016/j.ajhg.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Koide A, et al. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann Neurol. 2012;72:298–300. doi: 10.1002/ana.23620. [DOI] [PubMed] [Google Scholar]
- Kato M, Yamagata T, Kubota M, et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia. 2013;54:1282–1287. doi: 10.1111/epi.12200. [DOI] [PubMed] [Google Scholar]
- Bonanni P, Malcarne M, Moro F, et al. Generalized epilepsy with febrile seizures plus (GEFS+): clinical spectrum in seven Italian families unrelated to SCN1A, SCN1B, and GABRG2 gene mutations. Epilepsia. 2004;45:149–158. doi: 10.1111/j.0013-9580.2004.04303.x. [DOI] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- Kananura C, Haug K, Sander T, et al. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch Neurol. 2002;59:1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N, Guerrini R. Diffuse malformations of cortical development. Handb Clin Neurol. 2013;111:653–665. doi: 10.1016/B978-0-444-52891-9.00068-3. [DOI] [PubMed] [Google Scholar]
- Carvill GL, Regan BM, Yendle SC, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013;45:1073–1076. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke JR, Lal D, Reinthaler EM, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet. 2013;45:1067–1072. doi: 10.1038/ng.2728. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kato M, Osaka H, et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 2013;81:992–998. doi: 10.1212/WNL.0b013e3182a43e57. [DOI] [PubMed] [Google Scholar]
- Mulley JC, Hodgson B, McMahon JM, et al. Role of the sodium channel SCN9A in genetic epilepsy with febrile seizures plus and Dravet syndrome. Epilepsia. 2013;54:e122–e126. doi: 10.1111/epi.12323. [DOI] [PubMed] [Google Scholar]
- Kyndt F, Gueffet JP, Probst V, et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115:40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- Derry CP, Duncan JS, Berkovic SF. Paroxysmal motor disorders of sleep: the clinical spectrum and differentiation from epilepsy. Epilepsia. 2006;47:1775–1791. doi: 10.1111/j.1528-1167.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- Buiting K. Prader-Willi syndrome and Angelman syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:365–376. doi: 10.1002/ajmg.c.30273. [DOI] [PubMed] [Google Scholar]
- Robinson PN, Krawitz P, Mundlos S. Strategies for exome and genome sequence data analysis in disease-gene discovery projects. Clin Genet. 2011;80:127–132. doi: 10.1111/j.1399-0004.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- Klassen T, Davis C, Goldman A, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen EL, Depondt C, Cavalleri GL, et al. Exome sequencing followed by large-scale genotyping fails to identify single rare variants of large effect in idiopathic generalized epilepsy. Am J Hum Genet. 2012;91:293–302. doi: 10.1016/j.ajhg.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Lek M. The uncertain road towards genomic medicine. Trends Genet. 2012;28:303–305. doi: 10.1016/j.tig.2012.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.