Phosphorylation of a scaffold protein negatively regulates its function by affecting protein stability.
Abstract
Receptor of activated C kinase1 (RACK1) is a versatile scaffold protein that binds to numerous proteins to regulate diverse cellular pathways in mammals. In Arabidopsis (Arabidopsis thaliana), RACK1 has been shown to regulate plant hormone signaling, stress responses, and multiple processes of growth and development. However, little is known about the molecular mechanism underlying these regulations. Here, we show that an atypical serine (Ser)/threonine (Thr) protein kinase, WITH NO LYSINE8 (WNK8), phosphorylates RACK1. WNK8 physically interacted with and phosphorylated RACK1 proteins at two residues: Ser-122 and Thr-162. Genetic epistasis analysis of rack1 wnk8 double mutants indicated that RACK1 acts downstream of WNK8 in the glucose responsiveness and flowering pathways. The phosphorylation-dead form, RACK1AS122A/T162A, but not the phosphomimetic form, RACK1AS122D/T162E, rescued the rack1a null mutant, implying that phosphorylation at Ser-122 and Thr-162 negatively regulates RACK1A function. The transcript of RACK1AS122D/T162E accumulated at similar levels as those of RACK1S122A/T162A. However, although the steady-state level of the RACK1AS122A/T162A protein was similar to wild-type RACK1A protein, the RACK1AS122D/T162E protein was nearly undetectable, suggesting that phosphorylation affects the stability of RACK1A proteins. Taken together, these results suggest that RACK1 is phosphorylated by WNK8 and that phosphorylation negatively regulates RACK1 function by influencing its protein stability.
Receptor for activated C kinase1 (RACK1) is an evolutionarily conserved scaffold protein that was originally identified as a receptor for activated protein kinase C in mammalian cells (Mochly-Rosen et al., 1991; Ron et al., 1994). Subsequent studies indicated that RACK1 binds many other proteins, and consequently, RACK1 is now viewed as a versatile scaffold protein that regulates diverse cellular pathways in animals (McCahill et al., 2002; Adams et al., 2011; Ron et al., 2013). For example, human RACK1 scaffolds an ADP ribosylation factor GTPase Accelerating Protein and focal adhesion kinase to neuronal outgrowths to control focal adhesion kinase activity and consequently, cell adhesion (Dwane et al., 2014).
The first plant RACK1 gene was cloned from tobacco (Nicotiana tabacum) Bright Yellow-2 cells as an auxin-induced gene, arcA (Ishida et al., 1993). RACK1 homologs are found in all plant species, and both the protein sequences and the crystal structure of RACK1 are highly conserved in plants (Chen et al., 2006b; Guo et al., 2007; Ullah et al., 2008). Like its counterpart in mammals, plant RACK1 protein interacts with nearly 100 proteins that fall into many different functional categories (Guo et al., 2007; Klopffleisch et al., 2011; Olejnik et al., 2011; Kundu et al., 2013). RACK1 is involved in plant hormone signaling (McKhann et al., 1997; Perennes et al., 1999; Chen et al., 2006a, 2006b; Guo et al., 2009a, 2009b; Fennell et al., 2012), leaf and root development (Guo and Chen, 2008; Guo et al., 2009b), drought and salt stress responses (Ullah et al., 2008; Guo et al., 2009a), flooding stress (Komatsu et al., 2014), nodulation (Islas-Flores et al., 2011, 2012), seed germination (Komatsu et al., 2005; Islas-Flores et al., 2009; Zhang et al., 2014), hydrogen peroxide production (Zhang et al., 2014), innate immunity (Nakashima et al., 2008), plant response to fungal pathogens (Wang et al., 2014), association with ribosomes (Chang et al., 2005; Giavalisco et al., 2005), protein translation (Guo et al., 2011), and microRNA abundance (Speth et al., 2013). However, little is known about the molecular mechanism of action of RACK1.
RACK1 contains a seven-Trp-Asp repeat domain (WD40) similar to the heterotrimeric GTP-binding protein β-subunit (Ullah et al., 2008). We previously screened for Arabidopsis (Arabidopsis thaliana) RACK1 interacting partners (Klopffleisch et al., 2011) and found that RACK1B and RACK1C interact with WITH NO LYS KINASE8 (WNK8), a member of 10 Arabidopsis WNK family kinases (Nakamichi et al., 2002; Wang et al., 2008). WNKs are composed of an N-terminal kinase domain and a C-terminal regulatory domain. WNKs have an atypical displacement of a catalytic Lys residue within the kinase subdomain II (Xu et al., 2000; Huang et al., 2007). This Lys residue is conserved among all other kinases and essential for the coordination of ATP in the active center (Xu et al., 2000; Huang et al., 2007).
In this study, we report that RACK1 is a substrate of WNK8 and that phosphorylation negatively affects RACK1 function. Recombinant WNK8 physically bound and phosphorylated three Arabidopsis RACK1 proteins: RACK1A, RACK1B, and RACK1C. The phosphomimetic mutations on RACK1A abolished its expression at the protein level but not at the transcript level. These results reveal a regulatory system in which the action of RACK1 is controlled by phosphorylation and subsequent protein degradation.
RESULTS
WNK8 Interacts with RACK1
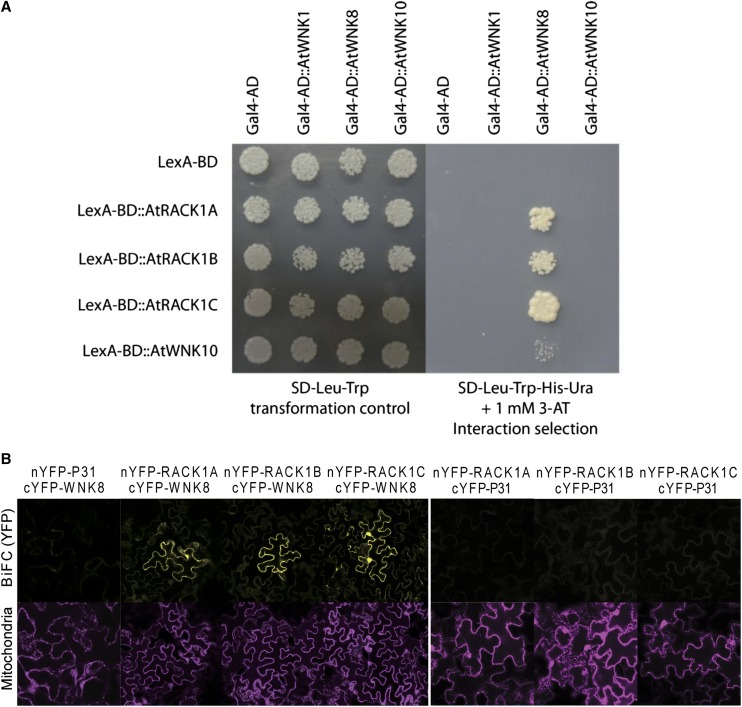
We previously reported interaction between WNK8 and RACK1 proteins (Klopffleisch et al., 2011). To analyze subclass specificity of the interaction, we tested complementation by yeast two-hybrid (Y2H) assay of the full-length open reading frame (ORF) of Arabidopsis WNK8 against each of three Arabidopsis RACK1 proteins: RACK1A, RACK1B, and RACK1C. We found that all three RACK1 proteins interacted with WNK8 (Fig. 1A). We also tested two other WNK family kinases, WNK1 and WNK10. WNK1 is the most divergent from WNK8, whereas WNK10 is the most similar to WNK8 at the amino acid sequence level (Urano et al., 2012). Neither WNK1 nor WNK10 interacted with RACK1 proteins in the Y2H assays (Fig. 1A), suggesting that, among WNK family kinases, the interaction between WNK8 and RACK1 proteins is specific.
