Two iron-bound flavidoxin-domain proteins mediate an oxygen-dependent alternative electron flow in the cyanobacterium Synechocystis under CO2-limited conditions.
Abstract
This study aims to elucidate the molecular mechanism of an alternative electron flow (AEF) functioning under suppressed (CO2-limited) photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. Photosynthetic linear electron flow, evaluated as the quantum yield of photosystem II [Y(II)], reaches a maximum shortly after the onset of actinic illumination. Thereafter, Y(II) transiently decreases concomitantly with a decrease in the photosynthetic oxygen evolution rate and then recovers to a rate that is close to the initial maximum. These results show that CO2 limitation suppresses photosynthesis and induces AEF. In contrast to the wild type, Synechocystis sp. PCC 6803 mutants deficient in the genes encoding FLAVODIIRON2 (FLV2) and FLV4 proteins show no recovery of Y(II) after prolonged illumination. However, Synechocystis sp. PCC 6803 mutants deficient in genes encoding proteins functioning in photorespiration show AEF activity similar to the wild type. In contrast to Synechocystis sp. PCC 6803, the cyanobacterium Synechococcus elongatus PCC 7942 has no FLV proteins with high homology to FLV2 and FLV4 in Synechocystis sp. PCC 6803. This lack of FLV2/4 may explain why AEF is not induced under CO2-limited photosynthesis in S. elongatus PCC 7942. As the glutathione S-transferase fusion protein overexpressed in Escherichia coli exhibits NADH-dependent oxygen reduction to water, we suggest that FLV2 and FLV4 mediate oxygen-dependent AEF in Synechocystis sp. PCC 6803 when electron acceptors such as CO2 are not available.
In photosynthesis, photon energy absorbed by PSI and PSII in thylakoid membranes oxidizes the reaction center chlorophylls (Chls), P700 in PSI and P680 in PSII, and drives the photosynthetic electron transport (PET) system. In PSII, water is oxidized to oxygen as the oxidized P680 accepts electrons from water. These electrons then reduce the cytochrome b6/f complex through plastoquinone (PQ) in the thylakoid membranes. Photooxidized P700 in PSI accepts electrons from the reduced cytochrome b6/f complex through plastocyanin or cytochrome c6. Electrons released in the photooxidation of P700 are used to produce NADPH through ferredoxin and ferredoxin NADP+ reductase. Thus, electrons flow from water to NADPH in the so-called photosynthetic linear electron flow (LEF). Importantly, LEF induces a proton gradient across the thylakoid membranes, which provides the driving force for ATP production by ATP synthases in the thylakoid membranes. NADPH and ATP serve as chemical energy donors in the photosynthetic carbon reduction cycle (Calvin cycle).
It recently has been proposed that, in cyanobacteria, the photorespiratory carbon oxidation cycle (photorespiration) functions simultaneously with the Calvin cycle to recover carbon for the regeneration of ribulose-1,5-bisphosphate, one of the substrates of Rubisco (Hagemann et al., 2013). Rubisco catalyzes the primary reactions of carbon reduction as well as oxidation cycles. However, the presence of a specific carbon concentration mechanism (CCM) in cyanobacteria had been thought to prevent the operation of photorespiration. CCM maintains a high concentration of CO2 around Rubisco so that the oxygenase activity of Rubisco is suppressed (Badger and Price, 1992). However, recent studies on mutants deficient in photorespiration enzymes have shown that photorespiration functions, particularly under CO2-limited conditions, in cyanobacteria as it does in higher plants (Eisenhut et al., 2006, 2008).
Decreased consumption of NADPH under CO2-limited or high-light conditions causes electrons to accumulate in the PET system. As a result, the photooxidation and photoreduction cycles of the reaction center Chls in PSI and PSII become uncoupled from the production of NADPH, inducing alternative electron flow (AEF) pathways (Mullineaux, 2014). In cyanobacteria, several AEFs that differ from those in higher plants are proposed to function as electron sinks (Mullineaux, 2014). Electrons accumulated in the PET system flow to oxygen through FLAVODIIRON1 (FLV1) and FLV3 proteins in PSI and the terminal oxidase, cytochrome c oxidase complex, and cytochrome bd-quinol oxidase (Pils and Schmetterer, 2001; Berry et al., 2002; Helman et al., 2003; Nomura et al., 2006; Lea-Smith et al., 2013). Cyanobacterial FLV comprises a diiron center, a flavodoxin domain with an FMN-binding site, and a flavin reductase domain (Vicente et al., 2002). In Synechocystis sp. PCC 6803, Helman et al. (2003) identified four genes encoding FLV1 to FLV4 and showed that FLV1 and FLV3 were essential for the photoreduction of oxygen by PSI. FLV1 and FLV3 were proposed to function as a heterodimer (Allahverdiyeva et al., 2013). FLV2/4 have been proposed to function in energy dissipation associated with PSII (Zhang et al., 2012). In addition, hydrogenases convert H+ to H2 with NADPH as an electron donor (Appel et al., 2000). Furthermore, Flores et al. (2005) suggested that the nitrate assimilation pathway functions in AEF when the cells live in medium containing nitrate.
To elucidate the physiological functions of these AEFs, evaluation of the presence and capacity of each AEF pathway is required. Therefore, in vivo analyses of electron fluxes are essential. We had found that an electron flow uncoupled from photosynthetic oxygen evolution functioned under suppressed (CO2-limited) photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803 but not in Synechococcus elongatus PCC 7942 (Hayashi et al., 2014), indicating that an AEF operated in Synechocystis sp. PCC 6803. This AEF was induced in high-[CO2]-grown Synechocystis sp. PCC 6803 during the transition from CO2-saturated photosynthesis to CO2-limited photosynthesis (Hayashi et al., 2014). In contrast, in Synechocystis sp. PCC 6803 grown at ambient CO2 concentration, AEF was detected immediately following the transition to CO2-limited photosynthesis (Hayashi et al., 2014), suggesting that AEF was already induced under ambient atmospheric conditions.
The expression of the AEF activity observed under CO2-limited photosynthesis required the presence of oxygen in Synechocystis sp. PCC 6803 (Hayashi et al., 2014). In Synechocystis sp. PCC 6803, FLV1/3 were proposed to catalyze the photoreduction of oxygen (Helman et al., 2003). However, Hayashi et al. (2014) found no evidence that FLV1/3 operated under CO2-limited photosynthesis: a mutant Synechocystis sp. PCC 6803 deficient in FLV1/3 maintained almost constant electron flux under CO2-limited photosynthesis after the transition from CO2-saturated conditions. Thus, the postulated photoreduction of oxygen by FLV1/3 was not responsible for the electron flux observed under CO2-limited photosynthesis in Synechocystis sp. PCC 6803.
