ABSTRACT
Adrenomedullin2 (ADM2) is reported to facilitate embryo implantation and placental development. Therefore, the current study was undertaken to identify if ADM2 has a functional role in ovary to facilitate its reproductive actions. This study shows that the expression of ADM2 is differentially regulated in rat estrous cycle and that ADM2 increases the synthesis and secretion of 17beta-estradiol accompanied with an increase in the expression of steroidogenic factor 1 (Sf1), estrogen receptor Esr1, and enzymes involved in steroidogenesis in equine chorionic gonadotropin (eCG)-treated rat ovaries. In addition, inhibition of endogenous ADM2 function in eCG-treated immature rats caused impaired ovulation. Furthermore, the mRNA expression of Adm2 and receptor activity modifying protein 3 is higher in the ovary on Day 18 compared to nonpregnant and pregnant rats on Day 22. ADM2-like immunoreactivity is localized in granulosa cells, blood vessels, oocytes, cumulous oophorus, and corpus luteum of pregnant ovaries, suggesting a potential role for ADM2 in the ovary. This is supported by the presence of ADM2-like immunoreactivity in the corpus luteum during pregnancy and a decline in aromatase immunoreactivity in corpus luteum on Day 9 of gestation in rats infused with ADM2 antagonist during implantation and decidualization phase. Taken together, this study suggests a potential involvement of ADM2 in the rat ovary in regulating synthesis of estradiol to support ovulation and facilitate efficient implantation and placental development for a successful pregnancy.
Keywords: ADM2/IMD, ovary, ovulation, pregnancy, steroidogenesis
INTRODUCTION
Adremedullin2 (ADM2)/Intermedin is a novel peptide that belongs to the calcitonin (CALCA)/calcitonin gene-related peptide (CALCB) family of peptides that has ∼28% structural homology to adrenomedullin (ADM) and <20% to CALCB. ADM2 exerts its effects through 7-transmembrane (7-TM) domain G-protein coupled receptor (GPCR), calcitonin receptor-like receptor (CALCRL) in combination with one of the receptor activity-modifying proteins (RAMPs), that is, RAMP1, RAMP2, or RAMP3 [1]. Unlike ADM and CALCB, ADM2 is shown to be a nonselective agonist for RAMPs but exhibits greater potency with CALCRL/RAMP1 and CALCRL/RAMP3 [1].
Adm2 mRNA is abundantly expressed in the rat ovary, uterus, stomach, kidney, brain, and pituitary gland [1, 2]. Previous studies showed that ADM2 has vasodilatory and hypotensive actions that were similar to or more potent than those of ADM and CALCB [1, 3]. Adm2 mRNA has been shown to be an estrogen-dependent regulator of prolactin release in pituitary [4] and possesses an estrogen response element in its sequence, suggesting functional endocrine or a paracrine role for ADM2 in regulating pituitary hormone secretion.
Recently we reported that plasma levels of immunoreactive ADM2 are elevated during pregnancy and that ADM2-induced vasodilation is greater during pregnancy in rats [5]. Further, we demonstrated that ADM2 is secreted by Day 6 human blastocyst and that blocking endogenous effects of ADM2 in early pregnant rat causes a decline in serum estradiol levels along with impaired embryo implantation rate and placental development [6–8]. Thus, a series of evidence suggest a potential role for this novel peptide in mediating early placentation to facilitate a successful pregnancy [5–7, 9, 10]. ADM2, CALCRL, and all three RAMPs are expressed in the rat ovary [11, 12]. A recent report shows that ADM2 is important for regulation of cumulus cells survival and maintenance of the tertiary structure of the cumulus oocyte complex [11, 12]. However, it is not known if ADM2 has a role in regulating ovarian functions to support the establishment and maintenance of pregnancy. Therefore, the objectives of this study were to 1) assess if mRNA expression of Adm2 varies with changes in the rat estrus cycle, 2) assess the effect of ADM2 on ovarian function as it relates to the rate of ovulation and steroidogenesis in the rat ovary, 3) identify changes in Adm2 expression and localize ADM2 immunoreactivity in pregnant rat ovary, and 4) assess the effects of inhibition of endogenous ADM2 activity on the expression of enzyme p450 aromatase protein in the corpus luteum that plays an important role in estradiol biosynthesis during pregnancy.
MATERIALS AND METHODS
Nonpregnant and timed pregnant rats (six in each group with a body weight of 250–300 gm) and 25-day-old immature female rats (10−12 rats in each group) were purchased from Harlan-Sprague Dawley. All the animals were given free access to food and water. The Animal Care and Use Committee at University of Texas Medical Branch approved all the procedures for our study. Nonpregnant rats were euthanized on the diestrous, proestrous, and estrous stages, and pregnant rats were euthanized on Days 9, 18, and 22 of gestation. Ovaries were collected, and total RNA was extracted using TRIzol method. RNA was treated with DNase1 before performing the reverse transcriptase polymerase chain reaction (RT-PCR). Immunohistolocalization of ADM2 protein were assessed in tissue sections of ovaries from pregnant rats euthanized on Day 18.
Inhibition of Ovulation
The peptide ADM217–47 is the only reported antagonist specific for ADM2 action in several tissues and cell lines, but the action of ADM2 via an unknown receptor cannot be ruled out [1–5]. In this study, we used ADM217–47 to study the effect of ADM2 on the rate of ovulation and the growth of ovaries. Prepubertal rats (28-days-old) were injected with 10 international units (subcutaneous) equine chorionic gonadotropin (eCG; Sigma-Aldrich) between 0930 and 1000 h. These rats typically have a luteinizing hormone (LH) surge around 56 h after the eCG injection that is followed by ovulation of about 10–15 oocytes/rat around 72 h [13, 14]. Rats were infused with either saline or ADM217–47 (200 μg/day; n = 10/group) 48 h after eCG treatment that was delivered at 10 μl/h through osmotic minipumps (Alza) inserted (subcutaneous) into the dorsum of the rats while animals were under anesthesia. Anesthesia consisted of a combination of 45 mg/kg of body weight ketamine (Fort Dodge Laboratories) and 5 mg/kg of body weight xylazine (Burns Veterinary Supply). The dose of antagonist chosen was based on our previously published study [15], and the antagonist effects were similar in the dose range of 125–750 μg/rat/day. The rats were euthanized in the morning of the next day, and the ovaries were dissected out, weighed, and analyzed for the number of corpora lutea as a measure of ovulation.
