Abstract
Isolation of Arabidopsis mutants that maintain stomata open all night long credits the existence of dedicated regulators for stomatal closure in darkness.
Stomata are mouth-like cellular complexes at the epidermis that regulate gas transfer between plants and atmosphere. In leaves, they typically open during the day to favor CO2 diffusion when light is available for photosynthesis, and close at night to limit transpiration and save water. Despite the importance of stomatal closure at night for plant fitness and ecosystem water fluxes (Caird et al., 2007), it remains unclear whether this dark response is simply a passive consequence of the absence of light stimulus, or an active process recruiting other mechanisms of stomatal closure or involving independent signaling events (Tallman, 2004; Kollist et al., 2014). Here, we report the isolation and characterization of five Arabidopsis (Arabidopsis thaliana) mutants that maintain stomata open the whole night and were named open all night long1 (opal1) to opal5. Importantly, stomata of the opal mutants closed normally in response to abscisic acid (ABA) and atmospheric CO2. We propose that dedicated regulators enforce nighttime stomatal closure.
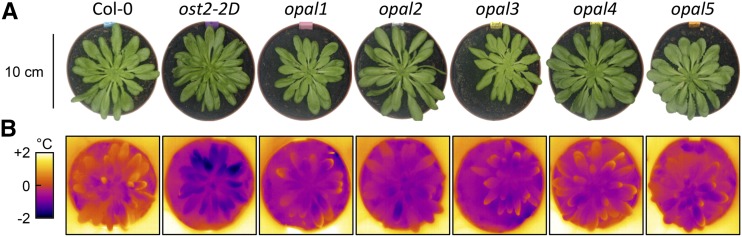
Transpiration drives evaporative cooling. Based on this property, thermal imaging has permitted screening for mutants impaired in leaf transpiration, and thus discovering new signaling players implicated in stomatal response to drought, atmospheric CO2, or light quality (Merlot et al., 2002; for review, see Negi et al., 2014). So far, no genetic screen has attempted to isolate mutants insensitive to darkness, a situation that plants encounter every night. Here, we screened a mutagenized population of Arabidopsis seedlings by imaging shoot temperature during the night period. Candidates with lower temperature than the wild type were selected, and 37 of them showed a heritable, cool phenotype in darkness (Supplemental Table S1). To avoid mutations with pleiotropic effects, we focused on the group of six cool mutants with similar growth as in the wild type (Fig. 1; Supplemental Fig. S1, A and B).
Figure 1.
Isolation and characterization of mutants with stomata open all night long. Six mutants were isolated from a thermography screen in dark conditions. A, Top-view pictures of wild-type and mutant (ost2-2D and opal1 to opal5) mature plants. B, False-color infrared images of the same plants after 18 h in darkness. The color scale is adjusted so that zero corresponds to the average rosette temperature of Col-0.
The six mutants maintained a stable cooler temperature throughout the nighttime and even when the night period was extended for several hours (tested up to 18 h of darkness; Fig. 1B). One of them exhibited an extremely cool phenotype, with a rosette temperature up to 2°C less than in the wild type. Segregation analysis revealed that the mutation was dominant. This prompted us to hypothesize that this mutant was allelic to open stomata2 (ost2), which was confirmed upon sequencing of the gene (Merlot et al., 2007). OST2 encodes the plasma membrane H+-ATPase AHA1, which drives the polarization of the plasma membrane that activates inward ion channels, thereby triggering water influx and thus stomatal opening. A dominant mutation in ost2-2D triggers constitutive activity of AHA1 and extreme stomatal opening, regardless of external stimuli (Merlot et al., 2007). The five other mutants showed a milder phenotype, with shoots being cooler by 0.5°C to 0.7°C compared with the wild type (Supplemental Fig. S1B). Backcrosses between the wild type and each mutant resulted in F1 plants showing wild-type temperature, whereas each F2 progeny segregated in a 3:1 hot:cool ratio (Supplemental Table S1). These data indicated that the causal mutations were single and recessive. Furthermore, all pairwise crosses between mutants generated plants with a wild-type temperature (data not shown), indicating that the mutations occurred in five distinct loci. These five mutants were therefore named opal1 to opal5.