In this study, we aimed to elucidate the molecular mechanism of the oxygen-dependent AEF functioning under CO2-limited photosynthesis in Synechocystis sp. PCC 6803. The possibility that FLV2 and FLV4 catalyze the photoreduction of oxygen under CO2-limited photosynthesis could not be excluded, given that AEF in high-[CO2]-grown Synechocystis sp. PCC 6803 was induced following the transition to CO2-limited photosynthesis (Hayashi et al., 2014). Both FLV2 and FLV4 are predicted to possess oxidoreductase motifs, similar to FLV1 and FLV3 (Helman et al., 2003; Zhang et al., 2012). Furthermore, the expression of two FLV genes (flv2 and flv4) was enhanced under low-[CO2] conditions (Zhang et al., 2009). Zhang et al. (2012) proposed that FLV2 and FLV4 did not donate electrons to oxygen on the basis of the finding that the Synechocystis sp. PCC 6803 mutants deficient in FLV1/3 showed no light-dependent oxygen uptake (Helman et al., 2003). However, Helman et al. (2003) cultivated Synechocystis sp. PCC 6803 strains deficient in FLV1 and FLV3 proteins under high-[CO2] conditions, and we cannot exclude the possibility that the FLV2 and FLV4 proteins were not produced in the studied cells. Taken together, it seems plausible that FLV2 and FLV4 mediate oxygen-dependent AEF following the transition to CO2-limited photosynthesis. To evaluate this possibility, we constructed Synechocystis sp. PCC 6803 mutants deficient in flv2 and flv4 and measured their oxygen evolution and Chl fluorescence simultaneously. The mutants showed suppressed LEF after transition to CO2-limited photosynthesis, similar to S. elongatus PCC 7942. We also tested the possibility that photorespiration functions as an electron sink under CO2-limited photosynthesis in Synechocystis sp. PCC 6803. A recent study revealed photorespiratory oxygen uptake in a flv1/3 mutant under CO2-depleted conditions (Allahverdiyeva et al., 2011). In this study, we found that the quantum yield of photosystem II [Y(II)] of mutants deficient in genes encoding proteins that function in photorespiration was similar to that of wild-type Synechocystis sp. PCC 6803. Thus, FLV2 and FLV4 appear to function in the oxygen-dependent AEF under CO2-limited photosynthesis in Synechocystis sp. PCC 6803. This inference is further supported by the lack of FLV2 and FLV4 homologs in the genome of S. elongatus PCC 7942 (Bersanini et al., 2014). In addition, we found oxygen-reducing activities of recombinant glutathione S-transferase (GST)-FLV4 fusion protein, similar to those of recombinant FLV3 protein (Vicente et al., 2002). In light of these results, we discuss the molecular mechanism of the oxygen-dependent AEF under CO2-limited photosynthesis and the physiological function of FLV proteins in Synechocystis sp. PCC 6803.
RESULTS
FLV2 and FLV4 Are Essential for AEF under CO2-Limited Photosynthesis in Synechocystis sp. PCC 6803
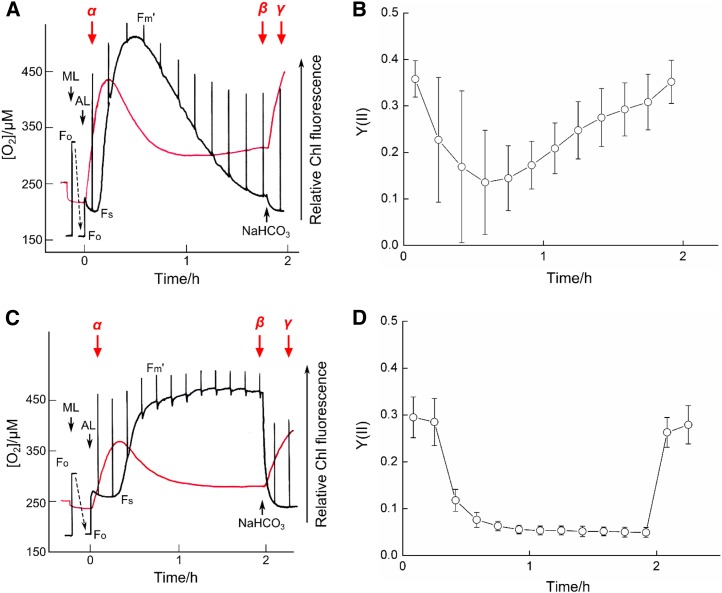
We monitored Chl fluorescence and the concentration of oxygen in medium containing Synechocystis sp. PCC 6803 and S. elongatus PCC 7942 cells (Fig. 1). During the measurements, the top of the reaction chamber was open to allow equilibration of the medium with air. Cyanobacteria were added after oxygen concentration in the medium had equilibrated with the concentration in air (about 250 µm at 25°C) in the dark. With both of the cyanobacterial strains (compare Fig. 1, A and C), the medium oxygen concentration quickly declined to a lower equilibrium due to respiratory oxygen consumption. Upon illumination with actinic light (200 µmol photons m−2 s−1), the relative Chl fluorescence increased from minimum to steady state and then decreased transiently (Fig. 1, A and C), while the electron flux in PSII [Y(II) = (Fm′ − Fs)/Fm′, where Fm′ = maximum variable fluorescence and Fs = steady-state fluorescence under actinic light] was high (Fig. 1, B and D). Actinic light started photosynthesis as indicated by the increase in oxygen concentration in the medium (Fig. 1, A and C). In both strains, medium oxygen concentration reached a peak when Fs had started to increase; Fs continued to rise while Y(II) decreased and oxygen concentration shifted toward a lower equilibrium (Synechocystis sp. PCC 6803, Fig. 1, A and B; S. elongatus PCC 7942, Fig. 1, C and D). Thereafter, the parameters developed differently in the two strains. Fs decreased continuously, paralleled by an increasing Y(II) in Synechocystis sp. PCC 6803 (Fig. 1, A and B). In contrast, Fs and Y(II) remained at high and low levels, respectively, in S. elongatus PCC 7942 (Fig. 1, C and D). Addition of CO2 (as NaHCO3) caused an increase in medium oxygen concentration and a decrease in Fs in both strains (Fig. 1, A and C) as well as a sharp increase of Y(II) in S. elongatus PCC 7942 (Fig. 1D). In separate experiments, we determined oxygen evolution rates at the three times marked in Figure 1 as α (CO2 in the medium not yet depleted by photosynthesis), β (medium CO2 at a low steady state controlled by photosynthetic CO2 consumption and diffusion from the atmosphere), and γ (high CO2 availability due to added NaHCO3; compare Supplemental Fig. S1). In both strains, oxygen evolution rates were similarly high at times α and γ, indicating CO2-saturated photosynthesis, and low at time β, indicating CO2-limited photosynthesis (Table I). Under these conditions, the gradual drop of Fs and the parallel increase of Y(II) (Fig. 1, A and B) in Synechocystis sp. PCC 6803 strongly suggested the operation of an AEF under CO2-limited photosynthesis.
Figure 1.