Ovary Explant Culture
Immature female rats were injected with 10 international units equine chorionic gonadotropin (eCG) to induce follicular growth. After 36 h, the rats were euthanized, ovaries removed, cut into fragments, and maintained as explants for 24 h in the presence or absence of ADM2 (10−8–10−9 M). Ovarian tissues were used either for RNA extraction or for steroid hormone extractions. Culture medium was also collected for measuring the steroid hormone secreted in the medium. The steroid contents in the ovarian tissues were expressed as pg/mg tissue or ng/mg tissue, and the total amount of steroids secreted into the medium was normalized to the weight of the tissue used for explant and expressed as total secretion (pg/mg and ng/mg tissue for estradiol and progesterone, respectively).
Radioimmunoassay
Radioimmunoassay for 17β-estradiol and progesterone was performed using a kit in accordance with manufacturer's instructions (DSL). Briefly, steroids were extracted with hexane:ethyl acetate (3:2 by volume). The organic extract was dried completely in a vacuum centrifuge (Savant SpeedVac; Savant Instruments Inc.) and reconstituted in assay buffer. For estradiol, the intraassay variation was 6% for both medium and extract, and the interassay variation was 10% in the medium and extract. For progesterone, the intraassay variation was 7% for both medium and extract, and the interassay variation was 12% in the medium and extract. The ranges of both the assays were 15–3000 pg/ml, and the assays were 100% specific for the respective steroid with 0% cross-reactivity to the other steroid hormone.
ADM217–47 Infusion Studies
Two adult female rats were paired with a male overnight. The next morning, the males were removed, and the females were assessed for the presence of sperm in the vaginal flush. Animals with positive sperm in the flushes are designated as Day 1 of gestation. Five pregnant rats were used in each experimental group. In our previous work, we showed that ADM2 antagonist (ADM217–47) infusion during peri-implantation period from Day 3 through Day 9 caused a decline in the estrogen levels in serum along with impaired implantation on Day 9. Therefore, we were interested to know if ADM2 had an effect on expression of aromatase enzyme, a key regulator in estradiol synthesis in the corpus luteum during pregnancy. We used rats infused with ADM217–47 from Day 3 to Day 9, covering the peri-implantation and decidualization period of pregnancy that precede the formation of placenta to study the effects on aromatase expression. Ovaries were collected and fixed in buffered formalin for immunohistochemistry.
RNA Extraction and RT-PCR
TRIzol reagent was used to isolate total RNA and protein from approximately 100 mg of rat ovary according to the manufacturer's protocol. The RNA was dissolved in 20 μl of RNase-free water containing DNase I buffer and 5 units of amplification grade DNase I. The DNase I was removed by phenol-chloroform extraction. The RNA was stored at −70°C until use. Using total RNA, first-strand complementary DNA (cDNA) was synthesized by reverse transcription in a 20 μl reaction volume containing 1× PCR buffer, 2 μg RNA, 5 mM MgCl2, 1 mM dNTP mixture, and random primers as described by the supplier (Ambion). For reverse transcription, samples were placed into a thermal cycler for 1 cycle at 42°C for 15 min, 99°C for 5 min, and 5°C for 5 min. The cDNA was stored at −20°C.
Semiquantitative PCR
Adm2, Calcrl, Ramp1, Ramp2, Ramp3, and 18S ribosomal (Rn18s) mRNA expression was assessed by semiquantitative PCR analysis with gene-specific primer (Table 1). Amplification of Rn18s was used as an endogenous control. Briefly, 2 μl of the cDNA was mixed with a PCR mixture containing 2.5 mM MgCl2, 1:10 dilution of 10× PCR buffer, 5 unit/100 μl of 1 mM dNTP mixture, and 0.2 μM of gene-specific forward and reverse primers as shown in Figure 1 in a total volume of 50 μl (Sigma-Aldrich). PCR reactions were carried out on GeneAmp PCR system 9700 (Perkin Elmer) with the following conditions: an initial denaturation step at 94°C for 5 min followed by 35 cycles of 30 sec at 94°C, 30 sec at 60°C, and 45 sec at 72°C. Reactions were terminated by a 7 min elongation step at 72°C. Total cycle number is chosen for each from the linear portions of the curve (data not shown). PCR products were visualized on 1.4% agarose gel containing 0.5 μg/ml ethidium bromide and run in 0.5× TBE buffer at 100 V for 1.5 h (Sigma-Aldrich). Gels were placed on an ultraviolet light box, imaged, and analyzed with the SigmaPlot software gel (SPSS Inc.). The identity of the amplified sequence was confirmed by sequence analysis of both DNA strands with gene-specific primers on a Perkin Elmer DNA sequencer.
TABLE1.
Sequences of primer pairs used for semiquantitative PCR analysis.
FIG. 1.