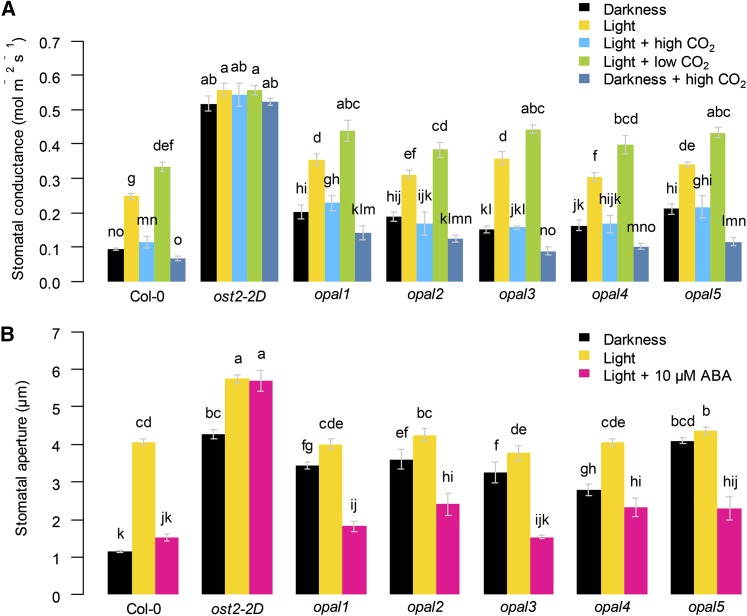
We confirmed the stomatal origin of the cooler temperature in the opal mutants. Because the mutants displayed an unaffected or lower stomatal density (Supplemental Fig. S1C), their cool phenotype was likely caused by a misregulation in guard cell functioning. Gas exchange was monitored on intact leaves of plants exposed to extended darkness. Compared with the wild type, stomatal conductance in darkness was 2 times higher in the opal mutants and 5 times higher in the extreme ost2-2D (Fig. 2A). Bioassays on epidermal strips confirmed that stomata of the opal mutants remain open in darkness (Fig. 2B). In light conditions, the opal mutants showed higher stomatal conductance than the wild type (Fig. 2A), whereas their stomatal aperture on epidermal strips was similar or only slightly increased (Fig. 2B), a discrepancy commonly observed for stomatal response to light, CO2, or ABA (Mott et al., 2008; Fujita et al., 2013; Pantin et al., 2013a). These results suggest that the mechanisms ruling nighttime stomatal closure also constrain daytime stomatal movements when in contact with the mesophyll.
Figure 2.
Stomatal response to dark, light, CO2, and ABA in the opal mutants. A, Gas exchange analysis on individual leaves attached to mature plants. Stomatal conductance to water vapor was measured in dark or light (500 µmol m−2 s−1) conditions at control (360 μL L−1), low (75 μL L−1), or high (2,000 μL L−1) CO2 concentration (41 ≤ n ≤ 4). B, Stomatal aperture was measured on epidermal peels in darkness or light (250 µmol m−2 s−1) with or without 10 µm ABA (36 ≤ n ≤ 6). Error bars are means ± se. Letters denote significant differences after a Kruskal-Wallis test (α = 0.05), with P values adjusted using the Benjamini and Hochberg method for multiple comparisons.