Development of photosynthetic parameters in media containing high-[CO2]-grown Synechocystis sp. PCC 6803 (A and B) and S. elongatus PCC 7942 (C and D). A and C, Time courses of medium oxygen concentration (red traces) and relative Chl fluorescence (black traces). Initially, media containing cyanobacteria (10 μg Chl mL−1) were kept dark; illumination with measuring light (ML) and actinic light (AL; 200 μmol photons m−2 s−1) started as indicated. The downward shift in the Chl fluorescence signal (Fo) after the start of ML illumination is due to increasing the instrument amplification from 200 to 100 mV. Chl fluorescence parameters are as defined in the text. NaHCO3 (final concentration, 10 mm) was added as indicated. α, β, and γ denote times at which oxygen evolution rates were determined in separate experiments. From about the time of maximum oxygen concentration to the addition of NaHCO3, photosynthesis can be considered CO2 limited. The experiments were performed four times, and representative data are shown. B and D, Time courses of Y(II), calculated for the corresponding measurements of Fm′ as (Fm′ − Fs)/Fm′. Values shown are means ± sd (n = 4).
Table I. Oxygen-exchange rates (µmol oxygen mg−1 Chl h−1) in the dark, under CO2-saturated and CO2-limited photosynthesis, and after the addition of NaHCO3 in Synechocystis sp. PCC 6803, S. elongatus PCC 7942, and the ∆flv2 and ∆flv4 mutants of Synechocystis sp. PCC 6803.
Oxygen-exchange rates were determined simultaneously with Chl fluorescence measurements under actinic light (200 μmol photons m−2 s−1) at the times marked as α, β, and γ in Figure 1 and Supplemental Figure S1. The reaction mixture contained cyanobacterial cells (10 μg Chl mL−1) and 50 mm HEPES-KOH (pH 7.5). Oxygen-exchange rates in the dark were determined before illuminating with measuring light. Negative values indicate oxygen uptake. Values are means ± sd of four independent experiments.
| Condition | Synechocystis sp. PCC 6803 | S. elongatus PCC 7942 | ∆flv2 | ∆flv4 |
|---|---|---|---|---|
| Dark | −12.6 ± 2.1 | −16 ± 4 | −11 ± 5 | −11 ± 3 |
| CO2-saturated photosynthesis (α) | 129 ± 8 | 90 ± 15 | 129 ± 9 | 129 ± 24 |
| CO2-limited photosynthesis (β) | 8 ± 5 | 3.6 ± 0.6 | 6 ± 4 | 7 ± 5 |
| Addition of NaHCO3 (γ) | 114 ± 11 | 68 ± 10 | 108 ± 15 | 111 ± 10 |
Fm′ increased during the transition (Fig. 1). The increase in Fm′ could be caused by a state transition from state II to state I (McConnell et al., 2002). However, low-temperature fluorescence spectra of Synechocystis sp. PCC 6803 and S. elongatus PCC 7942 showed no changes during the transition to CO2-limited photosynthesis (Miller et al., 1996; Hayashi et al., 2014). Thus, there is no evidence to support a state transition. The molecular mechanism for the increase in Fm′ remains unknown. Therefore, we focused on the responses of AEF to the electron sink limitation that occurred during the shift from CO2-saturated to CO2-limited photosynthesis.
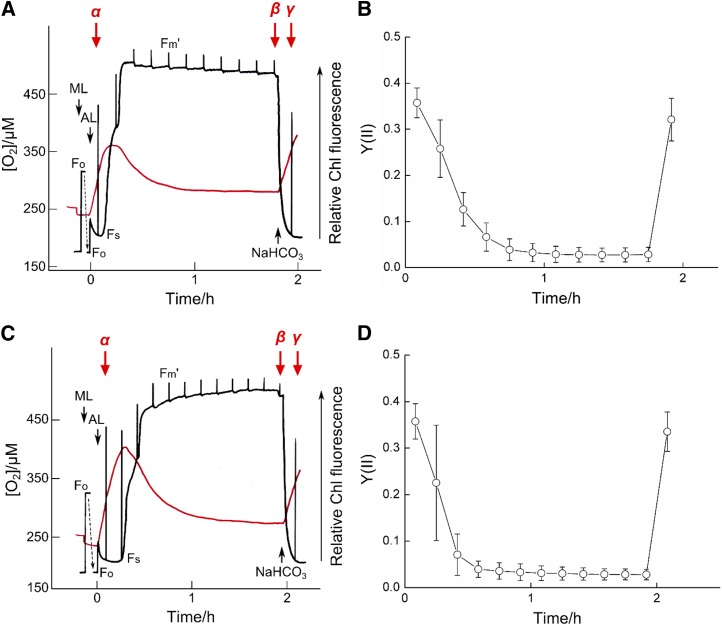
Synechocystis sp. PCC 6803, in which the transition from CO2-saturated to CO2-limited photosynthesis appeared to induce AEF, has four flv genes in its genome: flv1 (sll1521), flv2 (sll0219), flv3 (sll0550), and flv4 (sll0217). The activity of isozymes FLV2 and FLV4 seems to increase in response to low-[CO2] conditions (Zhang et al., 2009; Eisenhut et al., 2012). A double mutant deficient in flv1 and flv3 showed the same response of Y(II) to the transition from CO2-saturated to CO2-limited photosynthesis as wild-type Synechocystis sp. PCC 6803 (Hayashi et al., 2014). Furthermore, the genes encoding FLV2 and FLV4 are absent from the genome of S. elongatus PCC 7942 (Bersanini et al., 2014), while flv1 and flv3 are present (Synpcc7942_1810, ortholog of flv1; Synpcc7942_1809, ortholog of flv3). We hypothesized that FLV2 and FLV4 are involved in the AEF under CO2-limited photosynthesis and constructed mutants deficient in flv2 and flv4 (∆flv2 and ∆flv4, respectively; Supplemental Figs. S2 and S3). In contrast to the double mutant deficient in flv1/3 (Hayashi et al., 2014), neither ∆flv2 nor ∆flv4 showed any increase in Y(II) during the transition from CO2-saturated to CO2-limited photosynthesis (Fig. 2, B and D), while Fs increased and then maintained its level (Fig. 2, A and C). On addition of NaHCO3, Fs decreased and Y(II) recovered to values found under CO2-saturated photosynthesis. The oxygen evolution rates in ∆flv2 and ∆flv4 decreased from time α to time β and recovered at time γ (Table I). Thus, suppression of Y(II) in ∆flv2 and ∆flv4 was due to the limited capacity of the CO2-dependent electron sink, which could not be substituted by AEF. We concluded that in Synechocystis sp. PCC 6803, FLV2 and FLV4 are necessary for AEF under CO2-limited photosynthesis. FLV2 and FLV4 form heterodimers (Zhang et al., 2012), and deletion of flv4 suppresses the production of FLV2 (Eisenhut et al., 2012). Thus, deficiency in either of the two genes resulted in the complete loss of physiological function (Fig. 2).
Figure 2.
Development of photosynthetic parameters in media containing two high-[CO2]-grown mutants of Synechocystis sp. PCC 6803, ∆flv2 (A and B) and ∆flv4 (C and D). All further details are as given in Figure 1.