Figure 1.
WNK8 interacts with RACK1. A, Y2H assays. Yeasts expressing Gal4-AD and LexA-BD fusion proteins were grown on Synthetic Defined (SD)-Leu-Trp medium to validate efficient transformation or SD-Leu-Trp-His-Ura + 3-amino-1,2,4-triazole (3-AT) selection medium to test interactions of the indicated protein pairs. B, BiFC assay. Carboxyl terminal domain of yellow fluorescent protein (cYFP)-tagged WNK8 was expressed with amino-terminal domain of YFP (nYFP)-tagged RACK1A, RACK1B, or RACK1C in tobacco leaf epidermal cells. mCherry-mitochondrial marker was coinfected as the expression control. Complementation of split YFP and fluorescence of mCherry are shown. cYFP-tagged WNK8 expressed with nYFP-tagged P31 and nYFP-tagged RACK1A, RACK1B, and RACK1C expressed with cYFP-tagged P31 were used as negative controls.
To validate the interactions between WNK8 and RACK1 proteins in vivo, we used bimolecular fluorescence complementation (BiFC) by expressing YFP fragment-tagged test proteins in tobacco leaf epidermal cells (Fig. 1B). Consistent with the results of Y2H assays, WNK8 interacted with all three RACK1 proteins, whereas a soluble protein, P31, used as a control did not interact with WNK8 or RACK1 proteins.
WNK8 Phosphorylates RACK1
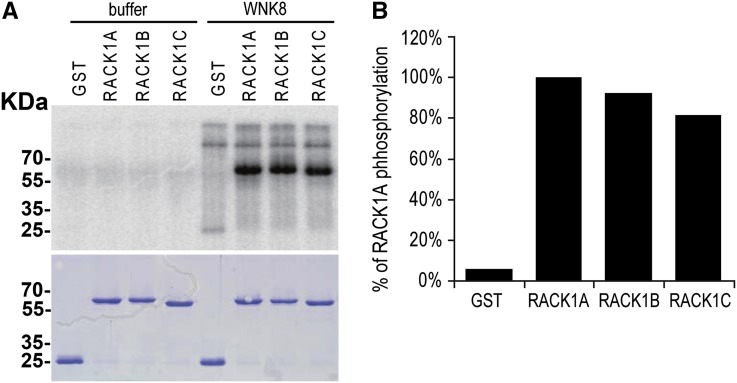
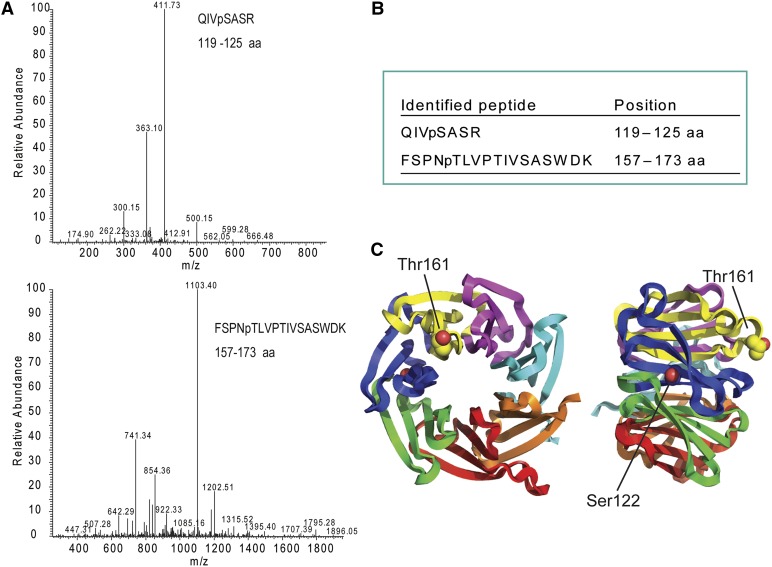
As shown above (Fig. 1), RACK1A, RACK1B, and RACK1C interacted with WNK8 in yeast (Saccharomyces cerevisiae) and in planta. WNK8 has been reported as a functional kinase through molecular and genetic studies (Hong-Hermesdorf et al., 2006; Tsuchiya and Eulgem, 2010; Urano et al.; 2012); therefore, we tested if WNK8 phosphorylates RACK1 proteins in vitro using recombinant proteins and radio-labeled [γ32P]ATP. WNK8 phosphorylated all three RACK1 proteins (Fig. 2). No substrate specificity was observed between the three RACK1 proteins. Phosphorylation sites were determined by MS (Fig. 3). Recombinant RACK1C protein phosphorylated by WNK8 contained two phosphorylated sites: Ser-122 and Thr-161 (Fig. 3, A and B). The Ser-122 on the side of blade 3 of RACK1 (Fig. 3C) is conserved in all three Arabidopsis RACK1 proteins (RACK1A, RACK1B, and RACK1C), both rice (Oryza sativa) RACK1 proteins (OsRACK1A and OsRACK1B), and RACK1 proteins from human, fruit fly, and yeast (Supplemental Fig. S1). Thr-161 of RACK1C (Thr-162 of RACK1A and Thr-161 of RACK1B) is present in all three Arabidopsis RACK1 proteins and one of two rice RACK1 proteins (OsRACK1A) but not conserved in nonplant species, indicating that the Thr-161 phosphorylation site is plant specific.
Figure 2.
WNK8 phosphorylates RACK1. A, Recombinant GST or GST-tagged RACK1A, RACK1B, or RACK1C protein was incubated with [γ-32P]ATP and GST-WNK8 for 6 h at room temperature. Phosphorylated proteins were then separated with SDS-PAGE and detected with a phosphoimage analyzer. Bottom shows Coomassie Blue staining of the gel. B, Quantification of phosphorylation level of RACK1 proteins.
Figure 3.
RACK1 phosphorylation sites. A, Liquid chromatography-tandem mass spectometry (LC-MS/MS) spectra of phosphorylated peptides of RACK1C. GST-RACK1C protein was phosphorylated by WNK8 and digested with trypsin. The digested sample was supplied to LC-MS/MS analysis. Peaks corresponding to phosphorylated RACK1 peptide were obtained from MS/MS analysis. B, Phosphorylated peptides identified by MS. RACK1C phosphorylated by WNK8 was used for MS analysis. Amino acid sequences and positions of two phosphorylated peptides are shown. pS, Phosphorylated Ser; pT, phosphorylated Thr. C, Phosphorylation sites on Arabidopsis RACK1A structure (Protein Data Bank ID code 3DM0; Ullah et al., 2008). Each WD40 repeat is drawn with different colors. Two phosphorylation sites identified by MS are drawn with space fill model. Note that Thr-162 of RACK1A corresponds to Thr-161 of RACK1C.