The sustained stomatal opening of opal mutants indicates that their phenotype prevails over transient or circadian effects. Opening stomata in darkness is a typical trait of several mutants affected in the regulation of photomorphogenesis. Photomorphogenesis in darkness is repressed by CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1), an E3 ubiquitin ligase that interacts with a large spectrum of photoreceptors. Although most photoreceptor mutants or overexpressors show the same basal stomatal aperture in the dark as in wild-type plants (Kinoshita et al., 2001; Ohgishi et al., 2004; Mao et al., 2005; Wang et al., 2010, 2014), down-regulation of COP1 activity induces constitutively open stomata in darkness (Mao et al., 2005; Wang et al., 2010). However, cop1 mutants show severe growth reduction (Mao et al., 2005) or altered stomatal patterning (Kang et al., 2009), ruling out COP1 as a possible candidate for the OPAL genes. Photomorphogenesis in darkness as well as stomatal closure are also controlled by the vacuolar H+-ATPase, a subunit of which is encoded by DE-ETIOLATED3 (DET3; Schumacher et al., 1999; Allen et al., 2000). The det3 mutant can be rescued by down-regulation of MYB61 (Newman et al., 2004), a gene coding for an R2R3-MYB transcription factor also involved in stomatal closure (Liang et al., 2005). The myb61 mutant shows enhanced stomatal conductance in the dark (Liang et al., 2005), but also pleiotropic developmental alterations (Romano et al., 2012), thereby decreasing MYB61’s chance as a candidate for the OPAL genes.
Stomatal response to darkness might recruit other mechanisms leading to stomatal closure, such as the pathways controlling ABA and CO2 responses (Tallman, 2004). In line with this, stomata of mutants severely impaired in ABA synthesis (aba type) or sensitivity (abi type) remain largely open in the dark (Leymarie et al., 1998; Pantin et al., 2013b), and mutants with defective ABA receptors (pyr/pyl/rcar) show deficient stomatal response to CO2 and darkness (Merilo et al., 2013). Moreover, disruption of the guard cell slow-type anion channel SLAC1 strongly decreases stomatal response to ABA, CO2, and darkness (Negi et al., 2008; Vahisalu et al., 2008; Merilo et al., 2013). Similarly, alteration of actin dynamics in guard cells of the high sugar response3 mutant reduces stomatal response to several closure stimuli, including ABA and darkness (Jiang et al., 2012). Thus, stimuli, such as darkness, ABA, and CO2, may promote stomatal closure through shared terminal molecular events, triggering solute movements and cytoskeleton rearrangements that result in guard cell deflation.
We, therefore, tested the possibility of opal mutants being impaired in stomatal sensitivity to ABA or CO2. ABA content in these lines did not significantly differ from the wild type (Supplemental Fig. S1E). Epidermal bioassays showed that stomata of the opal mutants close in response to 10 µm ABA, contrasting with ost2-2D (Fig. 2B). Moreover, the opal mutants had similar or even reduced levels of seed germination in the presence of ABA (Supplemental Fig. S1D). Thus, the opal mutants are neither strongly ABA deficient nor strongly ABA insensitive. We then probed opal responsiveness to contrasting CO2 concentrations. In the presence of light, the opal mutants showed intact responsiveness to both low and high CO2 (Fig. 2A). Likewise, in darkness, high CO2 triggered similar stomatal closure in the opal mutants as in the wild type, suggesting that these mutants are not impaired in CO2 signaling. Thus, the opal mutants clearly deviate from the classical behavior of mutants impaired in ABA or CO2 signaling pathways, although it still could be that the OPAL genes encode alternative components involved in guard cell ABA metabolism or remote signals related to mesophyll metabolism (Tallman, 2004; Lawson et al., 2014).