The GST-FLV4 Fusion Protein Reduces Oxygen to Water
In Synechocystis sp. PCC 6803, FLV2 and FLV4 are required for AEF under CO2-limited photosynthesis (Fig. 2), which also depends on the presence of oxygen in the flv1/3 double mutant (Hayashi et al., 2014). These facts suggested that FLV2 and FLV4 donate electrons to oxygen. To determine whether oxygen can accept electrons from FLV2 and FLV4 in vitro, we expressed recombinant GST-FLV2 and GST-FLV4 fusion proteins in Escherichia coli and tried to purify them by affinity chromatography (Supplemental Fig. S4). Unfortunately, GST-FLV2 could not be separated from a contaminant protein of about 60 to 65 kD (Supplemental Fig. S4). Therefore, we could only examine the GST-FLV4 protein in the following experiments.
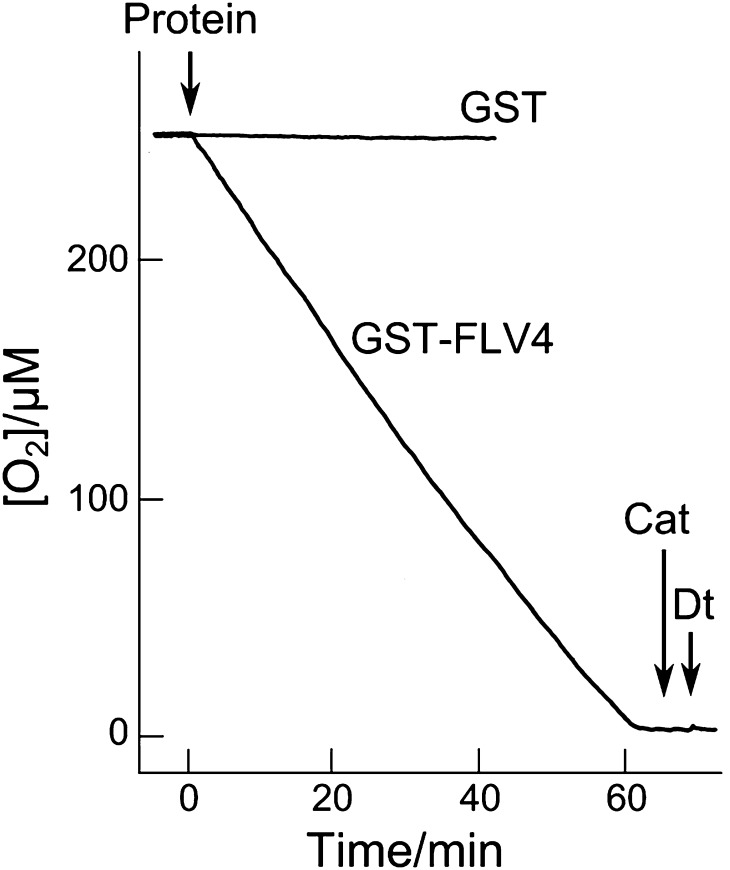
GST-FLV4 catalyzed the oxidation of NADH as monitored by the decrease in A340. The Km value for NADH was approximately 30 μm (Supplemental Fig. S5), similar to that of the recombinant FLV3 protein (Vicente et al., 2002). We could not obtain Km for the NADPH-dependent oxygen reduction, owing to the significantly lower activity. GST-FLV4 also reduced oxygen using NADH as the electron donor (Fig. 3). Addition of GST-FLV4 to a reaction mixture containing 50 mm Tris-HCl (pH 7.4) and 1 mm NADH stimulated the reduction of oxygen at the rate of 20.2 ± 1.5 min−1 (Fig. 3). No oxygen depletion was observed when GST protein was added to the reaction mixture as a control. When catalase was added, no oxygen evolved, indicating that GST-FLV4 reduces oxygen to water without generating reactive oxygen species (Fig. 3). Furthermore, addition of dithionite showed that oxygen was completely consumed (Fig. 3). The Km value for oxygen was below 10 μm. These results demonstrate the oxygen-reducing activity of GST-FLV4, which resembles that of the recombinant FLV3 protein (Vicente et al., 2002).
Figure 3.
Oxygen-reducing activities of recombinant GST and GST-FLV4 fusion protein. The reaction mixture contained 50 mm Tris-HCl (pH 7.4), 1 mm NADH, and the protein of interest (40 μg). Catalase (Cat; 200 units mL−1) and dithionite (Dt; 1 mm) were added as indicated. Purified GST protein served as a control.
Photorespiration Does Not Contribute to Y(II) under CO2-Limited Photosynthesis
In general, the oxygenation of ribulose-1,5-bisphosphate by Rubisco is presumed to be suppressed in cyanobacteria, because CCM maintains [CO2] high around Rubisco in the carboxysome (Badger and Price, 1992), but Synechocystis sp. PCC 6803 may be an exception (Eisenhut et al., 2006, 2008). Cyanobacterial photorespiration may operate through three routes, the canonical plant-like C2 cycle and two cyanobacteria-specific routes, the glycerate and decarboxylation pathways (Hagemann et al., 2013). Allahverdiyeva et al. (2011) demonstrated photorespiratory oxygen uptake in the flv1/3 double mutant by membrane inlet mass spectrometry. This opened the possibility that not only FLV2/4 but also photorespiration could be responsible for the increase in Y(II) under CO2-limited photosynthesis in Synechocystis sp. PCC 6803 (Fig. 1). We constructed the mutants ∆pgp, ∆glcD1/2, ∆gcvT, and ∆glyk, which were deficient in the genes encoding phosphoglycolate phosphatase (slr0458), glycolate dehydrogenase (sll0404 and slr0806), Gly decarboxylase T-protein (sll0171), and glycerate kinase (slr1840), respectively. These genes supposedly function in the canonical C2 cycle (Eisenhut et al., 2008). Inactivation of these genes in the mutants was confirmed by PCR analysis (Supplemental Figs. S2 and S3). All mutants showed the same Y(II) increase following the transition from CO2-saturated to CO2-limited photosynthesis as the wild-type Synechocystis sp. PCC 6803 (Supplemental Fig. S6; Supplemental Table S1), indicating that the C2 cycle was not involved in the Y(II) response.