Ser-122 Is the Dominant Phosphorylation Site of RACK1 by WNK8
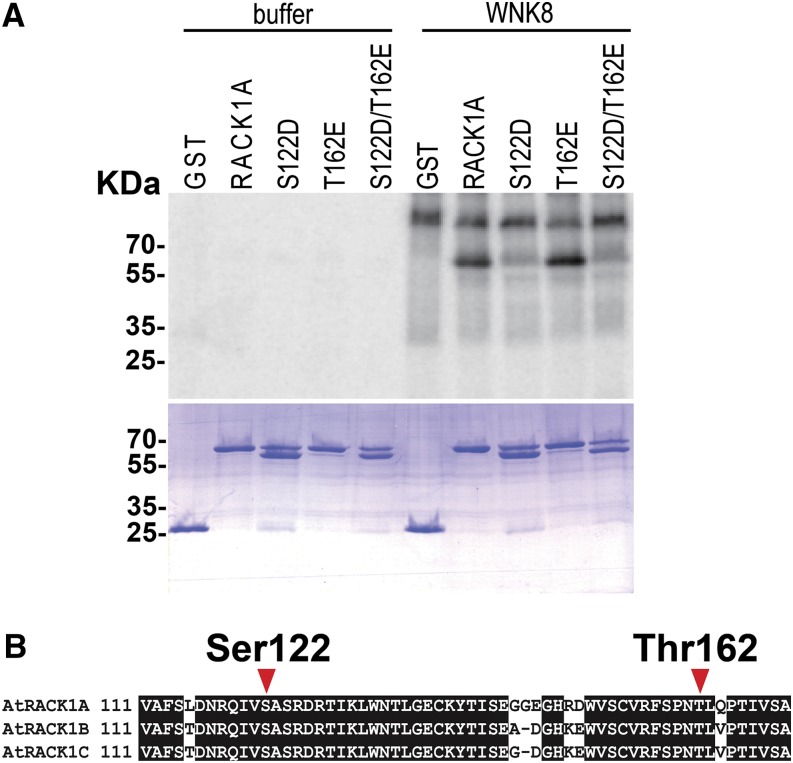
MS covered only 81% of the amino acids of RACK1 protein (Supplemental Fig. S2); therefore, we asked whether Ser-122 and Thr-161 represent major phosphorylation sites by using RACK1A mutant proteins (Fig. 4). We substituted Ser-122 and Thr-162 of RACK1A with Asp and Glu, respectively, to create two RACK1 phosphomimetic mutant proteins RACK1AS122D and RACK1AT162E, respectively, and tested the phosphorylation of the mutant proteins by WNK8. These two amino acid substitutions are commonly used as phosphomimetics that mimic the phosphorylated isoform. As shown in Figure 4, mutation of S122D abolished the phosphorylation of RACK1A protein, whereas the mutation of T162E did not affect the phosphorylation. To further test this, we created a mutant protein containing mutations at both Ser-122 and Thr-162 (RACK1AS122D/T162E). Similar to the RACK1AS122D single-mutant protein, the phosphorylation of the RACK1AS122D/T162E double mutant by WNK8 was abolished (Fig. 4). These results indicate that Ser-122 is the single major phosphorylation site of RACK1A by WNK8.
Figure 4.
Ser-122 is required and sufficient for the phosphorylation of RACK1. A, Recombinant GST-tagged RACK1A and the point mutants were incubated with [γ-32P]ATP and GST-WNK8 for 6 h at room temperature. Proteins were then separated by SDS-PAGE: S122D, RACK1AS122D; T162E, RACK1AT162E; and S122D/T162E, RACK1AS122D/T162E. Note that the Asp substitution at Ser-122 induced a partial cleavage of RACK1A protein, which resulted in a cleaved protein that was approximately 5 kD smaller than the native RACK1A protein. Neither the intact protein nor the cleaved protein was phosphorylated by WNK8. B, Partial amino acid sequence indicating the positions of Ser-122 and Thr-162. Thr-161 of RACK1C corresponds to Thr-161 of RACK1B and Thr-162 of RACK1A.
Phosphorylation Affects RACK1 Function
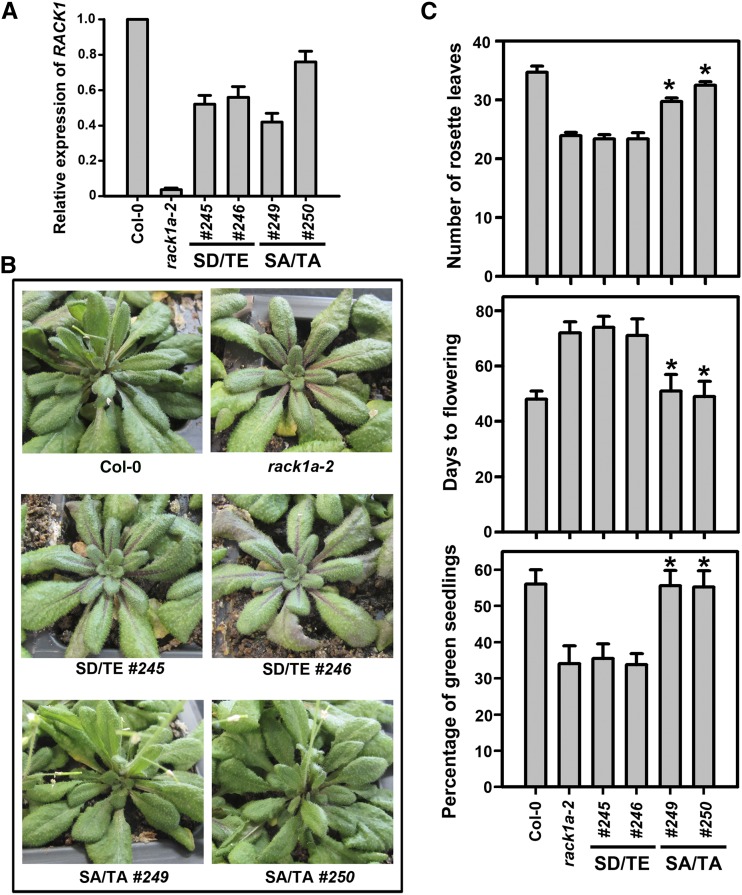
Having shown that RACK1 proteins are phosphorylated by WNK8 at Ser-122 and Thr-162, we investigated the biological consequence of RACK1 phosphorylation. We tested whether expression of the double phosphomimetic form, RACK1AS122D/T162E, rescues the rack1a mutant phenotypes. In addition, we created a double phosphorylation-dead isoform of RACK1A by substituting Ser-122 and Thr-162 with Ala (RACK1AS122A/T162A). The expression of the phosphomimetic form (RACK1AS122D/T162E) and the phosphorylation-dead form (RACK1AS122A/T162A) was driven by RACK1A’s native promoter (Guo et al., 2009a). PRACK1A::RACK1AS122D/T162E or PRACK1A::RACK1AS122A/T162A was transformed into the rack1-2 null mutant background. Quantitative reverse transcription (qRT)-PCR analysis indicated that both RACK1AS122D/T162E (lines 245 and 246) and RACK1AS122A/T162A (lines 249 and 250) were expressed in the transgenic lines (Fig. 5A). One of the characteristic phenotypes of the rack1a mutant, rosette leaf production (Chen et al., 2006a, 2006b), was used to determine genetic complementation. Under a 10-h-light/14-h-dark photoperiod (23°C) in the vegetative growth stage, the Columbia-0 (Col-0) wild type produced approximately 35 rosette leaves within 40 d, whereas the rack1a-2 mutant produced approximately 24 rosette leaves (Fig. 5, B and C). Expression of the phosphomimetic form, RACK1AS122D/T162E, had no effect on rosette leaf production in the rack1a-2 mutant background, indicating that RACK1AS122D/T162E is not able to rescue rack1-2 mutants. In contrast, expression of the phosphorylation-dead form, RACK1AS122A/T162A, resulted in near-full rescue of rosette leaf production phenotype of rack1a-2 mutants (Fig. 5C). Similarly, the late flowering and Glc hypersensitivity phenotypes of rack1a mutant were rescued by the expression of RACK1AS122A/T162A but not RACK1AS122D/T162E (Fig. 5C). These results imply that phosphorylation negatively affects RACK1A function and that the nonphosphorylated isoform of RACK1 is required for its function.