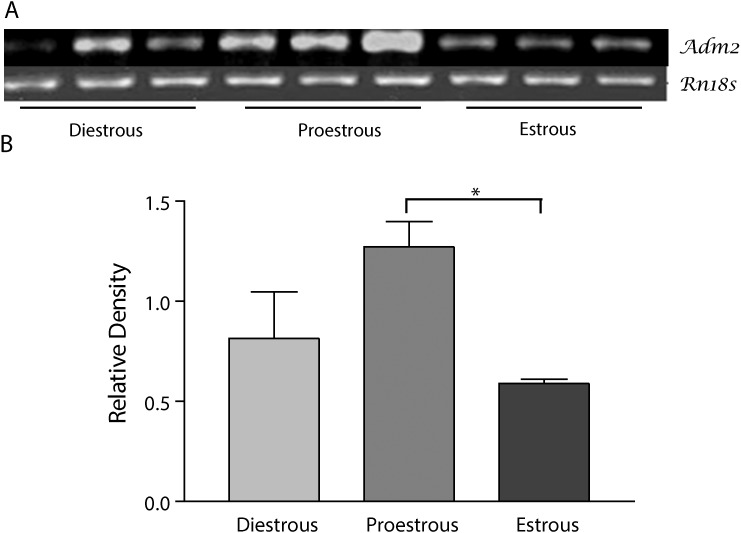
Adm2 mRNA expression in ovary in different stages of the rat estrous cycle. A) Top panel shows PCR product for Adm2 and Rn18s. B) Bottom panel shows the mean ± SEM of the ratios of ADM2 mRNA to Rn18s. Asterisks indicate significant difference compared to estrous phase (n = 6, *P < 0.05).
Real-Time PCR
Quantitative real-time RT-PCR using SYBR Green (Bio-Rad) was performed on a CFX96 Real-Time PCR Detection System (184-5096; Bio-Rad) for steroidogenic factor 1 (Sf1), 17β-dehydroxygenase 1(Hsd17b1), 3β-dehydroxygenase 1 (Hsd3b1), and 3β-dehydroxygenase 2 (Hsd3b2) genes using the specific primers (SABiosciences) listed in Table 2. Primers and probes for estrogen receptors Esr1 (assay identification no. Rn-00664737-m1) and Esr2 (assay identification no. Rn-00562610-m1) were from Applied Biosystems. Amplification of Gapdh served as an endogenous control. PCR amplification with Taqman probes were performed as per the manufacturer's instructions. PCR reactions with SYBR Green were incubated at 95°C for 10 min followed by 95°C for 30 sec (melt) and 60°C for 1 min (anneal/extend) for a total of 40 cycles. Negative control without cDNA was performed to test primer specificity. The relative gene expression was calculated by use of the threshold cycle (CT) Gapdh/CT target gene.
TABLE 2.
Sequences of primer pairs used for quantitative PCR analysis.
Polyclonal Antibodies
Antibodies for ADM2 used in this study were raised with the help of Alpha Diagnostics and have been characterized for their specificity and used in previous studies [9]. Antibody for p450 aromatase was obtained from Abcam.
Immunohistochemical Staining
Sections of rat ovary were heated for 10 min in sodium citrate buffer (pH 6.0) and then rinsed with PBS (0.1 M, pH 7.4). Endogenous peroxidase was blocked by 3% hydrogen peroxide for 10 min at room temperature. The slides were then washed with PBS (0.1 M, pH 7.4) and blocked with avidin-biotin solutions, each for 1 h at room temperature. The primary antibody for ADM2, p450 aromatase, or nonimmune serum/antibody diluent (1:100) was added to the slides and incubated at 4°C overnight. The slides were washed three times with 1% NGS-Triton for 10 min each on a shaker (300 rpm), and then the secondary anti-rabbit biotinylated antibody in a 1:1000 dilution was added and incubated for 45 min at room temperature. The slides were washed three times with 1% NGS-Triton and then PBS (0.1 M, pH 7.4) for 10 min each. The avidin-biotin reagent solution was added to the slides and incubated for 1.5 h at room temperature. After the slides were washed with 0.1 M PBS (three times for 10 min each), the 3,3′-diaminobenzidine solution was added and incubated for 3 min or until visible staining was attained. Then the slides were washed with 0.1 M PBS (three times for 3 min each), counterstained with hematoxylin for 1 min, and rinsed with deionized water (three times for 2 min each). The slides were dehydrated and viewed with an Olympus microscope with image ProPlus software.
Statistics
The experimental data were analyzed by Student t-test or one-way ANOVA. Bonferroni post hoc tests were used for comparisons made between multiple groups. Each RT-PCR experiment was done in triplicate. All the values are expressed as means ± SEM; P < 0.05 (*) were considered statistically significant.
RESULTS
Changes in the Expression of Adm2 mRNA in Cycling Rat Ovary
Figure 1 demonstrates that Adm2 mRNA expression varied with changes in estrous cycle in the rat ovary. Expression of Adm2 is significantly higher in proestrous compared to the estrous phase (n = 6, P < 0.05) whereas expression of Adm2 mRNA in proestrous shows a similar trend of increase, it does not reach significance compared to diestrous (P = 0.065).