DISCUSSION
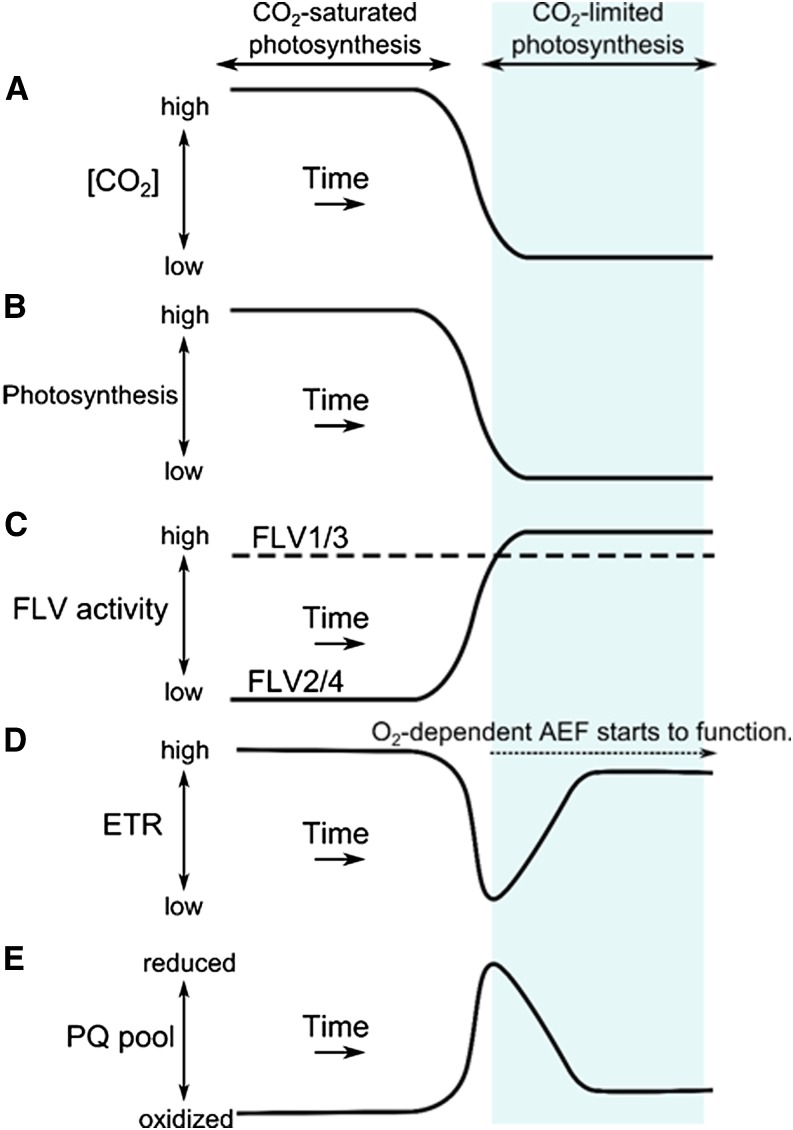
We aimed at elucidating the molecular mechanism of AEF under CO2-limited photosynthesis in Synechocystis sp. PCC 6803, reported in our previous study (Hayashi et al., 2014). On the transition from CO2-saturated to CO2-limited photosynthesis, LEF was transiently suppressed but then recovered to a value corresponding to the photosynthetic electron flux observed under CO2-saturated photosynthesis. This implied the induction of AEF under CO2-limited photosynthesis. We hypothesized that among the four FLV proteins in Synechocystis sp. PCC 6803, FLV2 and FLV4 could be involved because, first, the flv1/3 double mutant showed the same response as the wild type (Hayashi et al., 2014), and second, FLV2 and FLV4 were produced under low-[CO2] conditions (Zhang et al., 2009; Eisenhut et al., 2012). We confirmed that Synechocystis sp. PCC 6803 mutants deficient in FLV2 and FLV4 cannot maintain LEF during the transition to CO2-limited photosynthesis (Fig. 2), implying that FLV2 and FLV4 function in the AEF under CO2-limited photosynthesis. In summary (Fig. 4), we propose that under CO2-saturated photosynthesis, FLV1 and FLV3 operate (Helman et al., 2003; Allahverdiyeva et al., 2013; Hayashi et al., 2014) while FLV2 and FLV4 do not, whereas under CO2-limited photosynthesis, FLV2 and FLV4 operate in addition to FLV1 and FLV3 to mediate AEF, thus contributing to the alleviation of photooxidative stress (Zhang et al., 2009, 2012).
Figure 4.
Model of the responses of photosynthesis, FLV activity, electron transport rate (ETR), and oxidation state of the PQ pool to the shift from CO2-saturated to CO2-limited photosynthesis in Synechocystis sp. PCC 6803. This model presents an interpretation of the processes occurring in the experiment shown in Figure 1A. A, CO2 depletion by photosynthesis. Medium [CO2] decreases due to photosynthetic consumption following the start of illumination. B, Suppression of photosynthesis by photosynthesis-induced CO2 depletion. Photosynthetic CO2 consumption cannot be balanced by the diffusion of atmospheric CO2 into the medium. C, Activities of FLV proteins. FLV1 and FLV3 function under CO2-saturated and CO2-limited conditions, while FLV2 and FLV4 become functional as mediators of AEF only under CO2-limited conditions. D, Total ETR under CO2-saturated photosynthesis is driven mainly by photosynthesis. A similar rate is maintained under CO2 limitation by an oxygen-dependent AEF. E, Changes in the redox state of the PQ pool. Under CO2-limited photosynthesis, the oxidized state is preserved by the FLV2/4-mediated, oxygen-dependent AEF.
The most striking finding is that FLV2 and FLV4 together have the capacity to replace the steady-state photosynthetic electron flux observed under CO2-saturated photosynthesis (Figs. 1 and 2) by an AEF under CO2-limited conditions. In wild-type Synechocystis sp. PCC 6803 performing CO2-limited photosynthesis, Y(II) remained at 80% to 90% of that at CO2-saturated photosynthesis (Fig. 1). In Δflv2 and Δflv4 under CO2-limited conditions, Y(II) was only 5% to 10% of that at CO2-saturated photosynthesis, corresponding to the extent of the concomitant reduction of photosynthetic oxygen evolution (Fig. 2; Table I). These findings suggest that FLV2 and FLV4 are sufficient to maintain AEF activity under low [CO2].
Under CO2-depleted conditions, photorespiratory oxygen uptake in Δflv1/3 occurred at rates (about 50 μmol oxygen mg−1 Chl h−1) that exceeded the oxygen-photoreducing rate of FLV1/3 (approximately 30 μmol oxygen mg−1 Chl h−1; Allahverdiyeva et al., 2011), indicating that photorespiration can function as an electron sink under CO2-depleted as well as FLV1/3-depleted conditions (Helman et al., 2003; Allahverdiyeva et al., 2011). Unfortunately, we were unable to obtain evidence in support of this idea (Supplemental Fig. S6). On the other hand, our data are in agreement with the interpretation that photorespiration operates in wild-type cells. For example, ∆glcD1/2 and ∆gcvT mutants showed retarded growth (data not shown), as reported by Eisenhut et al. (2006, 2008). In addition, the initial Fm′ was lower in ∆glcD1/2 than in other strains (Supplemental Fig. S6), possibly owing to phycobilisome functions including state transition. A physiological role for cyanobacterial photorespiration has been proposed by Eisenhut et al. (2006, 2008), who reported that in Synechocystis sp. PCC 6803, photorespiration is not an electron sink but rather a scavenging system for toxic intermediates, notably glyoxylate (Eisenhut et al., 2008).