Figure 5.
Genetic complementation of rack1a mutants. A, qRT-PCR analysis of RACK1A transcripts. Total RNAs were isolated from rosette leaves of 4-week-old plants. Amplification of ACTIN8 was used as a control. Shown are means of three replicates ± se. SA/TA, RACK1AS122A/T162A; SD/TE, RACK1AS122D/T162E. B, Seven-week-old transgenic plants. Plants were grown under a 10-h-light/14-h-dark photoperiod. C, Rosette leaf production, days to flowering, and Glc sensitivity. Shown are means of a minimum 10 plants ± se for rosette leaf production and flowering assays. The percentages of green seedlings were recorded 10 d after seeds had been transferred to germination conditions with 6% Glc. At 1% Glc, the percentages of green seedlings for all genotypes were 100%. Shown are means of three biological replicates ± se. *, Significant difference from rack1a-2 mutant, P < 0.05.
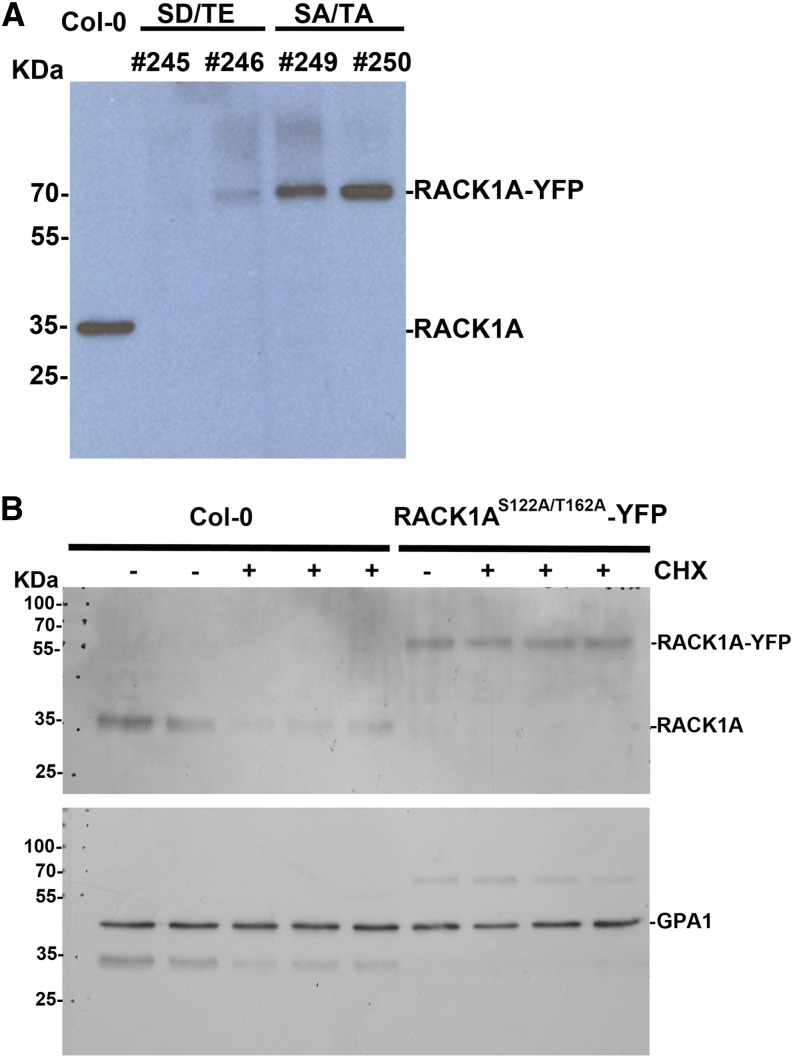
To further explore the possible mechanism of RACK1 phosphorylation, we examined whether phosphorylation affects the stability of RACK1 protein in plants. We used an anti-RACK1A peptide antibody to detect RACK1 protein in transgenic lines. Interestingly, we found that, although RACK1AS122A/T162A was detected at a similar level as wild-type RACK1A in Col-0, RACK1AS122D/T162E proteins were nearly undetectable (Fig. 6A). These results suggest that phosphorylation affects the stability of RACK1A proteins. Consistent with this view, we found that the phosphorylation-dead form, RACK1AS122A/T162A, is more stable than the wild-type RACK1A protein (Fig. 6B).
Figure 6.
Immunoblot analysis of RACK1A protein. A, RACK1A protein in wild-type (Col-0) and transgenic plants. Total proteins were extracted from leaves of 6-week-old plants and loaded to an SDS-PAGE gel. Anti-RACK1A peptide antibodies were used for immunoblot analysis. SA/TA, RACK1AS122A/T162A; SD/TE, RACK1AS122D/T162E. B, The stability of RACK1A protein. Total proteins were extracted from 1-week-old Arabidopsis seedlings of Col-0 and PRACK1A::RACK1AS122A/T162A-YFP transgenic plants (in rack1a-2 mutant background) grown in one-half-strength Murashige and Skoog liquid medium. Anti-RACK1A peptide antibodies were used for immunoblot analysis. The same membrane was blotted with anti-Arabidopsis GTP-binding protein α subunit1 (GPA1) antibodies as a loading control. Lanes 1 and 2, Col-0 without cycloheximide (CHX) treatment; lanes 3 to 5, Col-0 treated with 70 µM CHX for 6 h; lane 6, PRACK1A::RACK1AS122A/T162A-YFP without CHX treatment; and lanes 7 to 9, PRACK1A::RACK1AS122A/T162A-YFP treated with CHX for 6 h.