Based on the sensitivity of the opal mutants to ABA and CO2, we propose that stomatal response to darkness is at least partly independent from ABA or CO2 signaling pathways. Interestingly, lycophyte and fern stomata show low sensitivity to ABA and CO2 (Doi and Shimazaki, 2008; Brodribb et al., 2009; Brodribb and McAdam, 2011, 2013; Ruszala et al., 2011; McAdam and Brodribb, 2012a; Creese et al., 2014) but do respond to dark-light regime (Doi et al., 2006; Doi and Shimazaki, 2008; McAdam and Brodribb, 2012b; Creese et al., 2014). This may indicate that the dark response of stomata is a primitive regulatory backbone over which seed plants have evolved other signaling pathways to respond to an increasing number of stimuli (McAdam and Brodribb, 2012b; but see also Ruszala et al., 2011; Chater et al., 2013). Several pieces of evidence suggest that stomatal responsiveness has been evolutionary refined through an assembly of signaling modules that preexisted in ancestral clades. For instance, seed plants open their stomata in response to blue light, perception of which by phototropins triggers phosphorylation events that activate plasma membrane H+-ATPases (Takemiya et al., 2013). By contrast, ferns lack stomatal response to blue light, although they possess functional phototropins and plasma membrane H+-ATPases (Doi et al., 2006). This suggests that seed plants have evolved components able to bridge these signaling modules. Similarly, only angiosperms show stomatal closure in response to high CO2, which may result from a recent specialization of Ca2+ signaling in the guard cells of angiosperms (Brodribb and McAdam, 2013). According to this evolutionary framework, the dark response of stomata may be controlled by more primitive signaling events.
Plasma membrane depolarization through regulation of proton pumps seems to be a key step for stomatal response to darkness. The strong dark phenotype of ost2-2D (Merlot et al., 2007; this work) and a line overexpressing constitutively activated AHA2 in guard cells (Wang et al., 2014) show the necessity of down-regulating the activity of plasma membrane H+-ATPases to close stomata in darkness. The recent discovery that constitutive stomatal opening in the dark through overexpression of flowering regulators (FLOWERING LOCUS T, TWIN SISTER OF FT, CONSTANS, and GIGANTEA) is mediated by the activation of H+-ATPases (Kinoshita et al., 2011; Ando et al., 2013) further strengthens this proposition. Altogether, these data are consistent with in silico simulations showing stomatal opening in darkness upon constitutive activity of H+-ATPases, for instance, by abolishing the sensitivity of H+-ATPases to Ca2+ (Blatt et al., 2014). Regulators of the proton pumps (Fuglsang et al., 2007, 2014; Shimazaki et al., 2007), whose involvement in stomatal response to darkness remains largely unknown, seem therefore to be potential candidates underlying the opal mutants.
Downstream regulators of the guard cell solute balance also emerge as relevant candidates for the opal behavior. For instance, transport and metabolism of malate have been proven of particular importance for stomatal closure in darkness. Mutants defective in QUAC1, a guard cell malate transporter, show a slower rate of stomatal closure in response to light-dark transitions compared with the wild type, but similar steady-state stomatal conductance after dark adaptation (Meyer et al., 2010) or altered growth (Sasaki et al., 2010). By contrast, pck1, a mutant lacking an isoform of phosphoenolpyruvate carboxykinase involved in malate catabolism in guard cells, shows sustained open stomata in darkness and normal growth (Penfield et al., 2012). Importantly, apoplastic malate produced in the mesophyll has an opposite effect on guard cell movements (Araújo et al., 2011; Lawson et al., 2014). Therefore, effectors poising malate concentration within and around guard cells are key candidates for the opal phenotype, a stomatal trait naturally coselected with singular regulation of malate metabolism in Crassulacean acid metabolism plants.
Nighttime stomatal control is of evolutionary and ecological importance, but Francis Darwin’s early conclusion that “the biology of nocturnal closure is obscure” (Darwin, 1898) remains timely. The opal mutants reported here credit the existence of specific regulators leading to stomatal closure in darkness. Further characterization of these mutants may well shed some light on the dark side of stomatal behavior.
MATERIALS AND METHODS
Growth Conditions
For the primary screen, plants were grown in a growth chamber with a 14-h photoperiod, an irradiance of 180 µmol m−2 s−1, a temperature of 25°C, and a relative humidity (RH) of 65%. The other experiments were conducted on plants grown under an 8-h photoperiod at an irradiance of 270 µmol m−2 s−1, a temperature of 22°C:18°C (day:night) and an RH of 65%.