Supposedly, the electron acceptor for FLV2 and FLV4 is oxygen. Hayashi et al. (2014) reported that AEF activity under CO2-limited photosynthesis in Synechocystis sp. PCC 6803 cells requires oxygen. We showed that AEF requires FLV2 and FLV4 (Fig. 2) and that the GST-FLV4 fusion protein possesses oxygen-reducing activity (Fig. 3), similar to recombinant FLV3 (Vicente et al., 2002). These results suggest that FLV2 and FLV4 use oxygen as an electron acceptor in vivo and in vitro. In contrast, FLV2 and FLV4 interact with PSII and phycobilisomes (Zhang et al., 2012; Bersanini et al., 2014). Therefore, FLV2 and FLV4 may catalyze the photoreduction of oxygen to water associated with PSII under CO2-limited conditions in Synechocystis sp. PCC 6803. We did not succeed in preparing stable FLV2/4 heterodimers (data not shown). The molecular mechanism of FLV2/4 heterodimer function at PSII awaits its biochemical characterization in the future.
Under low-[CO2] conditions, ∆flv2 and ∆flv4 showed severe photoinhibition of PSII (Zhang et al., 2009, 2012). Moreover, an overexpression mutant of the flv4-2 operon in Synechocystis sp. PCC 6803 exhibited a more oxidized state of the PQ pool and a reduced production of singlet oxygen and showed resistance to photoinhibition of PSII (Bersanini et al., 2014). In wild-type Synechocystis sp. PCC 6803 under CO2-limited photosynthesis, Y(II) remained high (Figs. 1 and 2; Hayashi et al., 2014), a property expected to contribute to the survival of Synechocystis sp. PCC 6803 in nature, where CO2 assimilation would proceed under the CO2-limited photosynthesis condition.
We attribute the low capacity for AEF in S. elongatus PCC 7942 to the loss of FLV2 and FLV4. S. elongatus PCC 7942 and Synechocystis sp. PCC 6803 are β-type cyanobacteria, but in contrast to Synechocystis sp. PCC 6803, S. elongatus PCC 7942 does not harbor the gene for the orange carotenoid protein responsible for the nonphotochemical quenching of Chl fluorescence (Boulay et al., 2008). Synechocystis sp. PCC 6803 mutants deficient in orange carotenoid protein suffer from photoinhibition under low-[CO2] and high-light stress conditions (Wilson et al., 2006). The Synechocystis sp. PCC 6803 mutant deficient in FLV2/4 showed photoinhibition and growth inhibition under these conditions (Zhang et al., 2009, 2012). This suggests that S. elongatus PCC 7942 should lack tolerance against electron sink limitations such as those occurring under high light and low [CO2], in contrast to Synechocystis sp. PCC 6803. However, Bersanini et al. (2014) proposed that in cyanobacteria such as S. elongatus PCC 7942 that lack FLV2 and FLV4, photoinhibition is alleviated by the expression of an additional copy of the reaction center protein in PSII, D1, called D1:2, which enhances the tolerance against photo stress (Clarke et al., 1993). The relationship between this photoprotective system and FLV2/4 calls for further study.
Since the report of Asada and Badger (1984), the oxygen-dependent AEF (Mehler reaction in chloroplasts) has been quantitatively investigated in various photosynthetic organisms. In intact chloroplasts of higher plants, oxygen-dependent AEF amounted to 10% to 20% of the photosynthetic electron flux coupled to CO2 fixation (Badger et al., 2000). The proportion is as low as 1% to 10% according to a recent study, which would seem to suggest that the Mehler reaction is not a major electron sink in higher plants (Driever and Baker, 2011). In intact leaves of C3 plants, photorespiration could be a major electron sink under conditions of CO2-limited photosynthesis (Driever and Baker, 2011; Shirao et al., 2013). These conclusions cannot necessarily be extended to cyanobacteria and algae, which seem to have higher Mehler reaction activities than higher plants (Badger et al., 2000). In fact, the diatom Thalassiosira pseudonana showed oxygen photoreduction at a rate of 600 µmol mg−1 Chl h−1, corresponding to 49% of the electron flux through PSII (Waring et al., 2010). Similarly, the oxygen-dependent electron transport rate contributes 30% of the total electron flux in Symbiodinium sp., a group of symbiotic dinoflagellates of cnidarians, and FLV proteins might be involved (Roberty et al., 2014). In view of these reports and this study, algae and cyanobacteria appear to utilize oxygen-dependent AEFs other than photorespiration as major alternative electron sinks. Linking the physiological function of oxygen-dependent AEFs with their diversity among photosynthetic organisms is a fascinating topic for future investigations.
MATERIALS AND METHODS
Growth Conditions and Determination of Chl a
Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942 cells were cultured in BG-11 medium on a rotary shaker (100 rpm) under light/dark conditions (25°C, 16 h, 100 μmol photons m−2 s−1, fluorescent lamp/23°C, 8 h, dark) in 2% (v/v) CO2. For Chl quantification, cells were harvested and resuspended by vortexing in 1 mL of 100% (w/v) methanol. After incubation at room temperature for 5 min, the suspension was centrifuged at 10,000g for 5 min at room temperature. Chl a was determined by the method of Lee et al. (1998).
Generation of Mutants
To disrupt a gene (pgp, slr0458; glcD1, sll0404; glcD2, slr0806; gcvT, sll0171; glyk, slr1840; flv2, sll0219; and flv4, sll0217), the coding region was replaced with a cassette of the kanamycin resistance gene (Kanr) or the chloramphenicol resistance gene (Cmr) amplified from the pUC4-KIXX or pACYC184 vector, respectively (Nakahara et al., 2003), by PCR using appropriate f and r primer sets (listed in Supplemental Table S2). We constructed these mutants following the method of Sakurai et al. (2007). The regions upstream and downstream of each gene were amplified by PCR using two sets of primers: up f and up r, and dn f and dn r, respectively. The front ends of up r and dn f contained regions that complemented the sequences of the primers Kanr f and Kanr r or Cmr f and Cmr r (Supplemental Table S2). We used three fragments, up, Kanr or Cmr, and dn, which were then linked by successive PCRs to obtain a disruption cassette. Sato et al. (2005) generated 40 mutants of Synechocystis sp. PCC 6803 by this high-throughput method.
Transformation of Synechocystis sp. PCC 6803 was performed by the standard procedure (Williams, 1988). Transformants were selected on 0.5% (w/v) agar plates of BG-11 medium containing 20 μg mL−1 kanamycin or 30 μg mL−1 chloramphenicol. The double mutant ΔglcD1/2 was generated by transformation of the ΔglcD2 (slr0806) mutant with the mutated ΔglcD1 (sll0404). The resultant transformant was selected on 0.5% (w/v) agar plates of BG-11 medium containing 20 μg mL−1 kanamycin and 30 μg mL−1 chloramphenicol.