RACK1 Acts Downstream of WNK8
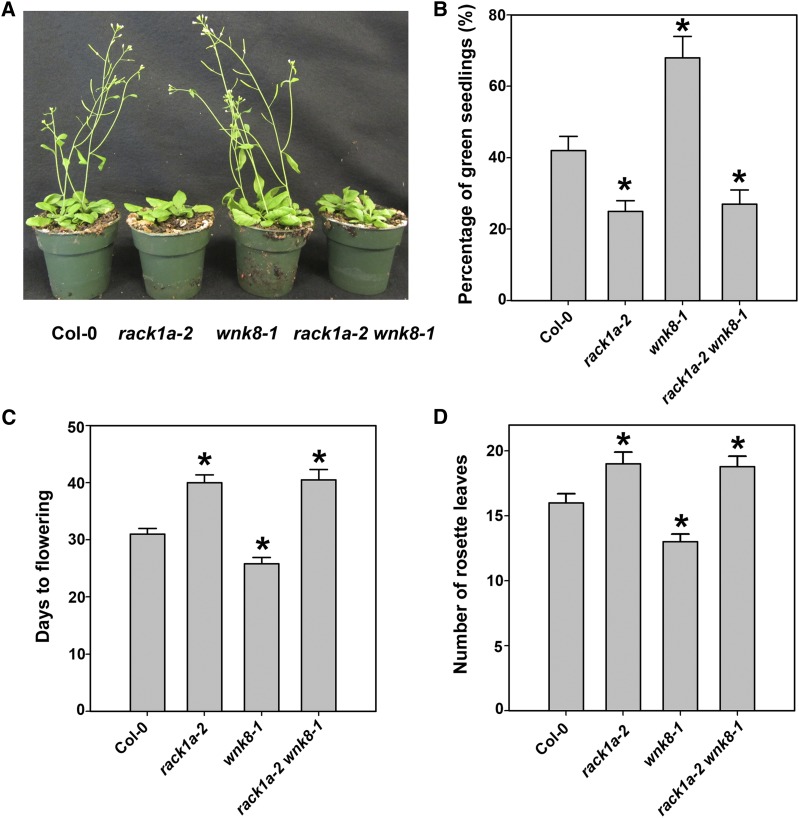
We next tested genetic interaction between RACK1A and WNK8. rack1a-2 (Chen et al., 2006b) and wnk8-1 (Wang et al., 2008), loss-of-function mutations in the corresponding genes (Chen et al., 2006b; Wang et al., 2008), were combined for epistasis analysis. rack1a mutants displayed pleiotropic phenotypes, such as impairment in plant hormone signaling, stress responses, and developmental processes (Chen et al., 2006b; Guo and Chen, 2008; Guo et al., 2009a, 2009b; Guo et al., 2011). In contrast, the adult wnk8 plants have wild-type morphology (Wang et al., 2008). Morphologically, the rack1a wnk8 double mutant phenocopied the rack1a single mutant (Fig. 7A), suggesting that the rack1a-2 null allele is epistatic to the wnk8-1 null allele. To further define the genetic relationship between RACK1A and WNK8, we analyzed one opposing developmental phenotype (flowering time) and one opposing conditional phenotype (Glc sensitivity) reported for rack1a-2 and wnk8-1 single mutants. The rack1a-2 mutant flowered later than the wild type (Chen et al., 2006b), whereas the wnk8-1 mutant flowered earlier (Wang et al., 2008; Park et al., 2011). The rack1a-2 mutant was hypersensitive to Glc (Fig. 7B), whereas wnk8-1 mutant was shown previously to be hyposensitive to Glc (Urano et al., 2012; Fu et al., 2014). These two opposing phenotypes of rack1a-2 and wnk8-1 single mutants provided an efficient way to determine the genetic relationship between rack1a-2 and wnk8-1. As shown in Figure 7, rack1a-2 wnk8-1 double mutant phenocopied rack1a-2 single mutant’s Glc hypersensitivity and late flowering phenotypes. Consistent with the flowering phenotypes, the transcripts of flowering marker genes CONSTANS (CO) and FLOWERING LOCUS T (FT; Putterill et al., 1995; Kardailsky et al., 1999; Kobayashi et al., 1999) were up-regulated in wnk8-1 single mutant but down-regulated in rack1a-2 single mutant and rack1a-2 wnk8-1 double mutant (Supplemental Fig. S3). These results indicate that the rack1a-2 null allele is epistatic to the wnk8-1 null allele and suggest that RACK1 and WNK8 work in the same pathway to regulate flowering and Glc responsiveness.
Figure 7.
rack1a wnk8 double mutants. A, Five-week-old rackla wnk8 double mutants. Plants were grown under a 14-h-light/10-h-dark photoperiod. B, Glc sensitivity phenotype determined by the percentage of green seedlings. Seeds from the Col-0 wild type and mutants were germinated on one-half-strength Murashige and Skoog medium with 1% or 6% Glc. The percentages of green seedlings were recorded 10 d after seeds had been transferred to germination conditions with 6% Glc. At 1% Glc, the percentages of green seedlings for all genotypes were 100%. Shown are means of three biological replicates ± se. C, Flowering phenotype determined by the number of days to flowering. Shown are means of a minimum of 10 plants ± se. D, Flowering phenotype determined by the number of rosette leaves upon bolting. Shown are means of a minimum of 10 plants ± se. *, Significant difference from Col-0, P < 0.05.
DISCUSSION
We showed that RACK1 proteins are phosphorylated by WNK8 and that the phosphorylation negatively affects RACK1 function. MS analysis revealed that Ser-122 and Thr-162 are phosphorylated sites and that these two sites are conserved in all three Arabidopsis RACK1 proteins (Figs. 3 and 4; Supplemental Fig. S1). Mutational analysis suggested that Ser-122 is necessary and sufficient for the phosphorylation of RACK1 proteins (Fig. 4). Ser-122 is conserved in RACK1 proteins from all species analyzed, including human, yeast, fruit fly, and plant, whereas Thr-162 is plant specific (Supplemental Fig. S1). Because a mutation at Thr-162 has a minimal effect on the phosphorylation level of RACK1A protein (Fig. 4), the impact of phosphorylation at Thr-162 is unknown. Our results also do not rule out the possibility that other phosphorylation sites may exist. For example, mammalian RACK1 is phosphorylated by protein Tyr kinases at Tyr-52, Tyr-228, and Tyr-246 (Chang et al., 2002; Kiely et al., 2009), and these sites are conserved in all plant RACK1 proteins examined (Supplemental Fig. S1).
RACK1 and WNK8 coordinate flowering and Glc responsiveness (Fig. 7). In addition to interacting with and phosphorylating RACK1 proteins, WNK8 interacts with and phosphorylates other proteins, such as subunit C of vacuolar H+-ATPase (Hong-Hermesdorf et al., 2006) and regulator of G-protein signaling1 (Urano et al., 2012).
Transgenic lines expressing the nonphosphorylatable form (RACK1AS122A/T162A) of RACK1A but not the phosphomimetic form (RACK1AS122D/T162E) rescued rack1a mutant (Fig. 5), suggesting that the phosphorylated RACK1A was functionally inactive. Furthermore, phosphorylation of RACK1A negatively affects its function, possibly by decreasing RACK1A protein stability (Fig. 6). However, we did not detect a difference in RACK1A protein abundance between wnk8 mutant and the wild type (Supplemental Fig. S4), implying that endogenous RACK1A protein is also subject to other forms of posttranslational modifications that counteract the impact of phosphorylation by WNK8. Nonetheless, we found that the phosphorylation-dead form, RACK1AS122A/T162A, is more stable than the wild-type RACK1A (Fig. 6B), supporting that phosphorylation affects the stability of RACK1A protein.
In summary, we provide biochemical evidence that RACK1 is a substrate of WNK8. Genetic analysis suggests that RACK1 acts downstream of WNK8 in the same pathway to regulate flowering and Glc responsiveness. Furthermore, phosphorylation negatively regulates the function of RACK1 protein by rendering the protein unstable. These findings provide unique insights into the molecular mechanism of action of RACK1 and open a unique window to dissect RACK1 signaling pathways.