Screen and Mutant Selection
An M2 population of Arabidopsis (Arabidopsis thaliana) seeds (ecotype Columbia-0 [Col-0]) mutagenized with ethyl methanesulfonate was purchased from Lehle Seeds. About 72,000 seeds were sown in pots (9 × 9 × 7 cm, 40 seeds per pot). Seedlings were screened 9 to 18 d after germination by thermal imaging in the growth chamber. The screen was performed in darkness about 2 h after light-to-dark transition. Individuals displaying cooler temperature than the wild type were selected as candidate mutants. The progeny of the fertile candidates was used to produce the M3 generation. M3 seedlings were probed again by thermal imaging to validate the heritability of the cool phenotype. All validated candidates were then backcrossed with the wild type, and the resultant F1 plants (M2 × Col-0) were self-crossed to obtain F2 seeds. Only the mutant lines with cool F2 seedlings and similar growth to the wild type were selected for additional backcrosses (Supplemental Table S1). Segregation in the F2 generation was analyzed with a χ2 for goodness of fit. The allelism tests between the isolated mutants were performed by genetic crosses covering all possible pairwise combinations. Each resulting F1 progeny was compared for rosette temperature with the wild type and its respective parent mutant lines. The temperature phenotype in darkness was checked 15 to 30 d after germination.
Thermal Imaging
Thermography screening was performed using an Inframetrics 760 infrared camera (Inframetrics) equipped with a Stirling-cooled scanning detector in the 3- to 5-µm spectral band. Image resolution was 256 × 256 pixels, and thermal sensitivity of the camera was such that noise-equivalent differential temperature was below 0.1°C. For subsequent phenotyping of the isolated candidates, thermal imaging was performed using a ThermaCam B20HS camera (FLIR Systems) equipped with an uncooled 320 × 240 microbolometer matrix detector in the 7- to 13-µm band having an improved sensitivity (noise-equivalent differential temperature below 0.05°C). These subsequent experiments were carried out in a dedicated darkroom under 25°C ± 1°C temperature, low RH (45% ± 5%), and low wind speed to ensure temperature contrast between lines.
Leaf Gas Exchange
For gas exchange analysis, single plants were grown in pots, and stomatal conductance to water vapor was measured using a LI-COR 6400 gas analyzer system (LI-COR Inc.) equipped with a clamp-on leaf cuvette (6400-40 Leaf Chamber Fluorometer; LI-COR Inc.). Measurements were performed on individual leaves attached to mature plants (40- to 50-d-old plants). Plants were dark adapted for 18 h, and stomatal conductance to water vapor was determined in the dark. After a 2-h light adaptation, stomatal conductance was measured again. The light-emitting diode source provided 500 μmol m−2 s−1 of irradiance using 10% blue and 90% red. Leaf temperature was maintained at 23.5°C, and leaf-to-air vapor pressure deficit was at 0.8 kPa. The effect of high and low CO2 was tested by increasing or decreasing atmospheric CO2 concentration from 360 to 2,000 or 75 μL L−1, respectively.
Stomatal Aperture Bioassays and Stomatal Density
Stomatal aperture and density were determined on epidermal peels of leaves from 21- to 28-d-old plants. Leaves were harvested at the end of the night period, and strips of the abaxial epidermis were processed as described by Merlot et al. (2007). Stomatal aperture was measured after subjecting epidermal peels to 2 to 3 h of darkness or light (250 µmol m−2 s−1). After light treatment, peels were incubated for 2 to 3 h with 10 µm ABA, and stomatal aperture was measured again. Each replicate was the average aperture of at least 60 stomata. Stomatal density was determined on abaxial and adaxial peels by counting stomata on a surface of 0.06 mm2.
Quantification of ABA
The ABA content in leaves of 5- to 6-week-old plants was determined from fresh material frozen in liquid nitrogen and freeze dried before extraction in 1 mL of sterile distilled water at 4°C overnight. ABA was quantified by an ELISA method using the Phytodetek enzyme immunoassay test kit (Agdia Inc.).
Germination Assays
Sensitivity of seed germination to exogenous ABA was tested on mature seeds of plants grown under identical conditions and harvested at the same time. Seeds were sterilized and plated on one-half-strength Murashige and Skoog medium with contrasting ABA concentrations. They were stratified at 4°C for 4 d and then transferred to a growth chamber under a temperature of 24°C and a 16-h photoperiod. Germination was scored according to radicle emergence after 3 d in the growth chamber.