Measurement of Oxygen Concentration
The uptake and evolution of oxygen were measured simultaneously with Chl fluorescence. The reaction mixture (2 mL) containing 50 mm HEPES-KOH (pH 7.5) and cyanobacterial cells (10 μg Chl mL−1) was illuminated with actinic light (red light, greater than 620 nm; 200 μmol photons m−2 s−1) at 25°C. During the measurements, the reaction mixture was stirred with a magnetic microstirrer. In one set of experiments, oxygen concentrations were monitored continuously with an oxygen electrode while the measuring cuvette remained open to allow the diffusion of oxygen and CO2 between the medium and the atmosphere (Hansatech; Hayashi et al., 2014). Typical results of this type of experiment are shown in Figures 1 and 2. In a second set of experiments, the top of the cuvette was temporarily (1–3 min) closed to determine the oxygen evolution rate. Typical data for the determination of oxygen evolution rate are shown in Supplemental Figure S1.
Measurement of Chl Fluorescence
The relative Chl fluorescence originating from Chl a was measured using a PAM-Chl fluorometer (PAM-101; Walz; Hayashi et al., 2014). Pulse-modulated excitation was achieved by a light-emitting diode lamp with peak emission at 650 nm. Modulated fluorescence was measured at the wavelength (>710 nm; Schott RG9 long-pass filter). The minimum Chl fluorescence was determined by illumination with measuring light. The Fs was monitored under actinic red light, and 1,000-ms pulses of saturated light (10,000 μmol photons m−2 s−1) were supplied at arbitrary intervals to determine the Fm′. Y(II) was defined as (Fm′ − Fs)/Fm′. The fluorescence terminology used in this study follows van Kooten and Snel (1990).
Cloning and Expression of Recombinant GST-FLV2/4 Fusion Proteins
The coding regions of FLV2 and FLV4 were obtained from the genomic DNA of Synechocystis sp. PCC 6803 using KOD-FX Neo (Toyobo) and subcloned via BamHI into pGEX4T-3 (GE Healthcare) using the In-Fusion HD cloning kit (Takara). The primers for these genes are listed in Supplemental Table S3. Recombinant proteins were produced in BL21 (Agilent Technology) host cells. Overnight cultures of the transformed BL21 cells in Luria-Bertani broth were used to inoculate (0.1% [v/v]) fresh Luria-Bertani broth, which was then incubated at 37°C until the A600 reached 0.5 to 1. Protein expression was evaluated at 15°C for 16 h in Luria-Bertani broth containing 0.1 mm isopropyl-β-d-thiogalactopyranoside.
Purification of Recombinant GST Fusion Proteins
Cells were harvested by centrifugation and resuspended in phosphate-buffered saline buffer (140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 1.8 mm KH2PO4, and 1 mm phenylmethylsulfonyl fluoride [pH 7.3]). After gentle sonication, the resulting crude extract was centrifuged at 15,000 rpm for 30 min at 4°C. The lysate was loaded onto GSTrap FF columns (GE Healthcare). Unbound proteins were removed by washing with phosphate-buffered saline buffer. Recombinant GST fusion proteins were then eluted with elution buffer (50 mm Tris-HCl, 10 mm reduced glutathione, and 1 mm phenylmethylsulfonyl fluoride [pH 8]). The purified recombinant protein was quantified using the Pierce 660-nm protein assay (Thermo Scientific). Fractions containing 0.1 µg of purified recombinant proteins were analyzed by SDS-PAGE and stained with Coomassie Brilliant Blue to evaluate the purity of the recombinant proteins (Supplemental Fig. S4). After electrophoresis, the proteins were electrotransferred to a polyvinylidene fluoride membrane and detected by GST-specific antibodies (Novagen; Supplemental Fig. S4). We used the purified GST protein as a control. The GST protein was expressed in BL21 containing a blank pGEX4T-3 vector.
Enzyme Assays
The NADH-oxidizing activity of recombinant GST-FLV4 fusion protein was evaluated in a reaction mixture (1 mL) containing 50 mm Tris-HCl (pH 7.4), 10 μg of GST-FLV4 protein, and various concentrations of NADH. The FLV-dependent oxidation of NADH was monitored as the decrease in A340 at 25°C, assuming an absorption coefficient of 6.22 mm cm−1.
The oxygen-reducing activities of the recombinant GST and GST-FLV4 proteins were evaluated in a reaction mixture (2 mL) containing 50 mm Tris-HCl (pH 7.4), 1 mm NADH, and the protein of interest (40 μg). GST protein was used as the control. GST- and GST-FLV-dependent oxygen reductions were measured with an oxygen electrode (Hansatech).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Determination of oxygen evolution rates during the development of photosynthetic parameters in the Synechocystis sp. PCC 6803 wild type.
Supplemental Figure S2. Maps of the insertion sites of antibiotic resistance cassettes in ∆pgp, ∆glcD1/2, ∆gcvT, ∆glyk, ∆flv2, and ∆flv4.
Supplemental Figure S3. DNA fragments amplified by PCR showing complete segregation of the inactivated genes pgp (slr0458), glcD1 (sll0404), glcD2 (slr0806), gcvT (sll0171), glyk (slr1840), flv2 (sll0219), and flv4 (sll0217).
Supplemental Figure S4. SDS-PAGE and western blot of GST, GST-FLV2, and GST-FLV4 proteins.
Supplemental Figure S5. Dependence of the NADH oxidation activity on the concentration of NADH in the reaction catalyzed by the recombinant GST-FLV4 protein.
Supplemental Figure S6. Development of photosynthetic parameters in media containing high-[CO2]-grown ∆pgp, ∆glcD1/2, ∆gcvT, and ∆glyk mutants of Synechocystis sp. PCC 6803.
Supplemental Table S1. Oxygen-exchange rates in the dark, under CO2-saturated and CO2-limited photosynthesis, and after the addition of NaHCO3, in four mutants of Synechocystis sp. PCC 6803 (∆pgp, ∆glcD1/2, ∆gcvT, and ∆glyk).
Supplemental Table S2. Oligonucleotides used for the disruption of the pgp, glcD1/2, gcvT, glyk, flv2, and flv4 genes.
Supplemental Table S3. Oligonucleotides used for the construction of FLV2 and FLV4 expression vectors.
Supplementary Material
Acknowledgments
We thank NAI, Inc. (http://www.nai.co.jp/) for the English language revision.
Glossary
- PET
photosynthetic electron transport
- PQ
plastoquinone
- LEF
linear electron flow
- CCM
carbon concentration mechanism
- AEF
alternative electron flow
- Chl
chlorophyll
- Y(II)
quantum yield of photosystem II
- Fs
steady-state fluorescence under actinic light
- Fm′
maximum variable fluorescence
Footnotes
This work was supported by the Japan Society for the Promotion of Science (Scientific Research grant no. 21570041 to C.M.) and the Ministry of Education, Culture, Sports, Science, and Technology in Japan (Scientific Research on Innovative Areas grant no. 22114512 to C.M. and A.M.).
Articles can be viewed without a subscription.