Effect of ADM2 on 17β-Estradiol and Progesterone Production in eCG-Treated Ovary Explants
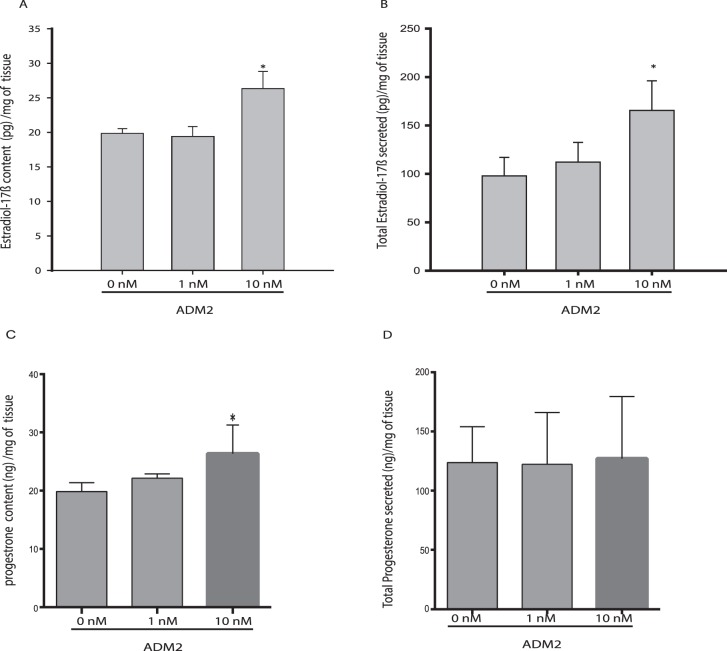
As shown, ADM2 increases the content (mean ± SEM) of 17β-estradiol in ovarian explant tissues (19.6 ± 0.68 pg/ml in control vs. 26.3 ± 2.27 pg/ml in treated; Fig. 2A) and its secretion in the conditioned medium (97.82 ± 19.08 pg/ml in control vs. 165 ± 30.5 pg/ml in treated; Fig. 2B) in the eCG-treated immature rats (P < 0.05), suggesting that ADM2 may enhance availability of estradiol for follicular growth in the rat ovary. However, although the progesterone content (mean ± SEM) is also increased by ADM2 treatment in these tissues (19.85 ± 0.68 ng/ml in control vs. 27.6 ± 3.007 ng/ml in treated, P < 0.05; Fig. 2C) no effect was observed in response to ADM2 on the progesterone levels in the conditioned medium of these ovary explants (Fig. 2D).
FIG. 2.
Effect of ADM2 on estradiol and progesterone levels in ovaries from eCG-treated immature rats. Estradiol levels in ovarian tissue (A) and in medium (B); progesterone levels in ovarian tissue (C) and in medium (D). The steroid contents in the ovarian tissues are expressed as pg/mg tissue, and the total amount of steroids secreted into the medium are expressed as total secretion (pg/mg tissue). Asterisks indicate significant difference compared to control (*P < 0.05, n = 6).
Effect of ADM2 on Expression of Genes Involved in the Regulation of Estradiol Production in the Ovary
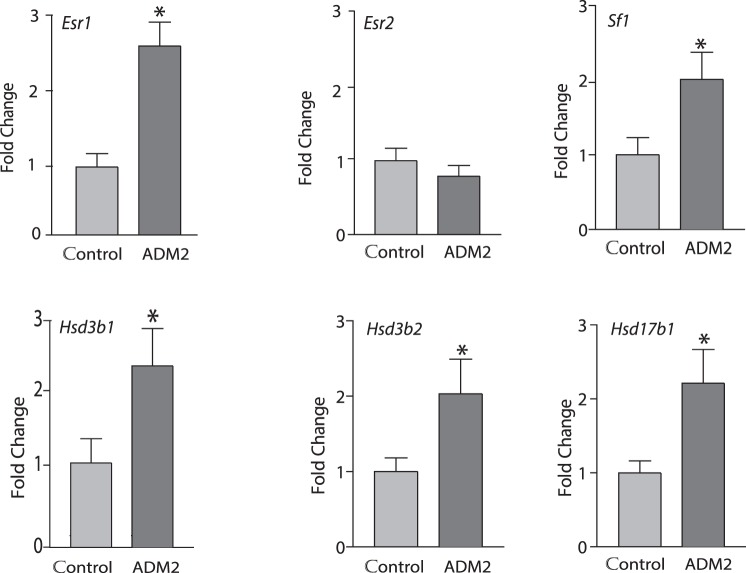
Figure 3 demonstrates that ADM2 treatment significantly increased the mRNA expression of Sf1, estrogen receptor alpha (Esr1), Hsd17b1, Hsd3b1, and Hsd3b2 during follicular growth in ovaries isolated from eCG treated rats (n= 5, P < 0.05).
FIG. 3.
Effect of ADM2 on expression of genes involved in the regulation of estrogen levels in ovary. Real-time PCR data on the ovary explants treated for 24 h in the presence or absence of 10−8 M ADM2. Ovaries were obtained from prepubertal rats at 48 h after eCG injection. Asterisks indicate significant difference compared to control (*P < 0.05, n = 6).
Effect of ADM2 Antagonist (ADM217–47) on the Number of Corpora Lutea and Weight of the Ovary
Involvement of ADM2 on the ovulatory function was assessed in eCG-treated immature rats. As shown in Figure 4A, infusion of the ADM2 antagonist (ADM217–47) to these rats caused a significant decline in the number of corpora lutea, suggesting that inhibition of endogenous ADM2 function results in impaired ovulation (P < 0.05). In these animals, the weight of the ovaries was also assessed as an additional measure for the ovulatory response. As shown in Figure 4B, inhibition of endogenous ADM2 function caused a significant decline in the weights of ovaries in the antagonist-treated group (P < 0.05).
FIG. 4.
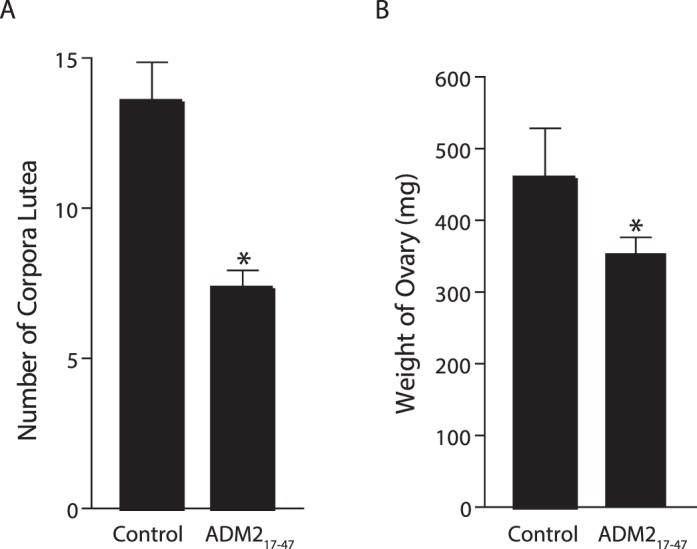
Effect of ADM217–47 infusion on the number of corpora lutea (A) and the weight of ovaries (B). Bars represent mean ± SEM values. Asterisks indicate significant difference compared to control (*P < 0.05, n = 10).