Statistical Analyses
Data analyses were performed using R 3.0.2 (R Core Team, 2013). Each replicate corresponds to one plant, except for germination, which was scored on 50 plants per replicate.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Additional phenotypic characterization of the opal mutants.
Supplemental Table S1. Isolation and characterization of mutants with low temperature in darkness.
Supplementary Material
Acknowledgments
We thank Sylvain Aubry (University of Zürich) and Danny Tholen (University of Natural Resources and Life Sciences) for insightful comments on this study, Alistair Hetherington and Jean-Charles Isner (University of Bristol) for helpful discussion, and Dominique Guilhem (Commissariat à l’Energie Atomique, Cadarache, France) for making available the Inframetrics thermal camera.
Glossary
- ABA
abscisic acid
- Col-0
Columbia-0
- RH
relative humidity
Footnotes
This work was supported by the Centre National de la Recherche Scientifique and the Commissariat à l’Energie Atomique et aux Energies Alternatives (for the FLIR camera), the European Union Marie Curie FP5 Research Training Network (program no. STRESSIMAGING HNRT–CT–2002–00254 to J.M.C., I.M.R., and B.G.), the Fundação para a Ciência e Tecnologia, Portugal (grant nos. SFRH/BPD/14498/2003 to J.M.C. and 34429/2006 to J.M.C.), and the CNRS and Provence Alpes Côte d'Azur Region, France (BDI fellowship to D.J.).
References
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Ando E, Ohnishi M, Wang Y, Matsushita T, Watanabe A, Hayashi Y, Fujii M, Ma JF, Inoue S, Kinoshita T (2013) TWIN SISTER OF FT, GIGANTEA, and CONSTANS have a positive but indirect effect on blue light-induced stomatal opening in Arabidopsis. Plant Physiol 162: 1529–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Osorio S, Usadel B, Fuentes D, Nagy R, Balbo I, Lehmann M, Studart-Witkowski C, Tohge T, et al. (2011) Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture. Plant Cell 23: 600–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Wang Y, Leonhardt N, Hills A (2014) Exploring emergent properties in cellular homeostasis using OnGuard to model K+ and other ion transport in guard cells. J Plant Physiol 171: 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2013) Unique responsiveness of angiosperm stomata to elevated CO2 explained by calcium signalling. PLoS ONE 8: e82057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Feild TS (2009) Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytol 183: 839–847 [DOI] [PubMed] [Google Scholar]
- Caird MA, Richards JH, Donovan LA (2007) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143: 4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Gray JE, Beerling DJ (2013) Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr Opin Plant Biol 16: 638–646 [DOI] [PubMed] [Google Scholar]
- Creese C, Oberbauer S, Rundel P, Sack L (2014) Are fern stomatal responses to different stimuli coordinated? Testing responses to light, vapor pressure deficit, and CO2 for diverse species grown under contrasting irradiances. New Phytol 204: 92–104 [DOI] [PubMed] [Google Scholar]
- Darwin F. (1898) Observations on stomata. Philos Trans R Soc Lond B Biol Sci 190: 531–621 [Google Scholar]
- Doi M, Shimazaki K (2008) The stomata of the fern Adiantum capillus-veneris do not respond to CO2 in the dark and open by photosynthesis in guard cells. Plant Physiol 147: 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Wada M, Shimazaki K (2006) The fern Adiantum capillus-veneris lacks stomatal responses to blue light. Plant Cell Physiol 47: 748–755 [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, et al. (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Kristensen A, Cuin TA, Schulze WX, Persson J, Thuesen KH, Ytting CK, Oehlenschlaeger CB, Mahmood K, Sondergaard TE, et al. (2014) Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J 80: 951–964 [DOI] [PubMed] [Google Scholar]
- Fujita T, Noguchi K, Terashima I (2013) Apoplastic mesophyll signals induce rapid stomatal responses to CO2 in Commelina communis. New Phytol 199: 395–406 [DOI] [PubMed] [Google Scholar]