References
- Allahverdiyeva Y, Ermakova M, Eisenhut M, Zhang P, Richaud P, Hagemann M, Cournac L, Aro EM (2011) Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J Biol Chem 286: 24007–24014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110: 4111–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel J, Phunpruch S, Steinmüller K, Schulz R (2000) The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch Microbiol 173: 333–338 [DOI] [PubMed] [Google Scholar]
- Asada K, Badger MR (1984) Photoreduction of 18O2 and H218O2 with concomitant evolution of 16O2 in intact spinach chloroplasts: evidence for scavenging of hydrogen peroxide by peroxidase. Plant Cell Physiol 25: 1169–1179 [Google Scholar]
- Badger MR, Price GD (1992) The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol Plant 84: 606–615 [Google Scholar]
- Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and Rubisco oxygenase. Philos Trans R Soc Lond B Biol Sci 355: 1433–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S, Schneider D, Vermaas WFJ, Rögner M (2002) Electron transport routes in whole cells of Synechocystis sp. strain PCC 6803: the role of the cytochrome bd-type oxidase. Biochemistry 41: 3422–3429 [DOI] [PubMed] [Google Scholar]
- Bersanini L, Battchikova N, Jokel M, Rehman A, Vass I, Allahverdiyeva Y, Aro EM (2014) Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in co-operation with phycobilisomes in cyanobacteria. Plant Physiol 164: 805–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay C, Abasova L, Six C, Vass I, Kirilovsky D (2008) Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria. Biochim Biophys Acta 1777: 1344–1354 [DOI] [PubMed] [Google Scholar]
- Clarke AK, Hurry VM, Gustafsson P, Oquist G (1993) Two functionally distinct forms of the photosystem II reaction-center protein D1 in the cyanobacterium Synechococcus sp. PCC 7942. Proc Natl Acad Sci USA 90: 11985–11989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever SM, Baker NR (2011) The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ 34: 837–846 [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Georg J, Klähn S, Sakurai I, Mustila H, Zhang P, Hess WR, Aro EM (2012) The antisense RNA As1_flv4 in the cyanobacterium Synechocystis sp. PCC 6803 prevents premature expression of the flv4-2 operon upon shift in inorganic carbon supply. J Biol Chem 287: 33153–33162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Kahlon S, Hasse D, Ewald R, Lieman-Hurwitz J, Ogawa T, Ruth W, Bauwe H, Kaplan A, Hagemann M (2006) The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiol 142: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci USA 105: 17199–17204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E, Frías JE, Rubio LM, Herrero A (2005) Photosynthetic nitrate assimilation in cyanobacteria. Photosynth Res 83: 117–133 [DOI] [PubMed] [Google Scholar]
- Hagemann M, Fernie AR, Espie GS, Kern R, Eisenhut M, Reumann S, Bauwe H, Weber APM (2013) Evolution of the biochemistry of the photorespiratory C2 cycle. Plant Biol (Stuttg) 15: 639–647 [DOI] [PubMed] [Google Scholar]
- Hayashi R, Shimakawa G, Shaku K, Shimizu S, Akimoto S, Yamamoto H, Amako K, Sugimoto T, Tamoi M, Makino A, et al. (2014) O2-dependent large electron flow functioned as an electron sink, replacing the steady-state electron flux in photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803, but not in the cyanobacterium Synechococcus sp. PCC 7942. Biosci Biotechnol Biochem 78: 384–393 [DOI] [PubMed] [Google Scholar]
- Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13: 230–235 [DOI] [PubMed] [Google Scholar]
- Lea-Smith DJ, Ross N, Zori M, Bendall DS, Dennis JS, Scott SA, Smith AG, Howe CJ (2013) Thylakoid terminal oxidases are essential for the cyanobacterium Synechocystis sp. PCC 6803 to survive rapidly changing light intensities. Plant Physiol 162: 484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Flores E, Herrero A, Houmard J, Tandeau de Marsac N (1998) A role for the signal transduction protein PII in the control of nitrate/nitrite uptake in a cyanobacterium. FEBS Lett 427: 291–295 [DOI] [PubMed] [Google Scholar]
- McConnell MD, Koop R, Vasil’ev S, Bruce D (2002) Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria: a structure-based model for the light state transition. Plant Physiol 130: 1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AG, Espie GS, Bruce D (1996) Characterization of the non-photochemical quenching of chlorophyll fluorescence that occurs during the active accumulation of inorganic carbon in the cyanobacterium Synechococcus PCC 7942. Photosynth Res 49: 251–262 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW. (2014) Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes. Biochim Biophys Acta 1837: 503–511 [DOI] [PubMed] [Google Scholar]
- Nakahara K, Yamamoto H, Miyake C, Yokota A (2003) Purification and characterization of class-I and class-II fructose-1,6-bisphosphate aldolases from the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 44: 326–333 [DOI] [PubMed] [Google Scholar]
- Nomura CT, Persson S, Shen G, Inoue-Sakamoto K, Bryant DA (2006) Characterization of two cytochrome oxidase operons in the marine cyanobacterium Synechococcus sp. PCC 7002: inactivation of ctaDI affects the PS I:PS II ratio. Photosynth Res 87: 215–228 [DOI] [PubMed] [Google Scholar]
- Pils D, Schmetterer G (2001) Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol Lett 203: 217–222 [DOI] [PubMed] [Google Scholar]
- Roberty S, Bailleul B, Berne N, Franck F, Cardol P (2014) PSI Mehler reaction is the main alternative photosynthetic electron pathway in Symbiodinium sp., symbiotic dinoflagellates of cnidarians. New Phytol 204: 81–91 [DOI] [PubMed] [Google Scholar]
- Sakurai I, Mizusawa N, Wada H, Sato N (2007) Digalactosyldiacylglycerol is required for stabilization of the oxygen-evolving complex in photosystem II. Plant Physiol 145: 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Ishikawa M, Fujiwara M, Sonoike K (2005) Mass identification of chloroplast proteins of endosymbiont origin by phylogenetic profiling based on organism-optimized homologous protein groups. Genome Inform 16: 56–68 [PubMed] [Google Scholar]
- Shirao M, Kuroki S, Kaneko K, Kinjo Y, Tsuyama M, Förster B, Takahashi S, Badger MR (2013) Gymnosperms have increased capacity for electron leakage to oxygen (Mehler and PTOX reactions) in photosynthesis compared with angiosperms. Plant Cell Physiol 54: 1152–1163 [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Vicente JB, Gomes CM, Wasserfallen A, Teixeira M (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun 294: 82–87 [DOI] [PubMed] [Google Scholar]
- Waring J, Klenell M, Bechtold U, Underwood GJC, Baker NR (2010) Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species. J Phycol 46: 1206–1217 [Google Scholar]
- Williams JGK. (1988) Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol 167: 766–778 [Google Scholar]
- Wilson A, Ajlani G, Verbavatz JM, Vass I, Kerfeld CA, Kirilovsky D (2006) A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18: 992–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4: e5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Eisenhut M, Brandt AM, Carmel D, Silén HM, Vass I, Allahverdiyeva Y, Salminen TA, Aro EM (2012) Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24: 1952–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.