Changes in Expression of Adm2, Calcrl, and Ramp mRNA with Gestational Age in Rat Ovary
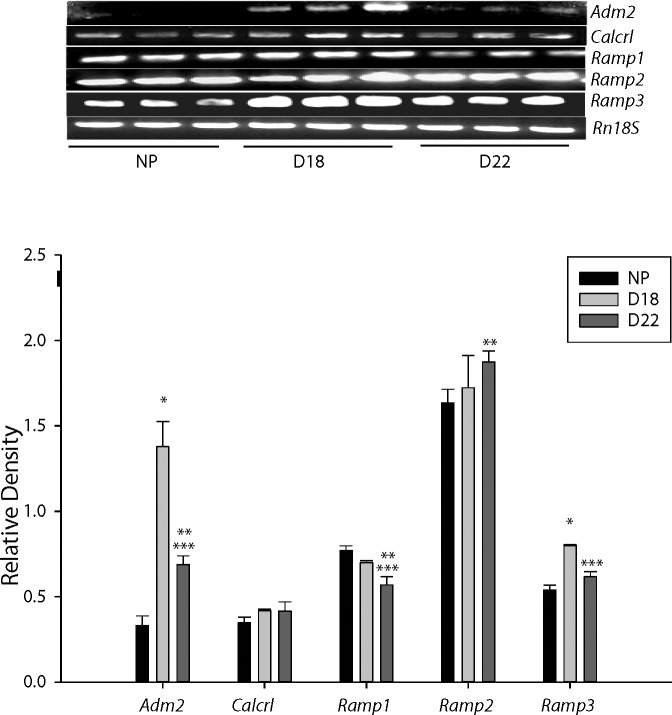
Figure 5 shows that expression of Adm2 mRNA in the rat ovary was highest on Day 18 of pregnancy compared to the nonpregnant rat and on Day 22 of pregnancy (P < 0.005). With advancing gestation, Adm2 levels in the ovary declined on Day 22 but still remained significantly higher compared to the nonpregnant rat (P < 0.05). Because ADM2 can function through the 7-TM GPCR CALCRL in the presence of any of the RAMPs, we further analyzed the expression of Calcrl, Ramp1, Ramp2, and Ramp3 mRNA in these ovaries. Calcrl expression remains similar throughout gestation whereas, Ramp1 mRNA expression significantly decreased on Day 22 compared to nonpregnant and Day 18 rats. Ramp2 mRNA expression is significantly higher on Day 22 compared to nonpregnant rat (P < 0.05) whereas the expression of Ramp3 and Adm2 were similar showing an increase on Day 18 followed by a decline on Day 22 in pregnant rat ovary.
FIG. 5.
Adm2, Calcrl, and Ramp mRNA in rat ovary. Top panel shows the 1.4% agarose gel photograph of RT-PCR product for Adm2, Calcrl, Ramp, Ram2, and Ramp3 mRNA amplified using rat gene-specific primers in ovaries collected from nonpregnant rats at diestrous (NP) and pregnant rats on Day 18 (D18) and Day 22 (D22) of gestation. Messenger RNA for Rn18s in the respective samples was also analyzed. Bottom panel shows the mean ± SEM of the ratios of specific mRNA levels to Rn18s. Asterisks indicate significant differences (*D18 vs. NP, **D22 vs. NP, and ***D22 vs D18; P < 0.005; n = 6).
Immunolocalization of ADM2 in Nonpregnant and Pregnant Rat Ovary
Immunolocalization of ADM2 immunoreactivity was assessed in nonpregnant and Day 18 pregnant rat ovaries. Figure 6 shows that ADM2-like immunoreactivity is lower in nonpregnant rat ovary compared to the pregnant ovary and is localized in granulosa cells, interstitial vasculature, oocytes, and corpus luteum (n = 3).
FIG. 6.