MATERIALS AND METHODS
Plant Materials
The rack1a-2 (Chen et al., 2006b) and wnk8-1 (Wang et al., 2008) mutants were described previously. The rack1a-2 wnk8-1 double mutant was generated by crossing wnk8-1 mutant with rack1a-2 mutant, and double mutants homozygous for the rack1a-2 and wnk8-1 loci were isolated by PCR genotyping.
Y2H Assays
Full-length ORFs of RACK1A, RACK1B, and RACK1C were cloned into LexA-BD vector pBTM117c (Wanker et al., 1997). Full-length ORFs of WNK1, WNK8, and WNK10 were cloned into Gal4-AD vector pGAD10 (GenBank accession no. U13188). Growth of yeast (Saccharomyces cerevisiae) on SD-Leu-Trp medium was used as a control of successful transformation, and growth of yeasts on selection medium SD-Leu-Trp-His-Ura plus 1 mm 3-amino-1,2,4-triazole was used to determine positive interactions between bait and prey proteins.
BiFC
The full-length ORFs of RACK1 proteins were cloned into pCL112 (N-terminal split-nYFP tag). Approximately 4- to 5-week-old tobacco (Nicotiana tabacum) leaves were infected with Agrobacterium tumifaciens harboring pCL113-WNK1, -WNK8, -WNK10, or -P31 (At3G01290, a negative control) and pCL112-RACK1A, -RACK1B, or -RACK1C to express split nYFP- and cYFP-tagged proteins. BiFC was performed as described previously (Klopffleisch et al., 2011) with a few modifications. An internal transformation control (mitochondrial marker; mCherry-mt-rk) was used to confirm infiltration of multiple plasmids (p19 gene silencing suppressor, mt-rk, and two BiFC halves). Leaf epidermis was imaged using a confocal laser-scanning microscope (LSM710; Zeiss) with an Apochromat 40× water-emersion objective (numerical aperture = 1.2). YFP and mCherry were excited by a 514-nm argon laser and a 560-nm diode laser, and their fluorescence was detected at 526 to 569 and 565 to 621 nm, respectively.
Recombinant Proteins
The full-length ORFs of RACK1 proteins were subcloned to pDEST15 (N-terminal glutathione S-transferase [GST] tag). Recombinant GST-tagged RACK1 proteins were expressed in ArcticExpress RP (Agilent Technologies) with 0.5 mm isopropylthio-β-galactoside at 12°C and solubilized in buffer A (90 mm Tris-HCl [pH 7.5], 100 mm NaCl, 1 mm EDTA, 5 mm 2-Mercaptoethanol, 1 mm phenylmethylsulfonyl fluoride [PMSF], 2 µg mL−1 leupeptin, 0.25 mg mL−1 lysozyme, and 0.2% [w/v] nonyl phenoxypolyethoxylethanol). The tagged proteins were captured from the soluble fraction with glutathione-Sepharose 4B (GE Healthcare), washed with buffer A containing 500 mm NaCl and 0.1% sodium cholate, and eluted with buffer B (100 mm Tris-HCl [pH 8.8], 200 mm NaCl, 5 mm dithiothreitol [DTT], 20 mm glutathione, 1 µg mL−1 leupeptin, and 1 mm PMSF). The purified proteins were dialyzed with buffer C (20 mm Tris-HCl [pH 7.5], 50 mm NaCl, 1 mm MgCl2, 1 mm EDTA, 1 mm DTT, and 1 mm PMSF) and stored at −80°C. The expression and purification of WNK8 kinase have been described previously (Urano et al., 2012).
Phosphorylation mutations of RACK1A (S122D, T162E, S122D/T162E, and S122A/T162A) were generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (Invitrogen). Mutant proteins (S122D, T162E, and S122D/T162E) were purified in the same manner as the wild-type RACK1 proteins described above.
In Vitro Phosphorylation Assay
Seventy-five picomoles of GST, GST-RACK1A, GST-RACK1B, or GST-RACK1C was incubated with 0.05 pmol of GST or GST-WNK8 in 15 µL of buffer D (50 mm Tris-HCl [pH 7.0], 10 mm MgCl2, 5 mm MnCl2, 2 mm DTT, 1 mm PMSF, and 1 µg mL−1 leupeptin) containing 0.2 mm [γ-32P]ATP for 6 h at room temperature. The reactions were terminated by adding Laemmli sample buffer, and proteins were separated by SDS-PAGE. Incorporation of 32P into proteins was visualized and quantified with a phosphoimage analyzer.
MS
GST-RACK1C protein was phosphorylated by WNK8 and analyzed by SDS-PAGE as described above. The gel band of GST-RACK1C was excised from the polyacrylamide gel, and the proteins in the band were digested using trypsin. The peptides were extracted, and the solution was lyophilized. A fraction of the sample was analyzed by matrix-assisted laser-desorption ionization time of flight/time of flight (MALDI-TOF/TOF), and the remaining was used for analysis by LC-electrospray ionization-MS/MS. A 90-min gradient was used, and MS analysis was performed with an LTQ Orbitrap XL mass spectrometer (ThermoFisher Scientific). MS spectra were searched using the MASCOT algorithm (Perkins et al., 1999) against the nonredundant National Center for Biotechnology Information database and the RACK1C-specific database with possible phosphorylation of Ser, Thr, and Tyr. The phosphorylated peptides were identified based on MASCOT results, and the phosphorylation sites were defined by manual inspection of the spectra.
Flowering Assay
The flowering phenotypes were determined by counting the number of rosette leaves upon bolting (florescence stem > 0.5 cm) and recording the number of days to bolting. Plants of Col-0, rack1-2, wnk8-2, and rack1-2 wnk8-1 were grown side by side under identical conditions of 23°C ± 1°C with a 14-h-light/10-h-dark photoperiod (approximately 125 µmol photons m−2 s−1). A minimum of 10 plants each genotype was used for the assay.
For RACK1 phosphorylation mutants, a 10-h-light/14-h-dark photoperiod (approximately 125 µmol photons m−2 s−1) was used for the flowering assay.
Glc Sensitivity Assay
Seeds from Col-0, rack1a-2, wnk8-1, rack1-2 wnk8-1, and RACK1 phosphorylation mutants were sown on one-half-strength Murashige and Skoog medium with 0.7% phytoagar plus 1% (w/v) or 6% (w/v) Glc, placed at 4°C for stratification for 2 d, and then transferred to germination conditions at 23°C ± 1°C with continuous light (approximately 100 µmol photons m−2 s−1). The percentages of green seedlings were scored 10 d after seeds had been transferred to germination conditions. Approximately 50 seeds were used for each genotype in each petri dish with three biological replicates.