- Jiang K, Sorefan K, Deeks MJ, Bevan MW, Hussey PJ, Hetherington AM (2012) The ARP2/3 complex mediates guard cell actin reorganization and stomatal movement in Arabidopsis. Plant Cell 24: 2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CY, Lian HL, Wang FF, Huang JR, Yang HQ (2009) Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 21: 2624–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, Kato Y, Ohnishi M, Nakano T, Inoue S, et al. (2011) FLOWERING LOCUS T regulates stomatal opening. Curr Biol 21: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203: 1064–1081 [DOI] [PubMed] [Google Scholar]
- Leymarie J, Lascève G, Vavasseur A (1998) Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Aust J Plant Physiol 25: 785–791 [Google Scholar]
- Liang YK, Dubos C, Dodd IC, Holroyd GH, Hetherington AM, Campbell MM (2005) AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Curr Biol 15: 1201–1206 [DOI] [PubMed] [Google Scholar]
- Mao J, Zhang YC, Sang Y, Li QH, Yang HQ (2005) From the cover: a role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc Natl Acad Sci USA 102: 12270–12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2012a) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2012b) Stomatal innovation and the rise of seed plants. Ecol Lett 15: 1–8 [DOI] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M, et al. (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162: 1652–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, et al. (2007) Constitutive activation of a plasma membrane H(+)-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Mustilli AC, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30: 601–609 [DOI] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martinoia E, Hedrich R (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Negi J, Hashimoto-Sugimoto M, Kusumi K, Iba K (2014) New approaches to the biology of stomatal guard cells. Plant Cell Physiol 55: 241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Newman LJ, Perazza DE, Juda L, Campbell MM (2004) Involvement of the R2R3-MYB, AtMYB61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant J 37: 239–250 [DOI] [PubMed] [Google Scholar]
- Ohgishi M, Saji K, Okada K, Sakai T (2004) Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc Natl Acad Sci USA 101: 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013a) The dual effect of abscisic acid on stomata. New Phytol 197: 65–72 [DOI] [PubMed] [Google Scholar]
- Pantin F, Renaud J, Barbier F, Vavasseur A, Le Thiec D, Rose C, Bariac T, Casson S, McLachlan DH, Hetherington AM, et al. (2013b) Developmental priming of stomatal sensitivity to abscisic acid by leaf microclimate. Curr Biol 23: 1805–1811 [DOI] [PubMed] [Google Scholar]
- Penfield S, Clements S, Bailey KJ, Gilday AD, Leegood RC, Gray JE, Graham IA (2012) Expression and manipulation of phosphoenolpyruvate carboxykinase 1 identifies a role for malate metabolism in stomatal closure. Plant J 69: 679–688 [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
- Romano JM, Dubos C, Prouse MB, Wilkins O, Hong H, Poole M, Kang KY, Li E, Douglas CJ, Western TL, et al. (2012) AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network. New Phytol 195: 774–786 [DOI] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y (2010) Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol 51: 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J (1999) The Arabidopsis det3 mutant reveals a central role for the vacuolar H(+)-ATPase in plant growth and development. Genes Dev 13: 3259–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Takemiya A, Sugiyama N, Fujimoto H, Tsutsumi T, Yamauchi S, Hiyama A, Tada Y, Christie JM, Shimazaki K (2013) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat Commun 4: 2094. [DOI] [PubMed] [Google Scholar]
- Tallman G. (2004) Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? J Exp Bot 55: 1963–1976 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FF, Lian HL, Kang CY, Yang HQ (2010) Phytochrome B is involved in mediating red light-induced stomatal opening in Arabidopsis thaliana. Mol Plant 3: 246–259 [DOI] [PubMed] [Google Scholar]
- Wang Y, Noguchi K, Ono N, Inoue S, Terashima I, Kinoshita T (2014) Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc Natl Acad Sci USA 111: 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.