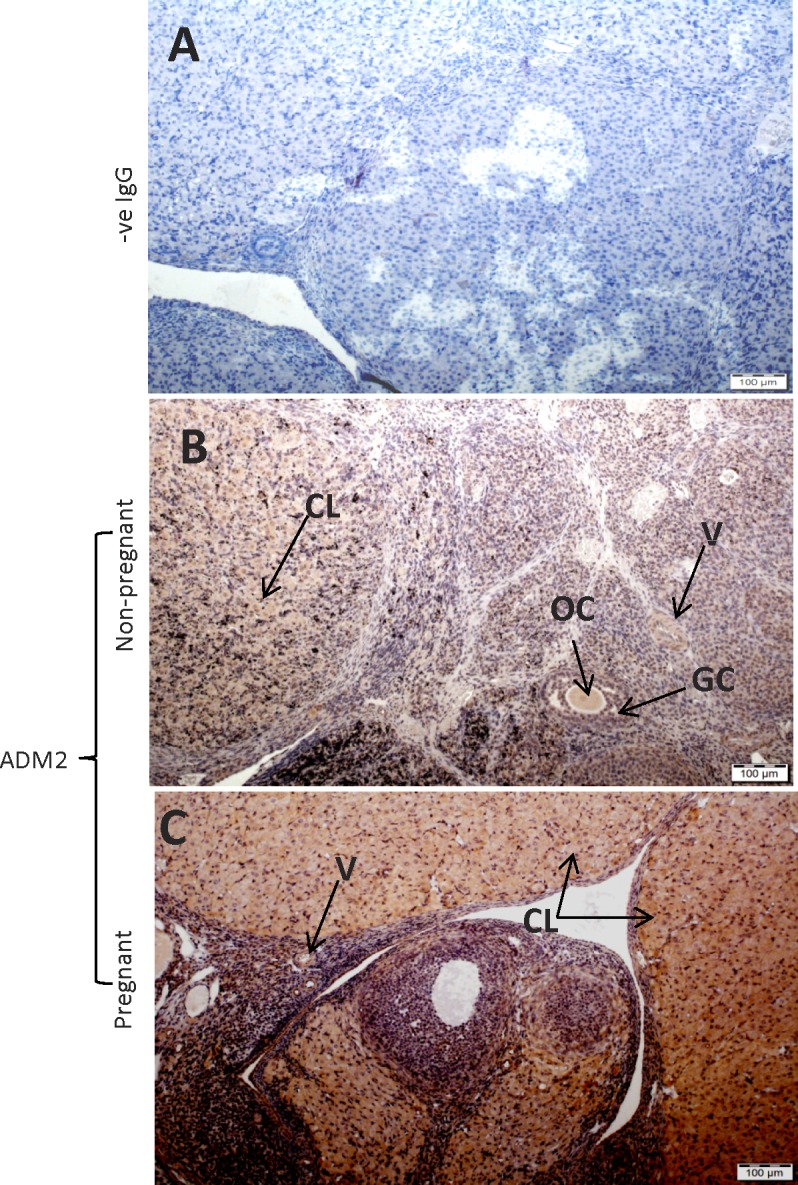
Immunohistochemical localization of ADM2 in nonpregnant and Day 18 pregnant rat ovary: The negative staining in control (A) and ADM2 staining in the ovaries of nonpregnant rat (B) and pregnant rat (C). GC, granulosa cells; OC, oocyte; V, blood vessels; CL, corpus luteum. Bars = 100 μm; n = 3.
Effect of ADM217–47 Infusion on Expression of Aromatase-Like Immunoreactivity in Pregnant Rat Ovary
Figure 7 shows that infusion of ADM2 antagonist (ADM217–47) caused a decline in the aromatase protein expression in Day 9 corpus luteum of treated ovaries (n = 3), suggesting a potential involvement of ADM2 in regulating ovarian estradiol production in pregnant rats.
FIG. 7.
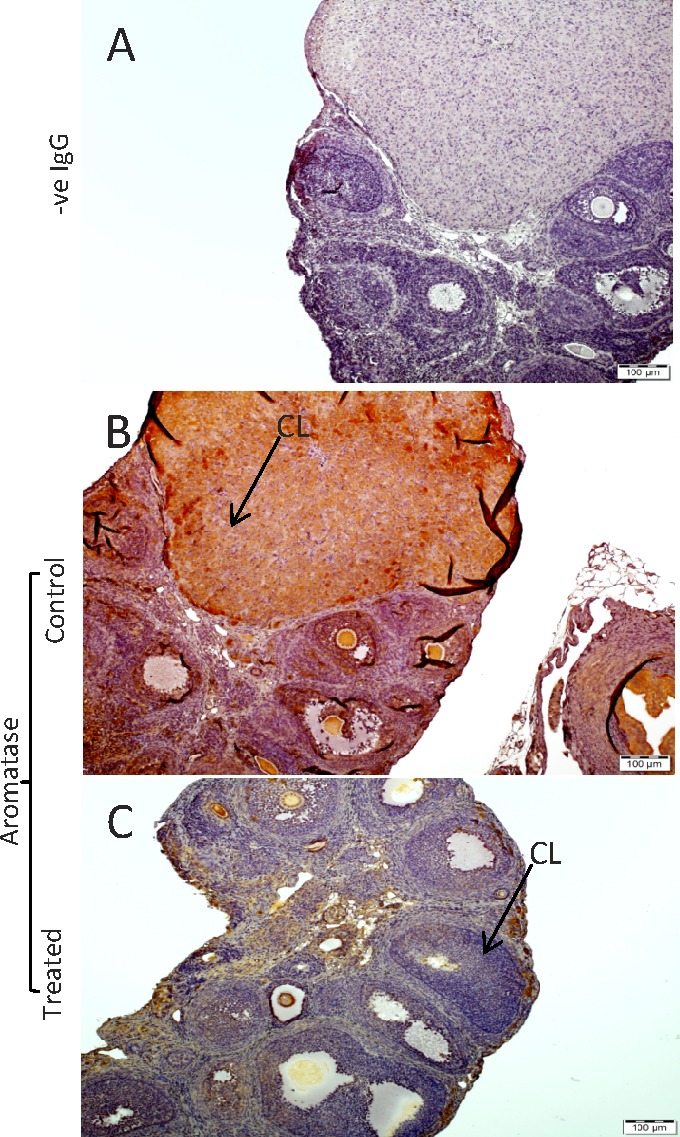
Immunohistochemical localization of immunoreactive ADM2 and p450 aromatase in Day 9 rat ovary. Pregnant rats were infused with ADM217–47 or vehicle (control) from Day 3 and euthanized on Day 9 followed by collection of ovaries for immunohistochemical studies. The negative staining in control (A) p450 aromatase immunoreactivity in corpora lutea (CL) of saline-treated rat (experimental control; B) and ADM217–47-treated rat (C). Bars = 100 μm; n = 3.