Genetic Complementation
The RACK1AS122D/T162E and RACK1AS122A/T162A mutant lines were generated as follows. The genomic DNA containing RACK1A promoter and RACK1A genomic sequence (Guo et al., 2009a) was cloned into the pENTR-D-TOPO vector (Invitrogen) and then transferred into the pGWB40 plant destination vector (Nakagawa et al., 2009), which fuses YFP at the carboxyl terminus of RACK1A protein. The QuikChange Lightning Site-Directed Mutagenesis Kit was used to introduce mutations at Ser-122 and Thr-162 of RACK1A protein. pGWB40 vectors containing PRACK1A::RACK1AS122D/T162E or PRACK1A:: RACK1AS122A/T162A were transformed into rack1a-2 mutant background through Agrobacterium tumifaciens-mediated transformation. The expression of RACK1A mutant genes was analyzed by qRT-PCR and immunoblot.
Genetic complementation of rack1 mutants was determined by counting the number of rosette leaves in a 40-d period. Plants of Col-0, rack1a-2, RACK1AS122D/T162E, and RACK1AS122A/T162A were grown side by side under identical conditions (23°C ± 1°C, 10-h-light/14-h-dark photoperiod, approximately 125 µmol photons m−2 s−1). A minimum of 10 plants of each genotype were used for the assay.
qRT-PCR
For qRT analysis of the expression of RACK1AS122D/T162E and RACK1AS122A/T162A transgene in rack1a-2 mutant background, total RNA was extracted from rosette leaves of 4-week-old plants using an Invisorb Spin Plant Mini Kit (Stratec Molecular). Two micrograms of total RNA was reversely transcribed to complementary DNA using Fermentas RevertAid Reverse Transcriptase (Thermo Scientific). The expression of ACTIN8 was used as a control.
For qRT analysis of the transcript levels of flowering marker genes FT and CO in rack1a-2 and wnk8-1 single and double mutants compared with those in Col-0, RNA was isolated from fully expanded rosette leaves of plants that had been grown for 51 d under an 8-h-light/16-h-dark photoperiod. qRT was performed by using StepOnePlus (Applied Biosystems), Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific), and complementary DNA corresponding to 80 ng of RNA in a total volume of 25 µL. The following cycling conditions were used for PCR: 10 min at 95°C and 35 cycles of 15 s at 95°C and 60 s at 60°C. The transcript level of FT and CO was normalized against that of ACTIN2. The sequences of FT and CO gene-specific primers used for qRT were the same as the ones reported by Park et al. (2011).
Immunoblot Analyses
The total protein lysates were prepared from rosette leaves of 6-week-old Arabidopsis (Arabidopsis thaliana) plants or 1-week-old seedlings in buffer E (50 mm Tris-HCl [pH 7.5], 50 mm NaCl, 5 mm EGTA, 2 mm DTT, 1% Triton-X100, and a protease inhibitor cocktail; P9599; Sigma). One milliliter of buffer E was added per 0.1 g of plant tissue. Twenty microliters of centrifuged protein extracts was subjected to SDS-PAGE followed by western blotting with anti-RACK1A antibody raised against the sequence K273VDLKAEAKADNSGPAAT291 in Arabidopsis RACK1A (Chang et al., 2005). Note that the epitope region is highly divergent among three Arabidopsis RACK1 proteins. In the protein stability assays, the same membrane was blotted with anti-GPA1 peptide antibodies (Chen et al., 2006a) as a loading control.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At1g18080 (RACK1A), At1g48630 (RACK1B), At3g18130 (RACK1C), At3g04910 (WNK1), At5g41990 (WNK8), and At1g64630 (WNK10).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Alignment of RACK1 amino acid sequences.
Supplemental Figure S2. Peptides detected in MS analysis.
Supplemental Figure S3. qRT-PCR analysis of the transcript level of flowering marker genes CO and FT.
Supplemental Figure S4. Immunoblot analysis of RACK1A protein in wnk8 mutant and wild-type background.
Supplementary Material
Acknowledgments
We thank Dr. David Smalley for determining phosphorylation sites of RACK1, Jing Yang and Ariko Urano for assistance in gene cloning and plant transformation, and Dr. Julia Bailey-Serres for providing anti-RACK1A antiserum.
Glossary
- BiFC
bimolecular fluorescence complementation
- Col-0
Columbia-0
- DTT
dithiothreitol
- MS
mass spectrometry
- ORF
open reading frame
- PMSF
phenylmethylsulfonyl fluoride
- qRT
quantitative reverse transcription
- Y2H
yeast two-hybrid
Footnotes
This work was supported by the National Institute of General Medical Sciences (grant no. R01GM065989), the U.S. Department of Energy (grant no. DE–FG02–05er15671), the National Science Foundation (grant nos. MCB–0723515 and MCB–0718202 to A.M.J.), the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory (to J.-G.C.), the Oak Ridge National Laboratory (managed by UT-Battelle, LLC for the U.S. Department of Energy under contract no. DE–AC05–00OR22725), the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (technical support through grant no. DE–FG02–05er15671 to A.M.J.), and the China Scholarship Council (scholarship to X.W.).
Articles can be viewed without a subscription.
References
- Adams DR, Ron D, Kiely PA (2011) RACK1, A multifaceted scaffolding protein: structure and function. Cell Commun Signal 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BY, Harte RA, Cartwright CA (2002) RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene 21: 7619–7629 [DOI] [PubMed] [Google Scholar]
- Chang IF, Szick-Miranda K, Pan S, Bailey-Serres J (2005) Proteomic characterization of evolutionarily conserved and variable proteins of Arabidopsis cytosolic ribosomes. Plant Physiol 137: 848–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Gao Y, Jones AM (2006a) Differential roles of Arabidopsis heterotrimeric G-protein subunits in modulating cell division in roots. Plant Physiol 141: 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Ullah H, Temple B, Liang J, Guo J, Alonso JM, Ecker JR, Jones AM (2006b) RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J Exp Bot 57: 2697–2708 [DOI] [PubMed] [Google Scholar]
- Dwane S, Durack E, O’Connor R, Kiely PA (2014) RACK1 promotes neurite outgrowth by scaffolding AGAP2 to FAK. Cell Signal 26: 9–18 [DOI] [PubMed] [Google Scholar]
- Fennell H, Olawin A, Mizanur RM, Izumori K, Chen JG, Ullah H (2012) Arabidopsis scaffold protein RACK1A modulates rare sugar d-allose regulated gibberellin signaling. Plant Signal Behav 7: 1407–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Lim S, Urano D, Tunc-Ozdemir M, Phan NG, Elston TC, Jones AM (2014) Reciprocal encoding of signal intensity and duration in a glucose-sensing circuit. Cell 156: 1084–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavalisco P, Wilson D, Kreitler T, Lehrach H, Klose J, Gobom J, Fucini P (2005) High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome. Plant Mol Biol 57: 577–591 [DOI] [PubMed] [Google Scholar]
- Guo J, Chen JG (2008) RACK1 genes regulate plant development with unequal genetic redundancy in Arabidopsis. BMC Plant Biol 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liang J, Chen JG (2007) RACK1: a versatile scaffold protein in plants? Int J Plant Dev Biol 1: 95–105 [Google Scholar]