DISCUSSION
The current study shows that the expression of ADM2 in the rat ovary varied according to the stages in the estrous cycle and suggests a potential steroidogenic role for ADM2 in the rat ovary to facilitate implantation and early placental development for a successful pregnancy. Ovaries play a major role in pregnancy by synthesizing and secreting estradiol and progesterone, which are important for a successful ovulation, implantation, and fetoplacental development [16–20]. Several growth factors and neuropeptides have been the subject of increasingly intense investigation for their roles in regulating the dynamic process of ovarian growth, oocyte maturation, and ovulation. Recently, the expression of ADM was demonstrated in the corpus luteum and granulosa cells, suggesting a potential involvement of ADM in follicular growth and development and differentiation of corpus luteum. [12, 21, 22]. One study has shown that Adm2, Calcrl, and Ramp mRNA are expressed in the rat ovary and that the ADM2-signaling pathway is present within the individual follicles [11]. Expression of Adm2 has been found to be elevated in oocytes from eCG-treated rats in parallel with the formation and maintenance of the cumulous cell oocyte complex as well as increases in the synthesis of estrogens in granulosa/cumulous cells [11]. These observations support our previous finding that the infusion of ADM2 antagonist resulted in decreased serum levels of estradiol in early gestation, suggesting an ADM2-mediated regulation of sex steroid hormones in rat pregnancy [6, 7]. This is also in line with the decline in the aromatase immunoreactivity in the ovaries of Day 9 pregnant rat infused with the ADM2 antagonist (Fig. 7). However, it is not known if ADM2 has a role in regulating ovarian functions to facilitate ovulation and early placentation. Higher expression of ADM2 at proestrus (Fig. 1) and the ADM2-induced increases in the synthesis and secretion of estradiol (Fig. 2A) observed in this study suggest a role for ADM2 in follicular maturation. In addition, although the amount of progesterone secreted into the medium of ovaries of eCG-treated rats was not affected, ADM2 treatment increased the content of progesterone in the ovary explants (Fig. 2C). In an earlier study, ADM had been shown to induce estradiol and progesterone synthesis in cultured rat ovarian follicles [23]. However, the involvement of steroidogenic enzymes in ADM- or ADM2-induced hormone production in the rat ovary is not known.
The orphan nuclear receptor SF1 is known to activate cytochrome p450 genes [24], and sf1 gene knockout studies have reported that SF1 regulates the expression of steroidogenic genes in steroid-producing cells and that ablation of SF1 leads to a loss in cell morphology and cell identity in the ovary [25]. In addition, Sf1 has also been shown to mediate the release of estradiol from granulosa cells [16]. The current study demonstrates a potential involvement of sf1 and steroidogenic enzymes Hsd17b1, Hsd3b1, and Hsd3b2 in ADM2-induced steroidogenesis in rat ovary (Fig. 3). The estradiol level peaks before ovulation and induces LH release from the pituitary to facilitate ovulation [16, 19, 20, 26, 27]. ADM2 induced increases in steroidogenic enzymes and factors that regulate these enzymes, and this suggests a potential endocrine/paracrine role for ADM2 in the rat ovary. Thus, the higher expression of ADM2 at proestrus and its involvement in ovarian steroidogenesis lead us to speculate that ADM2 may play a role in regulating ovulatory responses in the rat. The possible role of ADM2 in regulating ovulatory response is supported by our data presented in Figure 4, where the infusion of an ADM2 antagonist at proestrus resulted in a reduced rate of ovulation. Interestingly, there was a recent report that ADM treatment does not affect follicular growth or ovulation rate [23]. Haploinsufficiency of ADM in female rats also does not affect follicular growth or ovulation rate in the rat [28]. Because both ADM and ADM2 increase estradiol secretion, the effect of ADM2 on ovulation may be mediated by a mechanism not involving estradiol.
7-TM GPCR CALCRL, a common GPCR shared by CALCB family peptides, is reported to mediate ADM2 action in the presence of RAMP1, RAMP2, or RAMP3. ADM2 and all the receptor components are expressed in the rat ovary [11, 12]. However, it is not known whether the levels of ADM2 and its receptors in ovary are increased in the ovary during gestation. The expression levels of Adm2 and Ramp3 mRNA were significantly higher on Day 18 compared to nonpregnant and Day 22 pregnant rat ovary (Fig. 5). The simultaneous increases in Adm2 and Ramp3 mRNA levels suggest that RAMP3 (and AM2 receptors) may be important for ADM2 function in the ovary. A similar relationship had been shown earlier for Adm and Ramp3 mRNA levels in the rat ovary [2]. However, although the increase in RAMP3 may increase the ADM2 effect on steroidogenesis, the ADM2 effect on ovulation may not be mediated via ADM receptors because the effects of ADM2 and ADM on the rate of ovulation are different (as discussed earlier). There are actions of ADM2 not shared by ADM, for example, the inhibitory effect of ADM2 on growth hormone secretion [29], and there may be specific receptors for ADM2 other than those formed by CALCR and RAMPs that are also blocked by ADM217–47. Future studies are required to identify the specific receptor that mediates the effect of ADM2 in the ovary.
In summary, this study demonstrates that ADM2 immunoreactivity is localized in granulosa cells, oocytes, blood vessels, and corpus luteum in the rat ovary. Ovarian ADM2 mRNA expression changes in the estrous cycle and during gestation. ADM2 treatment stimulates an increase in the synthesis and secretion of ovarian estradiol with concomitant increases in the expression of Sf1, known to activate cytochrome p450 genes, Hsd17b1, Hsd3b1, Hsd3b2, and Esr1 in the ovary. Taken together, this study suggests that ovarian ADM2 may be involved in various aspects of ovarian function.
Footnotes
Supported from the National Institute of Health (NIH) through grants R01 HL58144. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 2004;556:53–58. doi: 10.1016/s0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- Chang CL, Roh J, Hsu SY. Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides. 2004;25:1633–1642. doi: 10.1016/j.peptides.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Lin CC, Roh J, Park JI, Klein C, Cushman N, Haberberger RV, Hsu SY. Intermedin functions as a pituitary paracrine factor regulating prolactin release. Mol Endocrinol. 2005;19:2824–2838. doi: 10.1210/me.2004-0191. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Ross GR, Yallampalli U, Yallampalli C. Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology. 2007;148:1727–1735. doi: 10.1210/en.2006-1105. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2006;75:940–947. doi: 10.1095/biolreprod.106.053322. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Elkins R, Balakrishnan M, Yallampalli C. Potential role of intermedin/adrenomedullin 2 in early embryonic development in rats. Regul Pept. 2011;170:65–71. doi: 10.1016/j.regpep.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havemann D, Balakrishnan M, Borahay M. Theiler, Jennings K, Endsley J, Phelps J, Hankins GD, Yallampalli C, Chauhan M. Intermedin/adrenomedullin 2 is associated with implantation and placentation via trophoblast invasion in human pregnancy. J Clin Endocrinol Metab. 2013;98:695–703. doi: 10.1210/jc.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod. 2009;81:777–783. doi: 10.1095/biolreprod.108.074419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Balakrishnan M, Yallampalli U, Endsley J, Hankins GD, Theiler R, Yallampalli C. Adrenomedullin 2/intermedin regulates HLA-G in human trophoblasts. Biol Reprod. 2011;85:1232–1239. doi: 10.1095/biolreprod.110.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Wang HS, Soong YK, Huang SY, Pai SY, Hsu SY. Regulation of oocyte and cumulus cell interactions by intermedin/adrenomedullin 2. J Biol Chem. 2011;286:43193–43203. doi: 10.1074/jbc.M111.297358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Tang F. O WS. Coexpression of adrenomedullin and its receptor component proteins in the reproductive system of the rat during gestation. Reprod Biol Endocrinol. 2010;8:130. doi: 10.1186/1477-7827-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar Y, Armstrong DT. Ability of progesterone to reverse anti-androgen (hydroxyflutamide)-induced interference with the preovulatory LH surge and ovulation in PMSG-primed immature rats. J Reprod Fertil. 1989;85:309–316. doi: 10.1530/jrf.0.0850309. [DOI] [PubMed] [Google Scholar]
- Dong Y-L, Gangula PRR, Fang L, Yallampalli C. Nitric oxide reverses prostaglandin-induced inhibition in ovarian progesterone secretion in rats. Hum Reprod. 1999;14:27–32. doi: 10.1093/humrep/14.1.27. [DOI] [PubMed] [Google Scholar]
- Witlin A, Li ZY, Wimalawansa SJ, Grady JJ, Grafe MR, Yallampalli C. Placental and fetal growth and development in late rat gestation is dependent on adrenomedullin. Biol Reprod. 2002;67:1025–1031. doi: 10.1095/biolreprod.101.002196. [DOI] [PubMed] [Google Scholar]
- Hanley NA, Ikeda Y, Luo X, Parker KL. Steroidogenic factor 1 (SF-1) is essential for ovarian development and function. Mol Cell Endocrinol. 2000;163:27–32. doi: 10.1016/s0303-7207(99)00237-3. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Duncan WC. The human corpus luteum: which cells have progesterone receptors? Reproduction. 2004;128:423–431. doi: 10.1530/rep.1.00051. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS. Ovulation: a multi-gene, multi-step process. Steroids. 2000;65:559–570. doi: 10.1016/s0039-128x(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Hickey GJ, Oonk RB, Hall PF, Richards JS. Aromatase cytochrome P450 and cholesterol side-chain cleavage cytochrome P450 in corpora lutea of pregnant rats: diverse regulation by peptide and steroid hormones. Endocrinology. 1989;125:1673–1682. doi: 10.1210/endo-125-3-1673. [DOI] [PubMed] [Google Scholar]
- Richards JS, Hedin L. Molecular aspects of hormone action in ovarian follicular development, ovulation, and luteinization. Annu Rev Physiol. 1988;50:441–463. doi: 10.1146/annurev.ph.50.030188.002301. [DOI] [PubMed] [Google Scholar]
- Abe K, Minegishi T, Ibuki Y, Kojima M, Kangawa K. Expression of adrenomedullin in the human corpus luteum. Fertil Steril. 2000;74:141–145. doi: 10.1016/s0015-0282(00)00585-9. [DOI] [PubMed] [Google Scholar]
- Abe K, Minegishi T, Tano M, Hirakawa T, Tsuchiya M, Kangawa K, Kojima M, Ibuki Y. Expression and effect of adrenomedullin on rat granulosa cell. Endocrinology. 1998;139:5263–5266. doi: 10.1210/endo.139.12.6524. [DOI] [PubMed] [Google Scholar]
- Li L. O WS, Tang F. Adrenomedullin in rat follicles and corpora lutea: expression, functions and interaction with endothelin-1. Reprod Biol Endocrinol. 2011;9:111. doi: 10.1186/1477-7827-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–4570. doi: 10.1242/dev.087247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS. Content of nuclear estradiol receptor complex in rat corpora lutea during pregnancy: relationship to estrogen concentrations and cytosol receptor availability. Endocrinology. 1975;96:227–230. doi: 10.1210/endo-96-1-227. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Hawkins RA, Stocker JF. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969;85:103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]
- Li M, Wu Y, Caron KM. Haploinsufficiency for adrenomedullin reduces pinopodes and diminishes uterine receptivity in mice. Biol Reprod. 2008;79:1169–1175. doi: 10.1095/biolreprod.108.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK. Intermedin/Adrenomedullin-2 inhibits growth hormone release from cultured, primary anterior pituitary cells. Endocrinology. 2006;147:859–864. doi: 10.1210/en.2005-0949. [DOI] [PubMed] [Google Scholar]