- Guo J, Wang J, Xi L, Huang WD, Liang J, Chen JG (2009a) RACK1 is a negative regulator of ABA responses in Arabidopsis. J Exp Bot 60: 3819–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wang S, Valerius O, Hall H, Zeng Q, Li JF, Weston DJ, Ellis BE, Chen JG (2011) Involvement of Arabidopsis RACK1 in protein translation and its regulation by abscisic acid. Plant Physiol 155: 370–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wang S, Wang J, Huang WD, Liang J, Chen JG (2009b) Dissection of the relationship between RACK1 and heterotrimeric G-proteins in Arabidopsis. Plant Cell Physiol 50: 1681–1694 [DOI] [PubMed] [Google Scholar]
- Hong-Hermesdorf A, Brüx A, Grüber A, Grüber G, Schumacher K (2006) A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett 580: 932–939 [DOI] [PubMed] [Google Scholar]
- Huang CL, Cha SK, Wang HR, Xie J, Cobb MH (2007) WNKs: protein kinases with a unique kinase domain. Exp Mol Med 39: 565–573 [DOI] [PubMed] [Google Scholar]
- Ishida S, Takahashi Y, Nagata T (1993) Isolation of cDNA of an auxin-regulated gene encoding a G protein β subunit-like protein from tobacco BY-2 cells. Proc Natl Acad Sci USA 90: 11152–11156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Flores T, Guillén G, Alvarado-Affantranger X, Lara-Flores M, Sánchez F, Villanueva MA (2011) PvRACK1 loss-of-function impairs cell expansion and morphogenesis in Phaseolus vulgaris L. root nodules. Mol Plant Microbe Interact 24: 819–826 [DOI] [PubMed] [Google Scholar]
- Islas-Flores T, Guillén G, Islas-Flores I, San Román-Roque C, Sánchez F, Loza-Tavera H, Bearer EL, Villanueva MA (2009) Germination behavior, biochemical features and sequence analysis of the RACK1/arcA homolog from Phaseolus vulgaris. Physiol Plant 137: 264–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Flores T, Guillén G, Sánchez F, Villanueva MA (2012) Changes in RACK1 expression induce defects in nodulation and development in Phaseolus vulgaris. Plant Signal Behav 7: 132–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kiely PA, Baillie GS, Barrett R, Buckley DA, Adams DR, Houslay MD, O’Connor R (2009) Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for insulin-like growth factor I-mediated regulation of focal adhesion kinase. J Biol Chem 284: 20263–20274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopffleisch K, Phan N, Augustin K, Bayne RS, Booker KS, Botella JR, Carpita NC, Carr T, Chen JG, Cooke TR, et al. (2011) Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol Syst Biol 7: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Komatsu S, Abbasi F, Kobori E, Fujisawa Y, Kato H, Iwasaki Y (2005) Proteomic analysis of rice embryo: an approach for investigating Galpha protein-regulated proteins. Proteomics 5: 3932–3941 [DOI] [PubMed] [Google Scholar]
- Komatsu S, Hiraga S, Nouri MZ (2014) Analysis of flooding-responsive proteins localized in the nucleus of soybean root tips. Mol Biol Rep 41: 1127–1139 [DOI] [PubMed] [Google Scholar]
- Kundu N, Dozier U, Deslandes L, Somssich IE, Ullah H (2013) Arabidopsis scaffold protein RACK1A interacts with diverse environmental stress and photosynthesis related proteins. Plant Signal Behav 8: e24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ (2002) The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol 62: 1261–1273 [DOI] [PubMed] [Google Scholar]
- McKhann HI, Frugier F, Petrovics G, de la Peña TC, Jurkevitch E, Brown S, Kondorosi E, Kondorosi A, Crespi M (1997) Cloning of a WD-repeat-containing gene from alfalfa (Medicago sativa): a role in hormone-mediated cell division? Plant Mol Biol 34: 771–780 [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Khaner H, Lopez J (1991) Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA 88: 3997–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Ishiguro S, Kimura T (2009) Gateway vectors for plant transformation. Plant Biotechnol 26: 275–284 [Google Scholar]
- Nakamichi N, Murakami-Kojima M, Sato E, Kishi Y, Yamashino T, Mizuno T (2002) Compilation and characterization of a novel WNK family of protein kinases in Arabiodpsis thaliana with reference to circadian rhythms. Biosci Biotechnol Biochem 66: 2429–2436 [DOI] [PubMed] [Google Scholar]
- Nakashima A, Chen L, Thao NP, Fujiwara M, Wong HL, Kuwano M, Umemura K, Shirasu K, Kawasaki T, Shimamoto K (2008) RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. Plant Cell 20: 2265–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik K, Bucholc M, Anielska-Mazur A, Lipko A, Kujawa M, Modzelan M, Augustyn A, Kraszewska E (2011) Arabidopsis thaliana Nudix hydrolase AtNUDT7 forms complexes with the regulatory RACK1A protein and Ggamma subunits of the signal transducing heterotrimeric G protein. Acta Biochim Pol 58: 609–616 [PubMed] [Google Scholar]
- Park HY, Lee SY, Seok HY, Kim SH, Sung ZR, Moon YH (2011) EMF1 interacts with EIP1, EIP6 or EIP9 involved in the regulation of flowering time in Arabidopsis. Plant Cell Physiol 52: 1376–1388 [DOI] [PubMed] [Google Scholar]
- Perennes C, Glab N, Guglieni B, Doutriaux MP, Phan TH, Planchais S, Bergounioux C (1999) Is arcA3 a possible mediator in the signal transduction pathway during agonist cell cycle arrest by salicylic acid and UV irradiation? J Cell Sci 112: 1181–1190 [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Ron D, Adams DR, Baillie GS, Long A, O’Connor R, Kiely PA (2013) RACK1 to the future—a historical perspective. Cell Commun Signal 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D (1994) Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G proteins. Proc Natl Acad Sci USA 91: 839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth C, Willing EM, Rausch S, Schneeberger K, Laubinger S (2013) RACK1 scaffold proteins influence miRNA abundance in Arabidopsis. Plant J 76: 433–445 [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Eulgem T (2010) The Arabidopsis defense component EDM2 affects the floral transition in an FLC-dependent manner. Plant J 62: 518–528 [DOI] [PubMed] [Google Scholar]
- Ullah H, Scappini EL, Moon AF, Williams LV, Armstrong DL, Pedersen LC (2008) Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana. Protein Sci 17: 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano D, Phan N, Jones JC, Yang J, Huang J, Grigston J, Taylor JP, Jones AM (2012) Endocytosis of the seven-transmembrane RGS1 protein activates G-protein-coupled signalling in Arabidopsis. Nat Cell Biol 14: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yu J, Zhu D, Chang Y, Zhao Q (2014) Maize ZmRACK1 is involved in the plant response to fungal phytopathogens. Int J Mol Sci 15: 9343–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu K, Liao H, Zhuang C, Ma H, Yan X (2008) The plant WNK gene family and regulation of flowering time in Arabidopsis. Plant Biol (Stuttg) 10: 548–562 [DOI] [PubMed] [Google Scholar]
- Wanker EE, Rovira C, Scherzinger E, Hasenbank R, Wälter S, Tait D, Colicelli J, Lehrach H (1997) HIP-I: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum Mol Genet 6: 487–495 [DOI] [PubMed] [Google Scholar]
- Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH (2000) WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801 [DOI] [PubMed] [Google Scholar]
- Zhang D, Chen L, Li D, Lv B, Chen Y, Chen J, XuejiaoYan, Liang J (2014) OsRACK1 is involved in abscisic acid- and H2O2-mediated signaling to regulate seed germination in rice (Oryza sativa, L.). PLoS ONE 9: e97120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.